Abstract
The pathogenesis of pulmonary hypertension has not been elucidated. We investigated the role of a circular ribonucleic acid, circDiaph3, in the proliferation and migration of pulmonary artery smooth muscle cells during pulmonary hypertension. CircDiaph3 overexpression in blood samples of patients with pulmonary hypertension was analyzed by real-time quantitative polymerase chain reaction. Subsequently, a rat model of pulmonary arterial hypertension was established under hypoxic conditions. Pulmonary artery smooth muscle cells were harvested from the rat model for subsequent experiments with small interfering ribonucleic acid-mediated knockdown of circDiaph3. In cell model, we found that PI3K, AKT, mTOR and insulin-like growth factor 1 signaling pathway (IGF1R) and smooth muscle cell marker genes (α-SMA, Vcam1) were significantly downregulated. The overexpression of Igf1r in pulmonary artery smooth muscle cells rescued the downregulated smooth muscle cell genes, IGF1R signaling pathway proteins, increased smooth muscle cell proliferation, and reduced apoptosis. CircDiaph3 regulates the PI3K/AKT/mTOR signaling pathway via IGF1R to inhibit apoptosis and promote proliferation of smooth muscle cells. Additionally, adenovirus-mediated in vivo inhibition of circDiaph3 was carried out in rats with pulmonary arterial hypertension, followed by harvesting of their pulmonary artery smooth muscle cells for subsequent experiments. Excessive proliferation of smooth muscle cells in the pulmonary artery has narrowed the pulmonary artery lumen, thereby causing pulmonary hypertension, and our results suggest that circDiaph3 has important value in the treatment of pulmonary hypertension.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Pulmonary arterial hypertension (PAH) is a chronic disease affecting the heart and lungs. PAH is caused by excessive proliferation and fibrosis in the wall of the pulmonary artery. Due to fibrosis, the vascular lumen of the pulmonary artery is reduced leading to an increased pulmonary vascular resistance (PVR) (Humbert et al. 2014). Consequently, pressure on the heart increases leading to heart failure. The global incidence of PAH is in several millions (Rich, et al. 2018). Some of the disease conditions leading to PAH include chronic obstructive pulmonary disease, thromboembolism, and heart disease (Hoeper et al. 2016).
The pathogenesis of PAH differs according to its etiology (Tuder et al. 2009). However, some of the common pathological events during PAH include excessive constriction and remodeling of the pulmonary artery, which leads to a narrowed lumen of the pulmonary artery, decreased pulmonary compliance, and increased right ventricular afterload (Hassoun 2021). Although pathological events, such as endothelial cell injury, excessive proliferation of endothelial cells, and invasion of fibroblasts, all occur in the PAH pathogenesis, the proliferation of pulmonary artery smooth muscle cells (PASMCs) is the most significant feature of PAH (Galiè et al. 2019). The pulmonary artery remodeling during PAH involves a chronic inflammatory response, which leads to an increase in the levels of interleukin (IL)-1, IL-8, IL-6, and matrix metallopeptidase 9 (Ou et al. 2020).
Circular ribonucleic acids (circRNAs) are a type of non-coding RNA. circRNAs are produced by backsplicing of protein-coding gene exons, sometimes with introns, in eukaryotic cells (Li et al. 2018). circRNAs are mainly present in the nucleus and cytoplasm, and their sequences are highly conserved and more stable than the linear RNAs (Gao et al. 2018). In addition, previous reports suggest that a variety of circRNAs are involved in the proliferation and migration of PASMCs, which could indicate their role in the development of PAH (Su et al. 2020).
In this study, we investigated the role of a circRNA, circDiaph3, in the proliferation and migration of pulmonary artery smooth muscle cells during pulmonary hypertension. We detected circDiaph3 in the blood of PAH patients. Our data from the primary culture of PASMCs of rats with PAH showed that circDiaph3 plays an important role in promoting the proliferation of PASMCs through insulin-like growth factor 1 receptor (IGF1R) and phosphatidylinositol-3-kinase/protein kinase B/mammalian target of Rapamycin (PI3K/AKT/mTOR) signaling pathway. In addition, our data also indicated that circDiaph3 regulates the expression of PASMC marker genes (α-SMA, Vcam1) required for proper development and functioning of PASMCs.
Methods
Patient description and sample collection
This study was approved by the Ethics Committee on Human Experiments of Bengbu Medical College (Protocol number 2022279), and all participants signed a consent form before inclusion in the study. Fresh blood samples were collected from 15 patients, who were diagnosed with PAH (pulmonary arterial pressure > 25 mmHg) between January 2022 and June 2022 at the Department of Cardiac Surgery of the First Affiliated Hospital of Bengbu Medical College of China. PAH was diagnosed according to echocardiographic, which was defined as a mean pulmonary arterial pressure ≥ 25 mmHg. Exclusion criteria were (a) patients who were younger than 18; (b) patients whose PAH was reversed after CHD correction; and (c) patients with incomplete clinical data. The procedure was conducted as per the guidelines outlined in the Declaration of Helsinki. Written informed consent was obtained from each patient participating in the study. The experimental protocol and informed consent were approved by the Ethics Committee of Bengbu Medical College (Bengbu, Anhui, China). All blood samples were stored at − 80 °C for subsequent experiments.
