Abstract
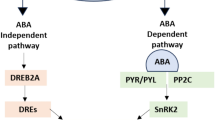
Drought stress is the main growth-limiting factor in pigeon pea production. Plant growth-promoting bacteria (PGPB) induce abiotic stress tolerance in several plants. However, the physiological and molecular changes with PGPB priming are not well understood in pigeon pea. The present study explored the potential of Firmibacteria (Bacillus azotoformans MTCC2953, Bacillus aryabhattai KSBN2K7, and Paenibacillus stellifer M3T4B6) to induce stress tolerance in pigeon pea under pot culture condition. Different physiological and biochemical parameters, including osmolytes, stress enzymes, and antioxidants, were evaluated under two stress conditions (50% and 25% field capacity) and an unstressed condition in pigeon pea. Under moisture stress conditions significant differences were observed in physiological and biochemical parameters between firmibacteria inoculated and control plants.The quantitative real-time polymerase chain reaction was performed to study the bacterial inoculation mediated expression of proline and drought-responsive genes in enhancing the drought tolerance in pigeon pea. Results showed that the inoculation of Bacillus aryabhattai upregulated the expression of drought-responsive genes (C. cajan_29830 and C. cajan_33874) and downregulated the expression of the proline gene by inducing the drought stress tolerance in inoculated plants compared with the uninoculated control plants. Therefore, Bacillus aryabhattai may be recommended for inducing drought stress tolerance and increasing the growth of pigeon pea under moisture stress conditions after field evaluation.


Similar content being viewed by others
References
Akhtar SS, Amby DB, Hegelund JN, Fimognari L, Großkinsky DK, Westergaard JC, Müller R, Moelbak L, Liu F, Roitsch T (2020) Bacillus licheniformis FMCH001 increases water use efficiency via growth stimulation in both normal and drought conditions. Front Plant Sci 11:297. https://doi.org/10.3389/fpls.2020.00297
Alami Y, Achouak W, Marol C, Heulin T (2000) Rhizosphere soil aggregation and plant growth promotion of sunflowers by an exopolysaccharide-producing Rhizobium sp. Strain isolated from sunflower roots. Appl Environ Microbiol 66:3393–3398. https://doi.org/10.1128/AEM.66.8.3393-3398.2000
Ali S, Kim WC (2018) Plant growth promotion under water: decrease of waterlogging-induced ACC and ethylene levels by ACC deaminase-producing bacteria. Front Microbiol 9:1096
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Chanratana M, Joe MM, Roy Choudhury A, Anandham R, Krishnamoorthy R, Kim K, Jeon S, Choi J, Choi J, Sa T (2019) Physiological response of tomato plant to chitosan-immobilized aggregated Methylobacterium oryzae CBMB20 inoculation under salinity stress. 3 Biotech 9:397. https://doi.org/10.1007/s13205-019-1923-1
Chaparro-Giraldo A, Barata R, Chabregas S, Azevedo RA, Silva-Filho MC (2000) Soybean leghemoglobin targeted to potato chloroplasts influences growth and development of transgenic plants. Plant Cell Rep 19:961–965. https://doi.org/10.1007/s002990000254
Chaves MM, Oliveira MM (2004) Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot 55:2365–2384. https://doi.org/10.1093/jxb/erh269
Chun SC, Paramasivan M, Chandrasekaran M (2018) Proline accumulation influenced by osmotic stress in arbuscular mycorrhizal symbiotic plants. Front Microbiol 9:2525. https://doi.org/10.3389/fmicb.2018.02525
Daryanto S, Wang L, Jacinthe PA (2015) Global synthesis of drought effects on food legume production. PLoS ONE. https://doi.org/10.1371/journal.pone.0127401
Demidchik V, Straltsova D, Medvedev SS, Pozhvanov GA, Sokolik A, Yurin V (2014) Stress-induced electrolyte leakage: the role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J Exp Bot 65:1259–1270
Devi NSA, Kumutha K, Anandham R, Krishnamoorthy R, Babu R, Gnanachitra M (2018) Plant growth promoting traits of Firmibacteria under drought stress. Res J Agric Sci 9:1294–1299
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Dickerson DP, Pascholati SF, Hagerman AE, Butler LG, Nicholson RL (1984) Phenylalanine ammonia-lyase and hydroxycinnamate: CoA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium carbonum. Physiol Plant Pathol 25:111–123
Du Jardin P (2015) Plant biostimulants: definition, concept, main categories and regulation. Sci Hortic 196:3–14
Egamberdieva D, Davranov K, Wirth S, Hashem A, Abd Allah EF (2017) Impact of soil salinity on the plant-growth–promoting and biological control abilities of root associated bacteria. Saudi J Biol Sci 24:1601–1608
Gagné-Bourque F, Bertrand A, Claessens A, Aliferis KA, Jabaji S (2016) Alleviation of drought stress and metabolic changes in timothy (Phleum pratense L.) colonized with Bacillus subtilis. Front Plant Sci 7:584
