Abstract
Cell cycle-specific cancer chemotherapy is based on the ability of a drug to halt, minimise or destroy rapidly dividing cells. However, their efficacy is limited by the emergence of a self-renewing cell pool called “cancer stem cells” (CSC). Choriocarcinoma is a tumour of trophoblastic tissue. We, in this study, analysed whether spheroids generated from doxorubicin-treated and non-treated choriocarcinoma cell lines exhibit markers of stem cells. Two choriocarcinoma cell lines, namely JEG-3 and BeWo, were used in this study. Spheroids were generated from doxorubicin-treated cells and the non-treated cells under non-adherent condition, followed by analysis of stem-cell markers’ expression, namely NANOG, OCT4 and SOX2. Immunofluorescence analysis suggested a general increase in the markers’ concentration in spheroids relative to the parental cells. RT-qPCR and immunoblots showed an increase in the stem-cell marker expression in spheroids generated from doxorubicin-treated when compared to non-treated cells. In spheroids, Sox2 was significantly upregulated in doxorubicin-treated spheroids, whereas Nanog and Oct4 were generally downregulated when compared to non-treated spheroids. Both 2D and 3D invasion assays showed that the spheroids treated with doxorubicin exhibited reduced invasion. Our data suggest that choriocarcinoma cell lines may have the potential to produce spheroidal cells, yet the drug-treatment affected the invasion potential of spheroids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several similarities have been shown between tumour invasion and trophoblast development. They both have the capacity to proliferate, differentiate and invade through the surrounding tissues by degrading the extracellular matrix (ECM), evading the immune response and establishing nutrient supply by angiogenesis (Ferretti et al. 2007; Lorena et al. 2021). However, the invasion of trophoblast cells into the uterine wall is a unique and controlled process, which is essential for foetal development (Louwen et al. 2012; Huppertz 2018). In gestational trophoblastic diseases such as choriocarcinoma, the trophoblast cells can become tumorigenic. In fact, choriocarcinoma is a malignant tumour of the uterus which originates in the foetal chorion. It is characterized by abnormal proliferation of trophoblast without the formation of chorionic villi (Greaves and Maley 2012; Kim et al. 2019). This gestational cancer is chemotherapy-sensitive but metastatic (Braga et al. 2019).
Chemotherapeutic drugs for cancer that target rapidly dividing cells are limited by their inability to differentiate between the physiologically rapidly dividing host cells and the cancer cells, the latter often results in the development of a self-renewing pool of resistant cells called cancer stem cells (CSCs) (Phi et al. 2018)). This small pool of cancer cells are resistant to the chemotherapeutic drugs. They contribute to recurrence and aggravation of cancers (Cho and Kim 2020). CSCs or stem-like cells are reported to be enriched in tumour-derived spheroids (Ishiguro et al. 2017). Tumour spheroids can be formed as a 3D in vitro culture system which can mimic the tumour microenvironment. This system has an added advantage of histologically mimicking in vivo tumour with tumour cell heterogeneity and ability to grow into tumours when mechanically dissociated (Wei et al. 2012; Ishiguro et al. 2017; Schultz et al. 2017). CSCs essentially express factors like SOX2, OCT4 and NANOG called stemness factors (Liu et al. 2013; Basati et al. 2020). SOX2 is a transcription factor belonging to the SOX B1 group of genes in the SOX (SRY related HMG box) family (Feng and Wen 2015). SOX2 expression has been implicated in various cancer stem cells. It has been reported to be involved in preservation of the development potential of a stem cell (Avilion et al. 2003), OCT4 is a member of the POSU family of transcription factors and an important pluripotency regulator. It is found to be essential for the formation of naïve epiblast and when the OCT4 genes are silenced in embryonic stems cells they lose their pluripotency and start differentiating and resulting in trophoblast like morphology (Hough et al. 2006; Radzisheuskaya and Silva 2014). NANOG is a homeobox containing a transcription factor which is involved in the maintenance of pluripotency in inner cell mass and in the undifferentiated embryonic stem cell propagation (Chambers et al. 2003; Rodda et al. 2005). The expression of these stemness markers in several cases positively correlates with the tumour progression and poor prognosis (You et al. 2017; Yang et al. 2018; Basati et al. 2020). In this study, we developed doxorubicin resistant spheroids from JEG-3 and BeWo cells in vitro, and explored their invasive potential both with and without pre-treating them with doxorubicin. We also compared the expressions of stemness factors such as NANOG, OCT4 and SOX2 in the parental and spheroidal cells (untreated and doxorubicin-treated) generated from the cell lines.