Establishment of animal models of PAH and adenovirus injection
Hypoxia-induced PAH model
A total of 32 healthy Sprague Dawley (SD) rats (specific pathogen-free grade), weighing 160–200 g were obtained from the Experimental Animal Center of Bengbu Medical College.8 rats were placed in a normoxic environment as sham group, and the other 24 rats were placed under hypoxic conditions (10% O2) for 30 days to induce PAH in rats, Twenty-four rats were divided into three groups with eight rats in each group (control, sh-NC, sh-circDiaph3 group). The heart and lung tissue samples were extracted from control rats and those with PAH under anesthesia (Isoflurane 50 mg/kg). Subsequently, these tissue samples were placed in cold phosphate-buffered saline (PBS) until further experiments.
All procedures, experiments, and housing of the animals were carried out according to current regulations and guidelines for animal welfare and to international principles of laboratory animal care, following the ARRIVE Guidelines Checklist as well. Ethics committee of Bengbu Medical College approved this animal study. The protocols were approved by the Ethics Review Board of Bengbu Medical College, Bengbu, Anhui Province, China (Protocol number 2022356).
PASMC primary cell culture and hypoxia treatment
PASMCs were isolated from the pulmonary arteries of rats with PAH and control rats. The isolated PASMCs were cultured with 10% fetal bovine serum at 37 °C in moist air containing 5% CO2 for all experiments. Immunofluorescence staining for α-smooth muscle actin (α-SMA) was used to ascertain the purity of cultured PASMCs.
The PASMCs of control rats with PAH were cultured in humid air containing 5% CO2 at 37 °C for 24 h. These cells were then cultured under hypoxic conditions in a hypoxia incubator (YCP-80/S, CHAOHONG, China), with 3% O2/5% CO2/92% N2.
Small interfering RNA (siRNA) transfection
The siRNA duplexes for gene silencing were obtained from HANBIO. The siRNA sequences are as follows: (1) circDiaph3 (si-circDiaph3) — 5′-GUUUGUAACCAUGUGUGUGUA-3′ and 5′-UACAGAGACAUGGUUACAAAC-3′; 2) scrambled siRNA as negative controls (si-Con) — 5′-UUCUCCGAACGUGUCACGUTT-3′ and 5′-ACGUGACACGUUCGGAGAATT-3′.
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of gene expression
The tissue and blood were weighed and dissolved in 1 ml TRIzol (15,596,018, Thermo Fisher Scientific, China). After centrifugation at 4 °C and 12,000 rpm for 10 min, the supernatant was collected and mixed with 0.5 mL isopropyl alcohol (2,019,121,060, Zhiyuan Chemical Reagent Co., LTD, China). The RNA pellets were centrifuged at 12,000 rpm at 4 °C for 5 min. The supernatant was discarded, and the RNA pellets were air-dried at 20 °C. The following primers were used in this study: IGF1R forward, 5′-GTCAAGGACGAGCCTGAAAC-3′ and IGF1R reverse, 5′-TGAGGCCTCGTTGAGAAACT-3′; CircDiaph3 forward, 5′-GCGACAGAAGAAAAAGGACACC-3′ and CircDiaph3 reverse, 5′-GTAGAAGCTGCCTTCCCATCT-3′; PI3K forward, 5′-CGAAACAAAGCCGAGAACCT-3′ and PI3K reverse, 5′-GACGCAATGTTTGACTTCGC-3′; AKT forward, 5′-CAGGTTCACCCAGTGACAAC-3′ and AKT reverse, 5′-CTCCTTCACCAGGATCACCT-3′; and mTOR forward, 5′-AGAACCACATGCCACACAGT-3′ and mTOR reverse, 5′-CTTTGGCATTTGTGTCCATC-3′.
Western blot analysis
The tissues and cells were weighed and incubated with 1 mL of RIPA cell lysate buffer (BL504a, Bio Sharp, USA). After centrifugation, the supernatant was collected, and sodium dodecyl sulfate (S8010, SolarBio, China) was added to the microspheres. Next, samples (30g) were loaded onto an acrylamide gel (5%). The samples were run in the stacking gel at 80 V for approximately 30 min. When the samples reached separating gel, the voltage was set at 120 V for 1 h to facilitate the protein separation process. Before the transfer, the filter paper and polyvinylidene fluoride (PVDF) membrane (IPVH00010, Millipore, USA) of the same size as the acrylamide gel were immersed in the transfer buffer for 5 min. The separated proteins were then transferred to the PVDF membrane at a constant current of 300 mA. The PVDF membrane with proteins was incubated overnight with diluted primary antibodies with slow shaking at 4 °C. The primary antibodies were washed next morning, and the PVDF membrane was incubated with horseradish peroxidase (HRP)-labeled secondary antibodies at room temperature for 2 h. The film was placed in an automatic exposure meter in a dark room, and the image was obtained under appropriate exposure conditions.
