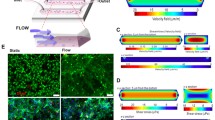
Abstract
Reactive oxygen species (ROS) are known to play an important role in the development of cancer, and many exogenous sources are believed to be related to the formation of ROS. For example, electric field is one of such factors reported to stimulate the production of ROS. Moreover, electric field is also shown to induce cell migration, a phenomenon termed electrotaxis. In this paper, a microfluidic chip was developed for studying the production of ROS and the migration in lung cancer cells under single or coexisting chemical/electrical stimulation. This chip has two unique features: (1) Five relative concentrations of 0, 1/8, 1/2, 7/8, and 1 are achieved in the culture regions; (2) five different strengths of EFs are produced inside these culture areas. Lung cancer cells were seeded inside this biocompatible chip for investigating their response to different concentrations of H2O2, a chemical stimulus known to increase the production of ROS. Then, the effects of honokiol, a chemical stimulus, in combination with electric field, a physical stimulus, on lung cancer cells were examined. Finally, lung cancer cell migration was investigated under single or combined honokiol/electric field treatments. The current microfluidic chip provides an in vitro platform mimicking the physiological condition where cells are under circulating conditions and are subject to controllable chemical/physical stimuli.








Similar content being viewed by others
References
Al-Majed AA, Neumann CM, Brushart TM, Gordon T (2000) Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci 20:2602–2608
Benzi G, Moretti A (1995) Are reactive oxygen species involved in Alzheimer’s disease? Neurobiol Aging 16:661–674
Bruzzese F, Rocco M, Castelli S, Di Gennaro E, Desideri A, Budillon A (2009) Synergistic antitumor effect between vorinostat and topotecan in small cell lung cancer cells is mediated by generation of reactive oxygen species and DNA damage-induced apoptosis. Mol Cancer Ther 8:3075–3087. doi:10.1158/1535-7163.MCT-09-0254
Cassarino DS et al (1997) Elevated reactive oxygen species and antioxidant enzyme activities in animal and cellular models of Parkinson’s disease. Biochim Biophys Acta 1362:77–86
Chen JJW et al (2001) Global analysis of gene expression in invasion by a lung cancer model. Cancer Res 61:5223–5230
Cheng JY, Wei CW, Hsu KH, Young TH (2004) Direct-write laser micromachining and universal surface modification of PMMA for device development. Sens Actuators B Chem 99:186–196. doi:10.1016/j.snb.2003.10.022
Cheng JY, Yen MH, Wei CW, Chuang YC, Young TH (2005) Crack-free direct-writing on glass using a low-power UV laser in the manufacture of a microfluidic chip. J Micromech Microeng 15:1147–1156. doi:10.1088/0960-1317/15/6/005
Cheng JY, Yen MH, Kuo CT, Young TH (2008) A transparent cell-culture microchamber with a variably controlled concentration gradient generator and flow field rectifier. Biomicrofluidics. doi:10.1063/1.2952290
Djamgoz MBA, Mycielska M, Madeja Z, Fraser SP, Korohoda W (2001) Directional movement of rat prostate cancer cells in direct-current electric field: involvement of voltage-gated Na+ channel activity. J Cell Sci 114:2697–2705
Duffy DC, McDonald JC, Schueller OJ, Whitesides GM (1998) Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal Chem 70:4974–4984. doi:10.1021/ac980656z
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247. doi:10.1038/35041687
Fried LE, Arbiser JL (2009) Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid Redox Signal 11:1139–1148
Gamboa OL, Pu J, Townend J, Forrester JV, Zhao M, McCaig C, Lois N (2010) Electrical estimulation of retinal pigment epithelial cells. Exp Eye Res 91:195–204. doi:10.1016/j.exer.2010.04.018
Hammerick KE, Longaker MT, Prinz FB (2010) In vitro effects of direct current electric fields on adipose-derived stromal cells. Biochem Biophys Res Commun 397:12–17. doi:10.1016/j.bbrc.2010.05.003
Harrison DJ, Manz A, Fan ZH, Ludi H, Widmer HM (1992) Capillary electrophoresis and sample injection systems integrated on a planar glass chip. Anal Chem 64:1926–1932
Hou HS, Tsai HF, Chiu HT, Cheng JY (2014) Simultaneous chemical and electrical stimulation on lung cancer cells using a multichannel-dual-electric-field chip. Biomicrofluidics 8:052007