Materials and methods
Cell lines culture
JEG-3 was cultured in EMEM medium (Lonza, UK), whereas BeWo was cultured in Ham’s F12 (Lonza, UK). Both media were supplemented with 10% (v/v) FBS (foetal bovine serum—FBS; Lonza, UK), 1% (v/v) penicillin/streptomycin (Lonza, UK), and 1% (w/v) L-glutamine (Lonza, UK). Cells were incubated at 37 °C in a humidified atmosphere of air containing 5% (v/v) CO2.
Generation of non-resistant and drug-resistant (Dox-resistant) spheroids
Spheroids were produced as reported previously (Balahmar et al. 2018). In brief, spheroid cells were grown under normal conditions for 72 h. After 72 h, cells were trypsinized and were seeded (5 × 106 cells/per flask) in an ultra-low attachment flask for 3–5 days. Spheroids were separated from single cells through gravity separation for 10 min and cultured as required. The same methodology was followed for producing treated spheroids with 250 ng/mL of doxorubicin. The expression of NANOG, OCT4 and SOX2 was later investigated in both untreated and treated spheroids using immunofluorescence, RT-qPCR, immunoblotting, 2D and 3D invasion assays.
Immunofluorescence
Spheroids (non-treated and doxorubicin-resistant) were collected and centrifuged at 500 × g for 10 min, after washing with 1X PBS (Sigma-Aldrich, UK). The collected spheroids were fixed in 4% formaldehyde for 30 min at room temperature and washed three times with 1X PBS. Nonspecific reactivity was blocked by incubating the cells in a blocking solution (1% BSA in PBS Tween20) (Sigma, UK) for one hour. Spheroids were incubated overnight at 4 °C with respective primary antibodies anti-Nanog (2 mg/ml; 1:50, OAAB11202 Aviva Systems Biology, San Diego, USA), anti-Oct4 (0.5 mg/mL; 1:200, ab18976 Abcam, Cambridge, UK) and anti-Sox2 (1 mg/mL; 1:500, ab97959 Abcam, Cambridge, UK). This was followed by the treatment with secondary antibody. After washing Spheroids with PBS transferred onto slides and mounted with two drops of VECTASHIELD® HardSet™ mounting medium, the boundaries were secured by means of a hydrophobic image barrier pen (Vector Laboratories, Burlingame, USA). Parental cells were grown onto sterile glass poly-l-lysine coated coverslips GG-18-PLL (neuVitro, USA, Germany), under the respective growth conditions.
RNA extractions and quantitative real-time PCR (qRT-PCR)
qRT-PCR was carried out to determine the mRNA expression of each marker of interest. RNeasy® Plus Mini-kit (Qiagen, Germany) was used to extract the total RNA from the harvested cells (both parental and spheroidal of JEG-3 and BeWo) according to manufacturer’s instructions. cDNA was then synthesized from RNA extracted using SuperScript™ II Reverse Transcriptase kit (Thermo Fisher (Invitrogen®), USA) according to manufacturer’s instructions. The primers were designed using the Primer 3® Input version 4 software. Details of primers (supplied by MWG Eurofin, Germany). The qRT-PCR was performed using the Rotor-Gene 6000 real-time PCR cycler (Qiagen, Germany) using SYBR green method. The mRNA expression of each target factors was normalised against averaged expression of the housekeeping genes [hypoxanthine phosphoribosyltransferase-1 (HPRT1) and TATA Box Binding Protein (TBP1)] in the same samples and 2−∆∆ Ct method was used for calculation.
Immunoblotting
The protein extraction of harvested cells was measured by the BCA protein assay according to the manufacturer’s protocol (Pierce, Rockford, USA). Cells were harvested with 300 μL RIPA buffer (Sigma-Aldrich, UK) with 0.2 mL protease inhibitor cocktail (Roche, UK), 0.2% (v/v) and 1 mM Na3VO4 (Sigma-Aldrich, UK) and the resulting cell lysate was boiled for 5 min at 95 °C followed by storing the lysate − 20 °C and used whenever needed. Protein samples (50 μg) were separated by SDS/PAGE in 10% or 12% polyacrylamide gel using a Bio-Rad Mini-Protean III system (Hercules, California, USA) which were then transferred to nitrocellulose membranes in a Bio-Rad Trans-Blot system; using electro-transfer buffer, membranes were blocked using 3% w/v BSA in 1X TBS-Tween20. Membranes were incubated overnight at 4 °C with respective primary antibodies anti-Nanog (2 mg/ml; 1:500, OAAB11202 Aviva Systems Biology, San Diego, US), anti-Oct4 (0.5 mg/ml; 1:500, ab18976 Abcam, Cambridge, UK) and anti-Sox2 (1 mg/ml; 1:1000, ab97959 Abcam, Cambridge, UK), followed by incubation with respective secondary antibodies (1:10,000, Sigma) for 1 h at room temperature. The Advanced Image Data Analysis Software (Fuji; version 3.52) was used for densitometry quantified of western blot analysis. Qualitative analysis was normalized in relation to β-actin expression.