Cell proliferation assay using cell counting Kit 8 (CCK8)
PASMCs isolated from rats with PAH and control rats were cultured in fetal bovine serum medium (CM-0122, ProCell, China). Subsequently, PASMCs were digested with trypsin (C0201, Beyotime, China) upon reaching the logarithmic growth phase, and collected with centrifugation. The cell pellet was then resuspended in the medium (with gentle mixing) to obtain a cell suspension with 5–10 × 104 cells/mL. Subsequently, 100 µL of cell suspension was added to each well of a 96-well flat-bottom plate. Wells on the edge of the plate were filled with sterile PBS. The cells were incubated in the plate (5%CO2, 37 °C) until a monolayer of cells covered the bottom of the well. These cells were then incubated overnight in a low-oxygen incubator. These cells were transfected with siRNA next morning, after observing their morphology under an inverted microscope. After 48 h of culture, 10-µL CCK8 (BB-4202–01, Bebo, China) was added to each well. After incubation for 1 h, the absorbance of each well was measured at 450 nm. Data were standardized with wells containing only the medium and CCK8 as controls.
Cell apoptosis assay
PASMCs adhered to the wall and were digested with trypsin without EDTA. After cell counting, 0.5 × 106 cells were collected and centrifuged at 1500 rpm for 3 min. The cell pellet was then washed twice with PBS by centrifuging at 1500 rpm for 3 min. After washing, the cells were resuspended in 100 µl 1 × Binding buffer. In this cell resuspension, 5-µL fluorescein isothiocyanate was added, and cells were incubated in the dark for 15 min. Afterward, 10-µL propidium iodide was added to the cell suspension, and the cells were incubated at room temperature for 5 min in the dark and then put on the FCM (CytoFLEX, BECKMAN, USA) for detection.
Cell cycle assay
The PASMCs were washed with cold PBS, followed by digestion with trypsin. The digested cells were centrifuged at 2000 rpm for 5 min and washed with cold PBS. Afterward, the supernatant was carefully removed, leaving behind a cell suspension of about 50 µL. Next, cells were resuspended into 0.3 mL PBS to obtain a single-cell suspension and 2.7 mL cold anhydrous ethanol was added to the cell suspension at a final concentration of 90%, and the cells were incubated at 4 °C overnight (18–24 h) to facilitate cell fixation. This was followed by washing the cells with cold PBS twice at 2000 rpm for 5 min. The cells were then resuspended in 500-µL PBS in a 1.5-mL Eppendorf tube and mixed gently to avoid cell clumping. Next, the cells were incubated with 20 μL RNase (storage concentration 25 mg/mL, PBS diluted to 1 mg/mL, 50 µg/mL), in a water bath at 37 °C for 30 min. The cells were then incubated with 400 µL of propidium iodide at 4 °C in darkness for 30–60 min and tested on the machine.
Hematoxylin–eosin staining (HE)
After dehydration, the specimens were embedded and sectioned (thickness 6 μm), soaked in xylene (10,023,418, China) for 3 times, 15 min each time, and then dehydrated in gradient 100% and 80% ethanol (10,009,218, China) for 5 min. The sections were rinsed once with pure water and then washed with alcohol to remove the section impurities. The cells were incubated with hematoxylin (BA-4041, BASO, China) for 5 min and washed three times with pure water. Samples were incubated with 1% hydrochloric acid ethanol solution for 3–5 s and washed with pure water. Aqueous lithium carbonate was added, and the mixture was incubated for 30 s before washing with pure water. After dehydration with 80% ethanol, the cells were stained with eosin for 30 s (BA-4022, BASO, China) and then soaked in xylene for 2 min.
Immunofluorescence assay
Tissues were fixed in 4% paraformaldehyde for 20 min and then soaked in 0.3% TritonX-100 (T9284, Sigma, USA) for 20 min at 20 °C. Tritonx-100 was removed, and tissues were washed with PBS. Goat serum (ZLI-9022, ZSBIO, USA) was incubated for 30 min, and the primary antibody was incubated for 60 min at 37 °C. The primary antibodies used were as follows: α-SMA (AF1032, Affinity, USA) and PCNA (AF0239, Affinity, USA). Afterward, the primary antibody was removed and the tissues were incubated with secondary antibody (A0516, Chinese Beyotime) at 37 °C in the dark for 30 min. Subsequently, the secondary antibody was removed, and the stained tissue was put into a fluorescence quencher and observed under a fluorescence microscope.
Statistical analysis
The continuous variables of the normal distribution were expressed as mean ± standard deviation, and the classification variables were expressed in terms of frequency or percentage. Chi-square tests or Fisher's exact tests were used to assess categorical variables, and multivariate analyses were performed using Cox proportional risk models. Subsequently, to determine whether there was a linear association between PAH and circDiaph3, linear regression analysis was performed to fit the clinical results. All analyses were performed using R (The Re Foundation for Statistical Computing, Vienna, Austria http://www.R-project.org), and Empower Stats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, USA) statistical packages are carried out. Statistical significance was set at P < 0.05.