Huang CW, Cheng JY, Yen MH, Young TH (2009) Electrotaxis of lung cancer cells in a multiple-electric-field chip. Biosens Bioelectron 24:3510–3516. doi:10.1016/j.bios.2009.05.001
Hughes-Alford SK, Lauffenburger DA (2012) Quantitative analysis of gradient sensing: towards building predictive models of chemotaxis in cancer. Curr Opin Cell Biol 24:284–291
Hulsmans M, Van Dooren E, Holvoet P (2012) Mitochondrial reactive oxygen species and risk of atherosclerosis. Curr Atheroscler Rep 14:264–276. doi:10.1007/s11883-012-0237-0
Kao YC et al. (2014) Modulating chemotaxis of lung cancer cells by using electric fields in a microfluidic device. Biomicrofluidics 8:024107
Korohoda W, Mycielska M, Janda E, Madeja Z (2000) Immediate and long-term galvanotactic responses of Amoeba proteus to DC electric fields. Cell Motil Cytoskel 45:10–26
Lee JH, Kim HL, Lee MH, You KE, Kwon BJ, Seo HJ, Park JC (2012) Asiaticoside enhances normal human skin cell migration, attachment and growth in vitro wound healing model. Phytomedicine 19:1223–1227
Lees JG et al (2013) Tropomyosin regulates cell migration during skin wound healing. J Invest Dermatol 133:1330–1339
Li J, Nandagopal S, Wu D, Romanuik SF, Paul K, Thomson DJ, Lin F (2011) Activated T lymphocytes migrate toward the cathode of DC electric fields in microfluidic devices. Lab Chip 11:1298–1304. doi:10.1039/C0lc00371a
Li J, Zhu L, Zhang M, Lin F (2012) Microfluidic device for studying cell migration in single or co-existing chemical gradients and electric fields. Biomicrofluidics 6:024121
Lin F, Baldessari F, Gyenge CC, Sato T, Chambers RD, Santiago JG, Butcher EC (2008) Lymphocyte electrotaxis in vitro and in vivo. J Immunol 181:2465–2471
Little CD (2013) Integrating cell and tissue motion patterns during early embryogenesis: How much “Cell Migration” really occurs? Faseb J 27(Meeting Abstract Supplement):312.3
Lo KY, Zhu Y, Tsai HF, Sun YS (2013) Effects of shear stresses and antioxidant concentrations on the production of reactive oxygen species in lung cancer cells. Biomicrofluidics 7:64108. doi:10.1063/1.4836675
Madhyastha H, Madhyastha R, Nakajima Y, Omura S, Maruyama M (2012) Regulation of growth factors-associated cell migration by C-phycocyanin scaffold in dermal wound healing. Clin Exp Pharmacol Physiol 39:13–19
Maizels Y, Rosenfeld YBZ, Oberman F, Fridlender Z, Yisraeli JK (2014) Roles for Vickz proteins in cell migration cancer progression and metastasis. Anticancer Res 34:6249
Manz A, Harrison DJ, Verpoorte EMJ, Fettinger JC, Paulus A, Ludi H, Widmer HM (1992) Planar chips technology for miniaturization and integration of separation techniques into monitoring systems—capillary electrophoresis on a chip. J Chromatogr 593:253–258
Mazzag BC, Zhulin IB, Mogilner A (2003) Model of bacterial band formation in aerotaxis. Biophys J 85:3558–3574
McCaig CD, Rajnicek AM, Song B, Zhao M (2005) Controlling cell behavior electrically: current views and future potential. Physiol Rev 85:943–978
McDonald JC, Whitesides GM (2002) Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc Chem Res 35:491–499. doi:10.1021/Ar010110q
Minc N, Chang F (2010) Electrical control of cell polarization in the fission yeast Schizosaccharomyces pombe. Curr Biol 20:710–716. doi:10.1016/j.cub.2010.02.047
Mori I, Ohshima Y (1995) Neural regulation of thermotaxis in Caenorhabditis elegans. Nature 376:344–348. doi:10.1038/376344a0
Nediani C, Raimondi L, Borchi E, Cerbai E (2011) Nitric oxide/reactive oxygen species generation and nitroso/redox imbalance in heart failure: from molecular mechanisms to therapeutic implications. Antioxid Redox Signal 14:289–331. doi:10.1089/ars.2010.3198
Nuccitelli R (2003) Endogenous electric fields in embryos during development, regeneration and wound healing. Radiat Prot Dosim 106:375–383
Popp F, Armitage JP, Schuler D (2014) Polarity of bacterial magnetotaxis is controlled by aerotaxis through a common sensory pathway. Nat Commun 5:5398
Pu J, McCaig CD, Cao L, Zhao ZQ, Segall JE, Zhao M (2007) EGF receptor signalling is essential for electric-field-directed migration of breast cancer cells. J Cell Sci 120:3395–3403. doi:10.1242/Jcs.002774
Roussos ET, Condeelis JS, Patsialou A (2011) Chemotaxis in cancer. Nat Rev Cancer 11:573–587
Roxanis I, Russell J, Lavery B (2013) Pattern of tumour cell migration as a prognostic parameter for lymph node metastasis in HER2-enriched breast cancer. Mod Pathol 26:65a–66a