Cell invasion assay (2D)
Cell invasion assay was carried out using the BD Falcon™ BioCoat tumour invasion systems (Corning, Arizona, USA) with fluoroBlock™ 96 well insert plate according to manufacturer’s guidelines. This was also compared with migration of cells through uncoated BD Falcon™ FluoroBlok™ 96 well insert plates (see Balahmar et al. 2018) for details). In this study, spheroid cells were used that have a total diameter above 8.0 μm. Therefore, the spheroids were disintegrated by using trypsin into single cells before performing the invasion assay. The number of cells invaded from the tumour invasion plate together with percentage invasion (see below for equation) was analysed using Image J software.
Cultrex® 96 well 3D spheroid basement membrane extract (BME) cell invasion assay
The monolayer cell invasion systems are commonly used to evaluate invasion of single cells. Therefore, this method in this research was not sufficient to study the invasion potential of spheroidal cells. Although the spheroids were artificially separated into single cells to suit the 2D invasion assay, it is not entirely appropriate to compare the invasive behaviour of untreated and doxorubicin-treated spheroids. Therefore, the 3D invasion assay was carried out according to the manufacturer’s guidelines (Amsbio, UK). All confocal images were analysed by Image J software to measure changes in the invasion area at 12, 24 and 48 h.
Statistical analyses
All data are presented as means ± SEM. Statistical significance was determined using a t test, a one-way ANOVA followed by Tukey’s and two-way ANOVA followed by Sidak’s test for multiple comparisons and for t tests p < 0.05 was considered statistically significant. (GraphPad Prism, version 6, California, USA).
Results
Generation of (non-resistant and doxorubicin-resistant) spheroids
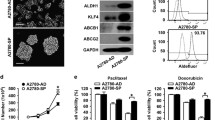
Both the cell lines were able to generate spheroids with and without the treatment of doxorubicin. To verify that spheroids (non-resistant and doxorubicin-resistant) are not just an aggregate of cells, we proceeded to disaggregate and split the spheroids into single cells by gravity separation and cultured them again under adherent conditions to assess their continued ability to self-grow. These cells attached and started to grow under normal conditions (Fig. 1).
Generation of non-resistant and doxorubicin-resistant spheroids from choriocarcinoma cell lines. The ability of JEG-3 and BeWo cells to generate non-resistant spheroids is shown in Panels A. Row 1 = Parental cells without treatment; Row 2 = Spheroidal cells produced from non-adherent 3D culture; Row 3 = the ability of spheroidal cells to regrow onto normal adherent 2D culture. Likewise, JEG-3 and BeWo have shown the ability to generate doxorubicin-resistant spheroids shown in Panel B. Row 1 = Parental cells without treatment; Row 2 = Parental cells treated with 250 ng/ml of doxorubicin. Row 3 = Spheroidal cells produced from non-adherent 3D culture; Row 4 = the ability of spheroidal cells treated to re-grow onto normal adherent 2D culture. Scale bar = 100 μm
Expression of Nanog, Oct4 and Sox2 in choriocarcinoma cell lines
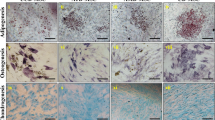
The presence of the stem cells was checked by studying the expression of stem-cell markers such as Nanog, Oct4 and Sox2. Spheroids that were generated from both cell lines showed positive staining for Nanog, Oct4 and Sox2 (Fig. 2B). In the case of BeWo, the parental cells (both non-resistant and doxorubicin-resistant) expressed Nanog and Sox2, whereas the expression of Oct4 was not observed in JEG-3 parental cells (both non-resistant and doxorubicin-resistant) (Fig. 2A). Yet the staining of Nanog and Sox2 was consistent in JEG-3 parental and spheroidal cells. The immunofluorescence Nanog, Oct4, Sox2 of the spheroidal cells (both non-resistant and doxorubicin-resistant) generated from JEG3 and BeWo are shown in Fig. 3. Interestingly, both doxorubicin-treated and untreated spheroidal cells showed immunoreactivities for all three stem cell markers.