Data were expressed as mean ± standard deviation and processed using SPSS 21.0 software (Chicago, IL, USA), and one-way ANOVA was used for comparison among multiple groups. Fisher test was used for comparison between the two groups. Statistical significance was set at P < 0.05.
Results
circDiaph3 expression was found to be significantly increased in the serum of PAH patients
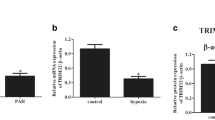
To determine the expression of circDiaph3, blood samples were collected from patients with PAH. qRT-PCR results showed that serum circDiaph3 expression was significantly upregulated in patients with PAH in comparison with that in normotensive patients (Supplementary Table 1). In addition, there was a linear relationship between pulmonary arterial pressure and circDiaph3 expression in patients with PAH (Supplementary Fig. 1). This indicates that circDiaph3 expression is closely related to PAH.
Establishment of a rat model of PAH and acquisition of primary cells
Rats were kept in a hypoxia incubator for 30 days, which resulted in the thickening and narrowing of the pulmonary artery. Subsequently, the thickened pulmonary arteries were harvested from the rats. The vascular endothelium of the pulmonary artery was removed, and PASMCs were isolated for primary culture (Fig. 1). To assess the cell purity for PASMCs, the cultured cells were immunostained for smooth muscle marker α-SMA, which showed that the cells were rich in PASMC.
circDiaph3 knockdown inhibited the proliferation and promoted cell death in PASMCs
SiRNA-mediated knockdown of circDiaph3 in the PASMC culture resulted in decreased cell proliferation and increased apoptosis of PASMCs (Fig. 2C, F) and decreased expression of smooth muscle cell-related proteins (α-SMA, Vcam1) detected by immunofluorescence staining (Fig. 2D), in comparison with knockdown with control siRNAs. Subsequently, we checked the expression of α-SMA, Vcam1, Igf1r, PI3K, Akt, and mTOR by qRT-PCR and found that the expression levels ofα-SMA, Vcam1, and Igf1r were significantly decreased in PASMCs post-siRNA-mediated knockdown circDiaph3 (Fig. 2A). Western blot results also showed decreased phosphorylation of AKT and mTOR and decreased protein levels of IGF1R, α-SMA, and VCAM1 (Fig. 2B, E).
circDiaph3 inhibits the proliferation of pulmonary artery smooth muscle cells (PASMCs). The expression levels of circDiaph3, Igf1r and PI3/Akt/mTOR pathway proteins in the cells were detected by qRT-PCR and Western blot. After that, the translation of circDiaph3 was manipulated by siRNA and the expression of Igf1r and PI3/Akt/mTOR pathway was observed A qRT-PCR analysis of circDiaph3, α-SMA, Vcam1, Igf1r, PI3K, Akt, and mTOR in PASMCs. C After circDiaph3 knockdown, PASMC cycle analysis was performed by flow cytometry. D Immunostaining of α-SMA and VCAM1 in PASMCs post-circDiaph3 knockdown. B, E Western blot analysis of α-SMA, VCAM1, and signaling proteins of IGF-1 pathway in PASMCs. F Flow cytometry-based detection of PASMC apoptosis of post-circDiaph3 knockdown. Data are presented as the mean ± standard deviation; *: compared with the control group, p < 0.05
circDiaph3 interacts with PI3K, AKT, and mTOR in the cytoplasm through IGF1R
CircDiaph3 knockdown resulted in reduced expression of α-SMA and VCAM1, and reduced phosphorylation of PI3K, AKT, and mTOR signaling mediators of the insulin-like growth factor 1 (IGF-1) pathway (Fig. 4A and B). To check whether Igf1r overexpression can rescue the effects of siRNA-mediated knockdown of circDiaph3, we transfected an overexpression plasmid of Igf1r in the PASMCs after circDiaph3 knockdown. Indeed, post-overexpression of Igf1r, the PASMCs showed increased cell proliferation, reduced cell death, and change in cycle stages (Fig. 3A, B and D). In addition, the expression of smooth muscle cell markers (α-SMA and VCAM1) was found to be rescued post-Igf1r overexpression (Fig. 3C, Fig. 4) in PASMCs. The overexpression of Igf1r also rescued the levels of phosphorylated PI3K, AKT, and mTOR (Fig. 4A, B) in the PASMCs.