Sato MJ, Ueda M, Takagi H, Watanabe TM, Yanagida T, Ueda M (2007) Input–output relationship in galvanotactic response of Dictyostelium cells. Biosystems 88:261–272. doi:10.1016/j.biosystems.2006.06.008
Shen JL et al (2010) Honokiol and magnolol as multifunctional antioxidative molecules for dermatologic disorders. Molecules 15:6452–6465
Shi JX, Liu HC, Yao F, Zhong CX, Zhao H (2014) Upregulation of mediator MED23 in non-small-cell lung cancer promotes the growth, migration, and metastasis of cancer cells. Tumor Biol 35:12005–12013
Shih JY et al (2001) Collapsin response mediator protein-1 and the invasion and metastasis of cancer cells. J Natl Cancer Inst 93:1392–1400. doi:10.1093/jnci/93.18.1392
Singh T, Katiyar SK (2013) Honokiol inhibits non-small cell lung cancer cell migration by targeting PGE(2)-mediated activation of beta-catenin signaling. PLoS ONE 8(4):e60749
Singh T, Prasad R, Katiyar SK (2013) Inhibition of class I histone deacetylases in non-small cell lung cancer by honokiol leads to suppression of cancer cell growth and induction of cell death in vitro and in vivo. Epigenetics 8:54–65
Sun YS, Peng SW, Lin KH, Cheng JY (2012) Electrotaxis of lung cancer cells in ordered three-dimensional scaffolds. Biomicrofluidics 6:014102
Tai G, Reid B, Cao L, Zhao M (2009) Electrotaxis and wound healing: experimental methods to study electric fields as a directional signal for cell migration. Methods Mol Biol 571:77–97. doi:10.1007/978-1-60761-198-1_5
Terry SC, Jerman JH, Angell JB (1979) Gas-chromatographic air analyzer fabricated on a silicon-wafer. IEEE Trans Electron Device 26:1880–1886
Tsai HF, Peng SW, Wu CY, Chang HF, Cheng JY (2012) Electrotaxis of oral squamous cell carcinoma cells in a multiple-electric-field chip with uniform flow field. Biomicrofluidics 6:034116
Tsai HF, Huang CW, Chang HF, Chen JJW, Lee CH, Cheng JY (2013) Evaluation of EGFR and RTK signaling in the electrotaxis of lung adenocarcinoma cells under direct-current electric field stimulation. PLoS ONE 8(8):e73418
Urbano JM, Dominguez-Gimenez P, Estrada B, Martin-Bermudo MD (2011) PS integrins and laminins: key regulators of cell migration during Drosophila embryogenesis. PLoS ONE 6(9):e23893
Vincent LG, Choi YS, Alonso-Latorre B, del Alamo JC, Engler AJ (2013) Mesenchymal stem cell durotaxis depends on substrate stiffness gradient strength. Biotechnol J 8:472–484
Wei CW, Cheng JY, Huang CT, Yen MH, Young TH (2005) Using a microfluidic device for 1 mu l DNA microarray hybridization in 500 s. Nucleic Acids Res 33:e78. doi:10.1093/nar/gni078
Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE (2001) Soft lithography in biology and biochemistry. Annu Rev Biomed Eng 3:335–373. doi:10.1146/annurev.bioeng.3.1.335
Wu SY, Hou HS, Sun YS, Cheng JY, Lo KY (2015) Correlation between cell migration and reactive oxygen species under electric field stimulation. Biomicrofluidics. doi:10.1063/1.4932662
Yan XL et al (2009) Lung cancer A549 cells migrate directionally in DC electric fields with polarized and activated EGFRs. Bioelectromagnetics 30:29–35. doi:10.1002/Bem.20436
Zanet J, Stramer B, Millard T, Martin P, Payre F, Plaza S (2009) Fascin is required for blood cell migration during Drosophila embryogenesis. Development 136:2557–2565
Zhang J et al (2011) Electrically guiding migration of human induced pluripotent stem cells. Stem Cell Rev. doi:10.1007/s12015-011-9247-5
Acknowledgments
This work was financially supported by the Ministry of Science and Technology of Taiwan under Contract No. MOST 104-2311-B-002-026 (K. Y. Lo), No. MOST 104-2112-M-030-002 (Y. S. Sun), and the National Taiwan University Career Development Project (103R7888) (K. Y. Lo). The authors would like to thank Dr. Ji-Yen Cheng for help in fabricating microfluidic chips. The authors also thank the Center for Emerging Material and Advanced Devices, National Taiwan University, for the cell culture room facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lo, KY., Wu, SY. & Sun, YS. A microfluidic device for studying the production of reactive oxygen species and the migration in lung cancer cells under single or coexisting chemical/electrical stimulation. Microfluid Nanofluid 20, 15 (2016). https://doi.org/10.1007/s10404-015-1683-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-015-1683-0