Expression of Nanog, Oct4 and Sox2 in placenta choriocarcinoma parental cells. Immunofluorescence photo-micrographs of Nanog, Oct4, Sox2 and with DAPI staining in choriocarcinoma parental cell lines of JEG3 and BeWo are shown in the left and the right panels, respectively. Note JEG-3 cells are not showing any immunoreactivity for Oct4. Objective magnification 20X. Scale bar = 50 μm
Expression of Nanog, Oct4 and Sox2 in placental choriocarcinoma spheroids. Immunofluorescence micrographs of Nanog, Oct4, Sox2 and with DAPI staining in spheroidal cells generated from choriocarcinoma cell lines (JEG-3and BeWo) are shown in the left and the right panels, respectively. The spheroidal cells of both choriocarcinoma cells are shown positive immunoreactivity for all stem cell markers. Objective magnification 20X. Scale bar = 50 μm
We further examined the expressions of these factors by qRT-PCR (mRNA) and immunoblotting (protein) in the parental cells and spheroids. The data showed that SOX2, OCT4 and NANOG exhibited a differential expression of mRNA patterns in parental and spheroidal cells (both untreated and treated). (Image provided as a supplement).
Further comparative analysis of their protein expressions is given in Fig. 4. Despite the individual differences, the expressions of the stem cell markers in spheroidal cells (both untreated and doxorubicin-treated) were higher than that of parental counterparts. In parental cells, treatment of doxorubicin had differential effect on the stem cell markers of the two cell lines. Nanog was slightly reduced (non-significant) in JEG-3, whereas it is significantly increased in the doxorubicin treated parental BeWo cells. Similarly, Oct4 was significantly increased in the parental treated JEG-3 cells, whereas its expression was significantly reduced in BeWo cells. Sox2 expression in significantly reduced in JEG-3 parental treated cells, whereas no significant difference was observed in BeWo cells. In spheroids, there was no significant difference in the expression of Nanog and oct4 in JEG-3 doxorubicin-treated spheroids, whereas both the proteins were significantly reduced in BeWo cells. Interestingly, the doxorubicin-treated spheroids of both JEG-3 and BeWo cells showed a significant increase in Sox2 expressions when compared with the non-treated spheroids.
Immunoblot of Nanog, Oct4 and Sox2 expression in choriocarcinoma cells. Immunoblots showing the expression of Nanog, Oct4 and Sox2 in panels A to C (left panels JEG-3 and BeWo right panels). Corresponding β-actin loading controls in JEG-3and BeWo untreated and treated choriocarcinoma parental cells and spheroids. Data represent mean ± SEM of three individual experiments, each performed in triplicate (***p < 0.001;**p < 0.01,*p < 0.05); P Parental; PT Parental treated; S Spheroid; ST Spheroid treated
The invasion ability of spheroids from choriocarcinoma cell lines
We also investigated the effects of doxorubicin on the invasive capacity of spheroidal cells originating from choriocarcinoma cell lines, using BD Bio-Coat tumour invasion assay kit. The number of cells invaded by untreated spheroids was found to be higher than that of doxorubicin-treated spheroids of both cell lines [see Fig. 5]. This was found to be significant in JEG-3 untreated spheroids [see Fig. 5; panel A (left)]. Also, spheroidal cells produced from these choriocarcinoma cells showed higher invasion potential than their parental cells. However, the number of cells invaded in the BeWo cell line was generally lower than JEG3.
Comparative cell invasion of choriocarcinoma cell lines. Panels A and B: Represent the number of JEG-3 and BeWo cells invaded at 24 h. Image was taken of the bottom coated membrane using an Olympus fluorescence microscope. Objective magnification 20X (Scale bar = 100 μm). Panels C and D: Represent the quantitative analysis of the number of cells invaded using ImageJ and GraphPad software. Statistical significance was determined using a t test. Data represent the mean ± SED of three individual experiments, each performed in triplicate (****p < 0.0001)
To further confirm these results, and to confirm that the spheroids themselves (without dispersing into single cells) retain the invasion capacity, we used the 3D spheroid BME cell invasion assays to compare the invasive potential of untreated and doxorubicin-treated spheroidal cells. In both choriocarcinoma cell lines, the size of the untreated spheroids was found to be larger than doxorubicin-treated spheroids (Fig. 6, A and B). In the case of JEG-3 cells, there was a significant increase in invasion potential observed in untreated spheroids compared to doxorubicin-treated spheroids at 12, 24 and 48 h. Similarly, in BeWo cells, the untreated spheroids showed a higher invasion potential than doxorubicin-treated spheroids at 12, 24 and 48 h. However, the doxorubicin-treated spheroids from both cell lines only produced a collection of spheroid-like bodies without any invasive properties. It could be observed from Fig. 6 that doxorubicin-treated spheroids did not show any signs of invasion even after 48 h, after which even the size started decreasing.