The cell cycle and the expression of related proteins were detected by flow cytometry, CCK8, qRT-PCR and Western blot. It further indicates that circDiaph3 inhibits pulmonary artery smooth muscle cell (PASMC) proliferation via ˆIgf1r and PI3K/AKT/mTOR signaling pathway. A Flow cytometry-based detection of PASMC apoptosis. B CCK8 assay for detecting PASMC proliferation. C qRT-PCR analysis of circDiaph3, α-SMA, Vcam1, Igf1r, PI3K, Akt, and mTOR in PASMCs. D PASMC cycle analysis performed by flow cytometry. Data are presented as the mean ± standard deviation (SD); *compared with the control group, p < 0.05
A rescue experiment was designed in the study to further prove that Igf1r overexpression rescues the effects of circDiaph3 knockdown in pulmonary artery smooth muscle cells (PASMCs) A, B Western blot analysis of α-SMA, Vcam1, and Igf1r signaling pathway proteins in PASMCs. C Immunostaining of α-SMA and VCAM1 in PASMCs. Data are presented as the mean ± standard deviation; *compared with the control group, p < 0.05
circDiaph3 plays an important role in rat models of pulmonary hypertension
To understand the role of circDiaph3 in vivo, we injected an adenovirus in the PAH rats to inhibit circDiaph3 expression. We divided the PAH rats into the following groups: uninjected controls, empty virus-injected group, and adenovirus-injected group. The adenovirus-injected animals showed reduced expression of circDiaph3 in the pulmonary artery, which indicated that the virus injection and circDiaph3 inhibition were working. The adenovirus-injected animals showed reduced expression levels of α-SMA and VCAM1 and reduced phosphorylation of PI3K, AKT, and mTOR (Fig. 5A, B); Supplement Fig. 2) in the isolated PASMCs. Additionally, hematoxylin and eosin (HE) and immunofluorescence staining showed that the proliferation of PASMCs decreased significantly after circDiaph3 inhibition (Fig. 5C).
A rat model of pulmonary hypertension was established and a virus was designed to inhibit the expression of circDiaph3. Adenovirus-mediated in vivo inhibition of circDiaph3 expression in pulmonary artery smooth muscle cells (PASMCs) isolated from rats with PAH A Western blot analysis of α-SMA, Vcam1, and Igf1r signaling pathway proteins in rat pulmonary artery specimens. B qRT-PCR analysis of circDiaph3, α-SMA, Vcam1, and genes encoding Igf1r signaling pathway proteins in rat pulmonary artery specimens. C Hematoxylin and eosin (HE) analysis and immunofluorescence staining of α-SMA in rat pulmonary artery specimens. Data are presented as the mean ± standard deviation; *compared with the control group, p < 0.05
Discussion
Previous studies have shown that PAH pathogenesis is closely related to the proliferation, apoptosis, and migration of PASMCs (Zhang et al. 2020). circRNAs have been shown previously to regulate genes and signaling pathways related to cell proliferation, apoptosis, and migration in PASMCs (Jiang et al. 2021). In this study, we discovered that the circDiaph3 expression was significantly upregulated in PAH patients. To confirm the role of circDiaph3 in PAH pathogenesis, we performed our studies on the following two models: (1) in vitro circDiaph3 knockdown model and (2) in vivo circDiaph3 inhibition model. Our data indicated that circDiaph3 controls PASMC proliferation and apoptosis via regulation of IGF-1 signaling (Fig. 6).
IGF-1, a member of the insulin/IGF family, plays a key role in the overall growth and metabolism of the organism and in cellular processes, such as proliferation, migration, differentiation, adhesion, and apoptosis (Macvanin et al. 2023; Steffensen, et al. 2019). IGF-1 is secreted by the liver and acts on the vascular system through para-hydroxybenzoic acid mechanism downstream of tyrosine kinase receptor IGF1R (Stanley et al. 2021)[15]. Multiple signaling effectors work downstream of IGF1R, including PI3K, PKB (also known as Akt), and extracellular signal-regulated kinase (ERK, also known as mitochondrial activated protein kinase [MAPK]) (Y. Sun et al. 2016a, b). Previous studies have suggested that IGF-1 can play a role in the development and diseases of the vascular and respiratory system. Under in vitro conditions, IGF-1 stimulation can promote smooth muscle cell (SMC) growth and inhibit cell apoptosis (Sun et al. 2016a, b). This was further confirmed by another study, which suggested that IGF-1 inhibits cell apoptosis in PASMCs by promoting the expression of p38, MAPK, and inducible nitric oxide synthase (Liu et al. 2020). The chronic expression of IGF-1 in the blood vessel wall can promote the contraction of arterial blood vessels. Experiments have shown that direct stimulation of aortic smooth muscle cells with IGF-1 in vitro can increase SMC differentiation markers such as: α-SMA, calcium Regulin and SM22α. (He et al. 2021). Stimulation of SMCs with IGF-1 can increase the expression of SMC differentiation markers, such as α-SMA and elastin, and can raise the calcium levels inside these cells (Shuang et al. 2018). Studies have also found that IGF-1 plays an important role in chronic lung diseases of premature infants and newborns, such as respiratory distress syndrome and bronchopulmonary dysplasia (Dong et al. 2012; Kheirollahi et al. 2022). However, the role of IGF-1 in PAH pathogenesis, including the mechanism underlying PASMC growth, and vascular remodeling during PAH has not been completely described earlier.
Researchers have found that some non-coding RNAs are involved in the pathogenesis of PAH (Zahid et al. 2020). circRNAs are a type of non-coding RNAs. Unlike linear non-coding RNAs, circRNAs are closed single-stranded RNA molecules formed via specific splicing mechanisms; hence, they are very stable, have longer half-life, and are resistant to exonucleases (A. Huang et al. 2020). The involvement of circRNAs during PAH progression still remains unclear. In our study, we have demonstrated an interplay between circDiaph3 and IGF1R during PAH pathogenesis. Our experiments showed that inhibition of circDiaph3 in PASMC reduced IGF-1 signaling, which negatively affected PASMC proliferation and expression of SMC marker genes, such as α-SMA and Vcam1. However, the overexpression of IGF1R in PASMCs rescued the PASMC proliferation and SMC marker gene expression, which confirmed the relationship between IGF-1 signaling and circDiaph3 during PAH progression.
