Abstract
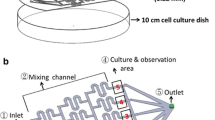
The ideal cancer therapeutic strategy is to inhibit the tumor with minimal influence on the normal tissue. Recently, applying an alternating electric field for inhibiting tumor was developed; but, it has not been adopted to be one of the regular therapeutic options. More basic scientific evidence is needed to clarify the efficacy and safety. In the current study, co-culturing cancer cells and normal cells under the electrical stimulation was conducted to provide evidence of this novel cancer therapy. A microfluidic cell culture biochip has been developed and consisted of nine culture chambers incorporating with stimulating electrodes. Cells cultured in the chamber received uniform electric field and cell viability was studied during the culture course. The electric field perturbs cell division and the correlation between cell proliferation rate and inhibition effect was studied among five cell lines, i.e., Huh7, HeLa, TW06, BM1, and HEL299. The results confirmed that cells with higher proliferation rate responded to a higher inhibition. In addition, co-culturing cancer cells and normal cells was conducted to mimic in vivo microenvironment that consists of both cancer and stromal cells. The cancer cells and normal cells were respectively transduced with green fluorescent protein and red fluorescent protein in order to differentiate the cells in a same culture chamber. During the culture course, the electric field was applied to the culture chamber and both cells simultaneously received the field. The results indicated that the growth of the cancer cells were inhibited while the normal cells were maintained. These results provided the evidence of the therapeutic efficacy and safety. Moreover, the microfluidic cell culture biochip could be used for the systematic and precise investigations of the cellular responses under the electrical stimulation.
Similar content being viewed by others
References
Kirson, E.D. et al. Disruption of Cancer Cell Replication by Alternating Electric Fields. Cancer Res. 64, 3288–3295 (2004).
Kirson, E.D. et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc. Natl. Acad. Sci. USA 104, 10152–10157 (2007).
Karanam, N.K. et al. Tumor-treating fields elicit a conditional vulnerability to ionizing radiation via the downregulation of BRCA1 signaling and reduced DNA double-strand break repair capacity in non-small cell lung cancer cell lines. Cell Death Dis. 8, e2711 (2017).
Gera, N. et al. Tumor treating fields perturb the localization of septins and cause aberrant mitotic exit. PLoS ONE 10, e125269 (2015).
Giladi, M. et al. Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci. Rep. 5, 18046 (2015).
Kim, E.H., Song, H.S., Yoo, S.H. & Yoon, M. Tumor treating fields inhibit glioblastoma cell migration, invasion and angiogenesis. Oncotarget 7, 65125–65136 (2016).
Stupp, R. et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: A randomized phase III trial of a novel treatment modality. Eur. J. Cancer 28, 2192–2202 (2012).
Lee Villano, L. et al. Delayed response and survival from NovoTTF-100A in recurrent GBM. Med. Oncol. 30, 338 (2013).
Wong, E.T. et al. Response assessment of NovoTTF-100A versus best physician’s choice chemotherapy in recurrent glioblastoma. Cancer Med. 3, 592–602 (2014).
Inui, T. et al. Case report: a non-small cell lung cancer patient treated with GcMAF, sonodynamic therapy and tumor treating field. Anticancer Res. 36, 3767–3770 (2016).
Plessa, M. et al. A phase I/II trial of Tumor Treating Fields (TTFields) therapy in combination with pemetrexed for advanced non-small cell lung cancer. Lung Cancer 81, 445–450 (2013).
Pless, M. & Weinberg, U. Tumor treating fields: concepts, evidence and future. Expert Opin. Investig. Drugs 20, 1099–1106 (2011).
Wang, C.C. et al. Asymmetric cancer-cell filopodium growth induced by electric-fields in a microfluidic culture chip. Lab Chip 11, 695–699 (2011).
Huang, C.W. et al. Electrotaxis of lung cancer cells in a multiple-electric-field chip. Biosens. Bioelectron. 24, 3510–3516 (2009).
Bai, H. et al. DC electric stimulation upregulates angiogenic factors in endothelial cells through activation of VEGF receptors. Cytokine 55, 110–115 (2011).
Zhao, M. et al. Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J. Cell Sci. 117, 397–405 (2004).
Lei, K.F., Lee, I.C., Liu, Y.C. & Wu, Y.C. Successful differentiation of neural stem/progenitor cells cultured on electrically adjustable indium tin oxide (ITO) surface. Langmuir 30, 14241–14249 (2014).
Fuhr, G., Glasser, H., Müller, T. & Schnelle, T. Cell manipulation and cultivation under a.c. electric field influence in highly conductive culture media. Biochim. Biophys. Acta-Gen. Subj. 1201, 353–360 (1994).
Lu, H., Schmidt, M.A. & Jensen, K.F. A microfluidic electroporation device for cell lysis. Lab Chip 5, 23–29 (2005).
Delinasios, J.G. et al. Proliferating fibroblasts and HeLa cells co-cultured in vitro reciprocally influence growth patterns, protein expression, chromatin features and cell survival. Anticancer Res. 35, 1881–1916 (2015).
Miki, Y. et al. The advantages of co-culture over mono cell culture in simulating in vivo environment. J. Steroid. Biochem. Mol. Biol. 131, 68–75 (2012).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lei, K.F., Hsieh, SC., Kuo, RL. et al. Co-Culturing Cancer Cells and Normal Cells in a Biochip under Electrical Stimulation. BioChip J 12, 202–207 (2018). https://doi.org/10.1007/s13206-018-2309-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-018-2309-x