3D Invasion of untreated and doxorubicin-treated spheroids of choriocarcinoma cell lines. Invasion pattern of untreated and treated JEG-3 and BeWo spheroids and corresponding quantitative analysis of invasion areas are shown in panels A and B, respectively. A two-way ANOVA followed by Sidak’s for multiple comparisons was carried out. Data represent the mean ± SEM of three individual experiments, each performed in quintuplicate ***p < 0.001; **p < 0.01). Objective magnification = 10X
Discussion
The emergence of self-regenerating heterogeneous groups of cancer stem cells has been hindering the long-term efficacy of many chemotherapeutic approaches. In fact, this is a selective pathophysiological adaptation of tumour cells in response to the drug itself (Czerwińska et al. 2018; Hepburn et al. 2019). Therefore, in vitro models to study the effects of chemotherapy on the production of CSC’s are warranted. We have previously shown that the transformed first trimester trophoblast cell lines were able to produce self-renewing pools of stem-like spheroidal cells (Balahmar et al. 2018). In this study, we checked the ability of resistant choriocarcinoma cells to produce spheroids, to express stem cell markers and to invade.
Choriocarcinoma is a type of gestational trophoblastic disease (GTD) which usually arises due to uncontrolled proliferation of trophoblastic cells (Smith et al. 2005). Studying choriocarcinoma is important because they are malignant and can cause secondary tumours in the liver, lungs and in very few cases brain (Naveen Kumar et al. 2019). Cancer cells have the ability to form 3D spheroids, which have been used to study pathogenesis (Bapat et al. 2005) and to determine the effect of drugs (Bapat et al. 2005) mimicking the pathological condition. The expression profiles of these 3D cultures have been found to more accurately mimic the physiological expression than the 2D cultures (Hirschhaeuser et al. 2010). The spheroids exhibit several properties of the solid tumours as they exhibit ECM secretion, cell to cell contact and other physiological features similar to the solid tumours (Rodrigues et al. 2018). The 3D spheroid model responds to cues based on stimuli such as multi-layered permeability barrier, hypoxic levels, proliferation zones, tumour heterogeneity and further similarities, which makes them a suitable model for studying the pathology and efficacy of drug under in vitro conditions (Sant and Johnston 2017).
In this study, JEG-3 and BeWo cells were used as a model system representing trophoblast tumours. We initially checked the ability of the cells to form 3D spheroids. Further the cell lines were treated with one of the common chemotherapeutic agents, doxorubicin, to investigate the abilities of these cells to survive and produce spheroids as a response to chemotherapy. When both cell lines were allowed to grow in ultra-low attachment, they produced spheroids. Moreover, spheroids generated from doxorubicin-resistant cells expressed various stem cell markers. Trophoblast cells have been reported to form spheroids in vitro by several authors (including our team) (Baal et al. 2009; Gonzalez et al. 2011; Balahmar et al. 2018). Spheroids are laden with cells that exhibit characteristics of stem cells (Bapat et al. 2005). Hence, we studied the status of various stem cell markers, such as NANOG, OCT4 and SOX2 in the drug-treated and non-treated spheroids of these two cell lines. These stem cell markers are the essential transcription factors for the maintenance of pluripotent embryonic stem cells. Previous reports show that these factors are also activated in tumour cells and the status of tumour aggressiveness is directly related with the expression of these factors (Gwak et al. 2017). In our study, the expression of Sox2 and Nanog was clearly observed in both the cell lines. However, the parental cells of JEG-3 did not show the expression of Oct4. Looijenga and Oosterhuis (2013) showed that some of the teratoma tumour cells (including choriocarcinoma cells) did not express Oct4. However, parental cells (untreated and treated) of BeWo exhibited immunofluorescence for Oct4. In the case of spheroids, Oct4 (together with other stem cell markers) expression is observed in both cell lines. This shows that these cell lines have the ability to undergo different forms of transformation in the expression of stem cell markers during spheroid generation.