The PI3K/AKT/mTOR signaling pathway is an important intracellular signal transduction pathway with a wide range of cell regulatory functions and plays an important role in cell proliferation and differentiation, glucose metabolism, insulin secretion and its signal transduction (Basile et al. 2022; J. Huang et al. 2022). PI3K can transmit signals to increase cyclin synthesis, thereby promoting the progression of G1 phase, shortening the cell cycle, and causing cell proliferation and activation. PI3K is involved in the biological effects of a variety of growth factors (Wang et al. 2022). PI3k-activated Akt can activate or inhibit its downstream target proteins, NF-κB, Forkhead, m TOR, etc. through phosphorylation to promote cell survival through a variety of pathways (Cai et al. 2019; Shao et al. 2022). PI3K/AKT/mTOR signaling pathway plays a crucial role in cell growth and differentiation (Chen et al. 2022; Tewari et al. 2022). The activation of PI3K/AKT/mTOR signaling pathway is closely related to the proliferation of vascular wall cells. Inhibition of Akt activation in cells can inhibit the growth of vascular smooth muscle cells induced by growth factors. Therefore, the activation of PI3K/AKT/mTOR signaling pathway is related to VSMCs phenotypic transformation and cell proliferation.
Pulmonary hypertension is a complex and life-threatening lung disease characterized by increased pulmonary vascular resistance, leading to increased pulmonary arterial pressure. Despite the available treatment methods, the prognosis of PAH is still poor because the underlying biological mechanisms are not fully understood. Therefore, it is particularly necessary to search for potential biomarkers to promote early diagnosis and individualized treatment. In the experiment, we induced pulmonary hypertension in rats through hypoxia. The advantage of this method is that it conforms to the pathological process of clinical patients, and the pathological results of pulmonary hypertension in rats are consistent with clinical patients.
Conclusion
Pulmonary hypertension is still incurable in clinical practice. In study, we investigated the role of circDiaph3 in the proliferation and migration of pulmonary artery smooth muscle cells during pulmonary hypertension. CircDiaph3 expression was analyzed in blood samples from patients with pulmonary hypertension and verified in PH rat model. We found that circDiaph3 increased smooth muscle cell proliferation and reduced apoptosis by regulating the PI3K/AKT/mTOR pathway through IGF1R. In conclusion, circDiaph3 plays an important role in promoting the proliferation of pulmonary artery smooth muscle cells in rats with pulmonary hypertension through IGF1R and PI3K/AKT/mTOR signaling pathways, suggesting the potential value of circDiaph3 in the treatment of pulmonary hypertension.
Our results provide a comprehensive understanding of the molecular mechanisms underlying PAH progression. However, our study still has some limitations. Although we have demonstrated that CircDiaph3 acts on IGF1R, its direct and cross-acting mechanisms still need to be further explored. In clinical studies, our baseline data collection of patients is still not perfect, and we will add more abundant and comprehensive baseline data of patients in future studies. We strongly believe that circDiaph3 can be used as molecular marker for PAH diagnosis and therapeutic target for developing therapeutic interventions in future.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- PAH:
-
Pulmonary arterial hypertension
- PVR:
-
Pulmonary vascular resistance
- PASMCs:
-
Pulmonary artery smooth muscle cells
- IGF1R:
-
Insulin-like growth factor 1 receptor
- (IL)-1:
-
Interleukin-1
- (IL)-8:
-
Interleukin-8
- (IL)-6:
-
Interleukin-6
- CHD:
-
Coronary heart disease
- SD:
-
Sprague Dawley
- PBS:
-
Phosphate-buffered saline
- α-SMA:
-
α-Smooth muscle actin
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- PVDF:
-
Polyvinylidene fluoride
- HRP:
-
Horseradish peroxidase
- CCK8:
-
Cell counting Kit 8
- HE:
-
Hematoxylin–eosin staining
- MAPK:
-
Mitochondrial activated protein kinase
- SMC:
-
Smooth muscle cell
- PI3K:
-
Phosphatidylinositol 3-kinase
- mTOR:
-
Mammalian target of rapamycin
- α-SMA:
-
Actin alpha 2
- VCAM-1:
-
Vascular cell adhesion molecule-1
References