The immunoblotting data suggest the expression of these stemness markers that are generally relatively higher in spheroidal cells compared to parental cells. In general, there is an increased expression of Nanog, Oct4 and Sox2 in the spheroids when compared with the parental cells. Since these three markers are termed as master regulators of stemness in the cell (Hepburn et al. 2019), it can be inferred that in spheroids the trophoblast cells and the choriocarcinoma cells undergo transition to form stem-like cells. Then, it was essential to ascertain that these CSC spheroidal cells are capable of invasion.
The data from 2D invasion suggested that doxorubicin-treated spheroids exhibited less invasion potential than the untreated spheroids. Overall, it is clear that the spheroids produced from both transformed trophoblast and choriocarcinoma cell lines were capable of 2D invasion. However, the invasive potential of spheroids will be better studied in 3D invasion systems (Vinci et al. 2015). Especially the fact that we wanted to compare the invasion potential of untreated and doxorubicin-treated cells. We wanted to check whether the spheroids per se (i.e. without dispersing into single cells) have the ability to invade. Untreated spheroids of both choriocarcinoma cell lines showed a distinct increase in the invading area with time. These data confirm that spheroids derived from untreated cells can retain, if not increase the potential to invade. However, doxorubicin-treated spheroids from both JEG-3 and BeWo cells have lost the ability to invade. The spheroid became distorted and did not show any invasion in either of the cell lines. This may be due to the persisting effect of the doxorubicin treatment in these cell lines.
The expression of NANOG, OCT4 and SOX2 is necessary for maintenance of stemness in various stem cells and are considered as master regulators of pluripotency (Leis et al. 2012; Hepburn et al. 2019). According to immunoblot data, spheroids have significantly higher expression of Nanog, Oct4 and Sox2 than the parental cells. Small changes in the expression of these three factors have been shown to produce different lineages in human embryonic stem cells (Wang et al. 2012). Kallas et al., (2014) showed that the regulation of SOX2 expression is essential for self-renewal in the population of embryonic stem cells. When the expression of Sox2 is decreased, cells start differentiating. In gastric cancer, higher Sox2 expression positively correlated with doxorubicin resistance and when SOX2 was inhibited using siRNA, the ability of the spheroid formation was significantly affected. This also made cells of spheroids more vulnerable to doxorubicin and reduced tumorigenicity in vivo (Tian et al. 2012). Therefore, the higher expression of SOX2 in resistant spheroids may confer the cells with doxorubicin resistance and ability to form spheroids. Apart from the development of stem cells/stem like cells, several other phenomenon have been put forward for the development of doxorubicin resistance including ABC transporters, activation of survival pathways, affecting epithelial-mesenchymal transition, besides mutations induced due to DNA-adduct formations (Cox and Weinman 2016; Micallef and Baron 2020). Our data also showed, in addition to doxorubicin resistance, that there was reduced invasion from drug-treated spheroids. This may be due to the persistent effect of doxorubicin on proliferation and invasion of the cells. Although spheroids exhibited reduced invasion, the stemness factors expression, especially SOX2 which is associated with promoting the invasion of cancer and aggressiveness of cancer warrants further studies to check whether the stemness factor revives the invasion of the spheroids on further prolonged exposure.
Conclusion
The spheroidal cells produced from both choriocarcinoma cells showed increased expression of the stem cell markers and enhanced invasion. The doxorubicin-resistant cells also exhibited the stem cell markers with reduced 3D invasion; further studies are warranted to study the nature of the stem-like cells and their suitability to further reveal how the trophoblast invasion is altered during the transformation into choriocarcinoma.
References
Avilion AA, Nicolis SK, Pevny LH et al (2003) Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev 17:126–140. https://doi.org/10.1101/GAD.224503
Baal N, Widmer-Teske R, McKinnon T et al (2009) In vitro spheroid model of placental vasculogenesis: does it work? Lab Investig 892(89):152–163. https://doi.org/10.1038/labinvest.2008.126
Balahmar RM, Boocock DJ, Coveney C et al (2018) Identification and characterisation of NANOG+/ OCT-4high/SOX2+ doxorubicin-resistant stem-like cells from transformed trophoblastic cell lines. Oncotarget. https://doi.org/10.18632/oncotarget.24151
Bapat SA, Mali AM, Koppikar CB, Kurrey NK (2005) Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res 65:3025–3029. https://doi.org/10.1158/0008-5472.CAN-04-3931
Basati G, Mohammadpour H, Emami Razavi A (2020) Association of high expression levels of SOX2, NANOG, and OCT4 in gastric cancer tumor tissues with progression and poor prognosis. J Gastrointest Cancer 51:41–47. https://doi.org/10.1007/S12029-018-00200-X/TABLES/3
Braga A, Mora P, de Melo AC et al (2019) Challenges in the diagnosis and treatment of gestational trophoblastic neoplasia worldwide. World J Clin Oncol 10:28. https://doi.org/10.5306/WJCO.V10.I2.28
Chambers I, Colby D, Robertson M et al (2003) Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113:643–655. https://doi.org/10.1016/S0092-8674(03)00392-1
Cho Y, Kim YK (2020) Cancer stem cells as a potential target to overcome multidrug resistance. Front Oncol. https://doi.org/10.3389/FONC.2020.00764
Cox J, Weinman S (2016) Mechanisms of doxorubicin resistance in hepatocellular carcinoma. Hepatic Oncol 3:57–59. https://doi.org/10.2217/hep.15.41
Czerwińska P, Mazurek S, Wiznerowicz M (2018) Application of induced pluripotency in cancer studies. Reports Pract Oncol Radiother 23:207–214. https://doi.org/10.1016/J.RPOR.2018.04.005
Feng R, Wen J (2015) Overview of the roles of Sox2 in stem cell and development. Biol Chem 396:883–891. https://doi.org/10.1515/hsz-2014-0317
Ferretti C, Bruni L, Dangles-Marie V et al (2007) Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Hum Reprod Update 13:121–141
Gonzalez M, Neufeld J, Reimann K et al (2011) Expansion of human trophoblastic spheroids is promoted by decidualized endometrial stromal cells and enhanced by heparin-binding epidermal growth factor-like growth factor and interleukin-1β. Mol Hum Reprod 17:421–433. https://doi.org/10.1093/MOLEHR/GAR015
Greaves M, Maley CC (2012) Clonal evolution in cancer. Nature 481:306. https://doi.org/10.1038/NATURE10762
Gwak JM, Kim M, Kim HJ et al (2017) Expression of embryonal stem cell transcription factors in breast cancer: Oct4 as an indicator for poor clinical outcome and tamoxifen resistance. Oncotarget 8:36305. https://doi.org/10.18632/ONCOTARGET.16750
Hepburn AC, Steele RE, Veeratterapillay R et al (2019) The induction of core pluripotency master regulators in cancers defines poor clinical outcomes and treatment resistance. Oncogene 3822(38):4412–4424. https://doi.org/10.1038/s41388-019-0712-y
Hirschhaeuser F, Menne H, Dittfeld C et al (2010) Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol 148:3–15. https://doi.org/10.1016/J.JBIOTEC.2010.01.012
Hough SR, Clements I, Welch PJ, Wiederholt KA (2006) Differentiation of mouse embryonic stem cells after RNA interference-mediated silencing of OCT4 and Nanog. Stem Cells 24:1467–1475. https://doi.org/10.1634/STEMCELLS.2005-0475
Huppertz B (2018) Trophoblast invasion: remodelling of spiral arteries and beyond. In: Saito S (ed) Preeclampsia comprehensive gynecology and obstetrics. Springer, Singapore. https://doi.org/10.1007/978-981-10-5891-2_3
Ishiguro T, Ohata H, Sato A et al (2017) Tumor-derived spheroids: relevance to cancer stem cells and clinical applications. Cancer Sci 108:283–289. https://doi.org/10.1111/cas.13155
Kallas A, Pook M, Trei A, Maimets T (2014) SOX2 is regulated differently from NANOG and OCT4 in human embryonic stem cells during early differentiation initiated with sodium butyrate. Stem Cells Int. https://doi.org/10.1155/2014/298163
Kim GS, Hwang KA, Choi KC (2019) A promising therapeutic strategy for metastatic gestational trophoblastic disease: engineered anticancer gene-expressing stem cells to selectively target choriocarcinoma (review). Oncol Lett 17:2576–2582. https://doi.org/10.3892/OL.2019.9911/HTML