Basile MS, Cavalli E, McCubrey J, Hernández-Bello J, Muñoz-Valle JF, Fagone P, Nicoletti F (2022) The PI3K/Akt/mTOR pathway: a potential pharmacological target in COVID-19. Drug Discovery Today 27(3):848–856. https://doi.org/10.1016/j.drudis.2021.11.002
Cai W, Zhang J, Yang J, Fan Z, Liu X, Gao W, Yang J (2019) MicroRNA-24 attenuates vascular remodeling in diabetic rats through PI3K/Akt signaling pathway. Nutr Metab Cardiovasc Dis NMCD 29(6):621–632. https://doi.org/10.1016/j.numecd.2019.03.002
Chen Q, Zhang H, Yang Y, Zhang S, Wang J, Zhang D, Yu H (2022) Metformin attenuates UVA-induced skin photoaging by suppressing mitophagy and the PI3K/AKT/mTOR pathway. Int J Mol Sci 23(13):6960. https://doi.org/10.3390/ijms23136960
Dong J, Carey WA, Abel S, Collura C, Jiang G, Tomaszek S, Wigle DA (2012) MicroRNA-mRNA interactions in a murine model of hyperoxia-induced bronchopulmonary dysplasia. BMC Genom 13:204. https://doi.org/10.1186/1471-2164-13-204
Galiè N, Channick RN, Frantz RP, Grünig E, Jing ZC, Moiseeva O, McLaughlin VV (2019) Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 53(1):1801889. https://doi.org/10.1183/13993003.01889-2018
Gao Y, Zhao F (2018) Computational strategies for exploring circular RNAs. Trends Genet 34(5):389–400. https://doi.org/10.1016/j.tig.2017.12.016
Hassoun PM (2021) Pulmonary arterial hypertension. N Engl J Med 385(25):2361–2376. https://doi.org/10.1056/NEJMra2000348
He H, Snowball J, Sun F, Na CL, Whitsett JA (2021) IGF1R controls mechanosignaling in myofibroblasts required for pulmonary alveologenesis. JCI Insight. https://doi.org/10.1172/jci.insight.144863
Hoeper MM, Humbert M, Souza R, Idrees M, Kawut SM, Sliwa-Hahnle K, Gibbs JSR (2016) A global view of pulmonary hypertension. Lancet Respir Med 4(4):306–322. https://doi.org/10.1016/s2213-2600(15)00543-3
Huang A, Zheng H, Wu Z, Chen M, Huang Y (2020) Circular RNA-protein interactions: functions, mechanisms, and identification. Theranostics 10(8):3503–3517. https://doi.org/10.7150/thno.42174
Huang J, Chen L, Wu J, Ai D, Zhang J-Q, Chen T-G, Wang L (2022) Targeting the PI3K/AKT/mTOR signaling pathway in the treatment of human diseases: current status, trends, and solutions. J Med Chem 65(24):16033–16061. https://doi.org/10.1021/acs.jmedchem.2c01070
Humbert M, Lau EMT, Montani D, Jaïs X, Sitbon O, Simonneau G (2014) Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation 130(24):2189–2208. https://doi.org/10.1161/CIRCULATIONAHA.114.006974
Jiang Y, Liu H, Yu H, Zhou Y, Zhang J, Xin W, Zhu D (2021) Circular RNA Calm4 regulates hypoxia-induced pulmonary arterial smooth muscle cells pyroptosis via the Circ-Calm4/miR-124–3p/PDCD6 Axis. Arterioscler Thromb Vasc Biol 41(5):1675–1693. https://doi.org/10.1161/ATVBAHA.120.315525
Kheirollahi V, Khadim A, Kiliaris G, Korfei M, Barroso MM, Alexopoulos I, El Agha E (2022) Transcriptional profiling of insulin-like growth factor signaling components in embryonic lung development and idiopathic pulmonary fibrosis. Cells. https://doi.org/10.3390/cells11121973
Li X, Yang L, Chen LL (2018) The biogenesis, functions, and challenges of circular RNAs. Mol Cell 71(3):428–442. https://doi.org/10.1016/j.molcel.2018.06.034
Liu L, Guo H, Song A, Huang J, Zhang Y, Jin S, Yang P (2020) Progranulin inhibits LPS-induced macrophage M1 polarization via NF-кB and MAPK pathways. BMC Immunol 21(1):32. https://doi.org/10.1186/s12865-020-00355-y
Macvanin M, Gluvic Z, Radovanovic J, Essack M, Gao X, Isenovic ER (2023) New insights on the cardiovascular effects of IGF-1. Front Endocrinol 14:1142644. https://doi.org/10.3389/fendo.2023.1142644
Ou M, Zhang C, Chen J, Zhao S, Cui S, Tu J (2020) Overexpression of MicroRNA-340-5p inhibits pulmonary arterial hypertension induced by APE by downregulating IL-1β and IL-6. Mol Ther Nucleic Acids 21:542–554. https://doi.org/10.1016/j.omtn.2020.05.022
Rich S, Haworth SG, Hassoun PM, Yacoub MH (2018) Pulmonary hypertension: the unaddressed global health burden. Lancet Respir Med 6(8):577–579. https://doi.org/10.1016/s2213-2600(18)30268-6
Shao W, Wang S, Wang X, Yao L, Yuan X, Huang D, Xue H (2022) miRNA-29a inhibits atherosclerotic plaque formation by mediating macrophage autophagy via PI3K/AKT/mTOR pathway. Aging 14(5):2418–2431. https://doi.org/10.18632/aging.203951
Shuang T, Fu M, Yang G, Wu L, Wang R (2018) The interaction of IGF-1/IGF-1R and hydrogen sulfide on the proliferation of mouse primary vascular smooth muscle cells. Biochem Pharmacol 149:143–152. https://doi.org/10.1016/j.bcp.2017.12.009
Stanley TL, Fourman LT, Zheng I, McClure CM, Feldpausch MN, Torriani M, Grinspoon SK (2021) Relationship of IGF-1 and IGF-binding proteins to disease severity and glycemia in nonalcoholic fatty liver disease. J Clin Endocrinol Metab 106(2):e520–e533. https://doi.org/10.1210/clinem/dgaa792
Steffensen LB, Conover CA, Oxvig C (2019) PAPP-A and the IGF system in atherosclerosis: what’s up, what’s down? Am J Physiol Heart Circ Physiol 317(5):H1039–H1049. https://doi.org/10.1152/ajpheart.00395.2019
Su H, Wang G, Wu L, Ma X, Ying K, Zhang R (2020) Transcriptome-wide map of m(6)A circRNAs identified in a rat model of hypoxia mediated pulmonary hypertension. BMC Genom 21(1):39. https://doi.org/10.1186/s12864-020-6462-y
Sukhanov S, Higashi Y, Shai SY, Snarski P, Danchuk S, D’Ambra V, Delafontaine P (2018) SM22α (smooth muscle protein 22-α) promoter-driven IGF1R (insulin-like growth factor 1 receptor) deficiency promotes atherosclerosis. Arterioscler Thromb Vasc Biol 38(10):2306–2317. https://doi.org/10.1161/atvbaha.118.311134
Sun M, Ramchandran R, Chen J, Yang Q, Raj JU (2016a) Smooth muscle insulin-like growth factor-1 mediates hypoxia-induced pulmonary hypertension in neonatal mice. Am J Respir Cell Mol Biol 55(6):779–791. https://doi.org/10.1165/rcmb.2015-0388OC
Sun Y, Kang L, Li J, Liu H, Wang Y, Wang C, Zou Y (2016b) Advanced glycation end products impair the functions of saphenous vein but not thoracic artery smooth muscle cells through RAGE/MAPK signalling pathway in diabetes. J Cell Mol Med 20(10):1945–1955. https://doi.org/10.1111/jcmm.12886
Tewari D, Patni P, Bishayee A, Sah AN, Bishayee A (2022) Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: a novel therapeutic strategy. Semin Cancer Biol. https://doi.org/10.1016/j.semcancer.2019.12.008
Tuder RM, Abman SH, Braun T, Capron F, Stevens T, Thistlethwaite PA, Haworth SG (2009) Development and pathology of pulmonary hypertension. J Am Coll Cardiol 54(1 Suppl):S3-s9. https://doi.org/10.1016/j.jacc.2009.04.009
Wang S, Duan J, Liao J, Wang Y, Xiao X, Li L, Shao M (2022) LncRNA H19 inhibits ER stress induced apoptosis and improves diabetic cardiomyopathy by regulating PI3K/AKT/mTOR axis. Aging 14(16):6809–6828. https://doi.org/10.18632/aging.204256
Zahid KR, Raza U, Chen J, Raj UJ, Gou D (2020) Pathobiology of pulmonary artery hypertension: role of long non-coding RNAs. Cardiovasc Res 116(12):1937–1947. https://doi.org/10.1093/cvr/cvaa050
Zhang M, Xin W, Yu Y, Yang X, Ma C, Zhang H, Zhu D (2020) Programmed death-ligand 1 triggers PASMCs pyroptosis and pulmonary vascular fibrosis in pulmonary hypertension. J Mol Cell Cardiol 138:23–33. https://doi.org/10.1016/j.yjmcc.2019.10.008
Funding
This work was supported by Anhui Science and Technology Research Project (1804h08020280) and Projects of Natural Science Research in Colleges and Universities of Anhui Province (KJ2021A0820, 2022AH051531).
Author information
Authors and Affiliations
Contributions
GL & SZ: Conceptualization, Methodology, Resources, and Writing—Original Draft. SY&CS: Formal analysis, Methodology, Resources, and Investigation. CS and WD: Conceptualization, Writing—Review & Editing, Project administration, and Funding acquisition. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval and consent to participate
All procedures, experiments, and housing of the animals were carried out according to current regulations and guidelines for animal welfare and to international principles of laboratory animal care, following the ARRIVE Guidelines Checklist as well. Ethics committee of Bengbu Medical College approved this animal study. The protocols were approved by the Ethics Review Board of Bengbu Medical College, Bengbu, Anhui Province, China (Protocol number 2022356). This study was approved by the Ethics Committee on Human Experiments of Bengbu Medical College (Protocol number 2022279), and all participants signed a consent form before inclusion in the study.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, G., Zhang, S., Yang, S. et al. CircDiaph3 influences PASMC apoptosis by regulating PI3K/AKT/mTOR pathway through IGF1R. 3 Biotech 13, 342 (2023). https://doi.org/10.1007/s13205-023-03739-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-023-03739-0