Leis O, Eguiara A, Lopez-Arribillaga E et al (2012) Sox2 expression in breast tumours and activation in breast cancer stem cells. Oncogene 3111(31):1354–1365. https://doi.org/10.1038/onc.2011.338
Liu J, Ma L, Xu J et al (2013) Spheroid body-forming cells in the human gastric cancer cell line MKN-45 possess cancer stem cell properties. Int J Oncol 42:453–459. https://doi.org/10.3892/IJO.2012.1720/HTML
Looijenga LHJ, Oosterhuis JW (2013) Pathobiology of germ cell tumors—applying the gossip test! Int J Dev Biol 57:289–298. https://doi.org/10.1387/IJDB.130025LL
Lorena C, Jaime G, Eugenia M, Andrea L (2021) Autophagy process in trophoblast cells invasion and differentiation: similitude and differences with cancer cells. Front Oncol. https://doi.org/10.3389/fonc.2021.637594
Louwen F, Muschol-Steinmetz C, Reinhard J et al (2012) A lesson for cancer research: placental microarray gene analysis in preeclampsia. Oncotarget 3(8):759–773
Micallef I, Baron B (2020) Doxorubicin: an overview of the anti-cancer and chemoresistance mechanisms. Ann Clin Toxicol 3:1031
Naveen Kumar M, Babu RL, Patil RH et al (2019) Protein kinases orchestrate cell cycle regulators in differentiating BeWo choriocarcinoma cells. Mol Cell Biochem 452:1–15. https://doi.org/10.1007/S11010-018-3407-8/FIGURES/10
Phi LTH, Sari IN, Yang Y-G et al (2018) Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells Int. https://doi.org/10.1155/2018/5416923
Radzisheuskaya A, Silva JCR (2014) Do all roads lead to Oct4? The emerging concepts of induced pluripotency. Trends Cell Biol 24:275–284. https://doi.org/10.1016/J.TCB.2013.11.010
Rodda DJ, Chew JL, Lim LH et al (2005) Transcriptional regulation of Nanog by OCT4 and SOX2. J Biol Chem 280:24731–24737. https://doi.org/10.1074/JBC.M502573200
Rodrigues T, Kundu B, Silva-Correia J et al (2018) Emerging tumor spheroids technologies for 3D in vitro cancer modeling. Pharmacol Ther 184:201–211. https://doi.org/10.1016/j.pharmthera.2017.10.018
Sant S, Johnston PA (2017) The production of 3D tumor spheroids for cancer drug discovery. Drug Discov Today Technol 23:27–36
Schultz I, De P, Mello A, Israel B (2017) Cervical cancer stem-like cells: systematic review and identification of reference genes for gene expression; cervical cancer stem-like cells: systematic review and identification of reference genes for gene expression. Artic Cell Biol Int. https://doi.org/10.1002/cbin.10878
Smith HO, Kohorn E, Cole LA (2005) Choriocarcinoma and gestational trophoblastic disease. Obstet Gynecol Clin North Am 32:661–684. https://doi.org/10.1016/j.ogc.2005.08.001
Tian T, Zhang Y, Wang S et al (2012) Sox2 enhances the tumorigenicity and chemoresistance of cancer stem-like cells derived from gastric cancer. J Biomed Res 26:336–345. https://doi.org/10.7555/JBR.26.20120045
Vinci M, Box C, Eccles SA (2015) Three-dimensional (3D) tumor spheroid invasion assay. J vis Exp. https://doi.org/10.3791/52686
Wang Z, Oron E, Nelson B et al (2012) Distinct lineage specification roles for NANOG, OCT4, and SOX2 in human embryonic stem cells. Cell Stem Cell 10:440–454. https://doi.org/10.1016/j.stem.2012.02.016
Wei B, Han XY, Qi CL et al (2012) Coaction of spheroid-derived stem-like cells and endothelial progenitor cells promotes development of colon cancer. PLoS ONE 7:e39069. https://doi.org/10.1371/JOURNAL.PONE.0039069
Yang F, Zhang J, Yang H (2018) OCT4, SOX2, and NANOG positive expression correlates with poor differentiation, advanced disease stages, and worse overall survival in HER2+ breast cancer patients. Onco Targets Ther 11:7873. https://doi.org/10.2147/OTT.S173522
You L, Guo X, Huang Y (2017) Correlation of cancer stem-cell markers OCT4, SOX2, and NANOG with clinicopathological features and prognosis in operative patients with rectal cancer. Yonsei Med J 59:35–42. https://doi.org/10.3349/YMJ.2018.59.1.35
Acknowledgements
We highly thank the Ministry of Higher Education of the Kingdom of Saudi Arabia for this project grant (Funding reference: S10886).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Balahmar, R.M., Deepak, V. & Sivasubramaniam, S. Doxorubicin resistant choriocarcinoma cell line derived spheroidal cells exhibit stem cell markers but reduced invasion. 3 Biotech 12, 184 (2022). https://doi.org/10.1007/s13205-022-03243-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-022-03243-x