Gaxiola RA, Li J, Undurraga S, Dang LM, Allen GJ, Alper SL, Fink GR (2001) Drought-and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc Natl Acad Sci USA 98:11444–11449
Ghosh D, Sen S, Mohapatra S (2017) Modulation of proline metabolic gene expression in Arabidopsis thaliana under water-stressed conditions by a drought-mitigating Pseudomonas putida strain. Ann Microbiol 67:655–668. https://doi.org/10.1007/s13213-017-1294-y
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica. https://doi.org/10.6064/2012/963401
Gontia-Mishra I, Sapre S, Sharma A, Tiwari S (2016) Amelioration of drought tolerance in wheat by the interaction of plant growth-promoting rhizobacteria. Plant Biol 18:992–1000. https://doi.org/10.1111/plb.12505
Greive CM, Grattan SR (1983) Rapid assay for determination of water-soluble quaternary amino compounds. Plant Soil 70:303–307
Gusain YS, Singh US, Sharma AK (2015) Bacterial mediated amelioration of drought stress in drought tolerant and susceptible cultivars of rice (Oryza sativa L.). Afr J Biotechnol 14:764–773. https://doi.org/10.5897/AJB2015.14405
Harrington JT, Mexal JG, Fisher JT (1994) Volume displacement provides a quick and accurate way to quantify new root production. Tree Plant Notes 45:121–124
Heidari M, Golpayegani A (2012) Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J Saudi Soc Agri Sci 11:57–61
Hura T, Grzesiak S, Hura K, Thiemt E, Tokarz K, Wędzony M (2007) Physiological and biochemical tools useful in drought-tolerance detection in genotypes of winter triticale: accumulation of ferulic acid correlates with drought tolerance. Ann Bot 100:767–775
Hura T, Hura K, Grzesiak S (2008) Contents of total phenolics and ferulic acid, and PAL activity during water potential changes in leaves of maize single-cross hybrids of different drought tolerance. J Agron Crop Sci 194:104–112
Ilangumaran G, Smith DL (2017) Plant growth promoting rhizobacteria in amelioration of salinity stress: a systems biology perspective. Front Plant Sci 8:1768
Islam MR, Gregorio GB (2013) Progress of salinity tolerant rice variety development in Bangladesh. SABRAO J Breed Genet 45:21–30
Jha Y, Subramanian RB (2014) PGPR regulate caspase-like activity, programmed cell death, and antioxidant enzyme activity in paddy under salinity. Physiol Mol Biol Plants 20:201–207. https://doi.org/10.1007/s12298-014-0224-8
Kohler J, Hernández JA, Caravaca F, Roldán A (2008) Plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungi modify alleviation biochemical mechanisms in water-stressed plants. Funct Plant Biol 35:141–151
Manoj KP, Kumar V, Kumar M, Shrivastava N, Varma A (2016) Rhizosphere microbes: potassium solubilization and crop productivity-present and future aspects. Potassium solubilizing microorganisms for sustainable agriculture. Springer, pp 315–325
Mansour MMF, Ali EF (2017) Evaluation of proline functions in saline conditions. Phytochemistry 140:52–68
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42:565–572
Mellacheruvu S, Tamirisa S, Vudem DR, Khareedu VR (2016) Pigeonpea hybrid-proline-rich protein (CcHyPRP) Confers biotic and abiotic stress tolerance in transgenic rice. Front Plant Sci 6:1167
Miller KJ, Wood JM (1996) Osmoadaptation by rhizosphere bacteria. Annu Rev Microbiol 50:101–136
Mishra A, Mishra KB, Höermiller II, Heyer AG, Nedbal L (2011) Chlorophyll fluorescence emission as a reporter on cold tolerance in Arabidopsis thaliana accessions. Plant Signal Behav 6:301–310
Nadeem SM, Zahir ZA, Naveed M, Ashraf M (2010) Microbial ACC-deaminase: prospects and applications for inducing salt tolerance in plants. Crit Rev Plant Sci 29:360–393. https://doi.org/10.1080/07352689.2010.524518
Naylor D, DeGraaf S, Purdom E, Coleman-Derr D (2017) Drought and host selection influence bacterial community dynamics in the grass root microbiome. The ISME J 11:2691
Sandhya VS, Ali SZ, Grover M, Reddy G, Venkateswarlu B (2010) Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul 62:21–30
Santos-Medellín C, Edwards J, Liechty Z, Nguyen B, Sundaresan V (2017) Drought stress results in a compartment-specific restructuring of the rice root-associated microbiomes. Mbio 8:e00764-e817. https://doi.org/10.1128/mBio.00764-17
Sarath GD, Gutsche AR, Heng-Moss TM, Higley LG, Burd JD (2014) Physiological and biochemical responses of resistant and susceptible wheat to injury by Russian wheat aphid. J Econ Entomol 100:1692–1703
Sarma RK, Saikia R (2014) Alleviation of drought stress in mung bean by strain Pseudomonas aeruginosa GGRJ21. Plant Soil 377:111–126. https://doi.org/10.1007/s11104-013-1981-9
Selvakumar G, Panneerselvam P, Ganeshamurthy AN (2012) Bacterial mediated alleviation of abiotic stress in crops In: bacteria in agrobiology: stress management. Springer, pp 205–224
Simova-Stoilova L, Demirevska K, Petrova T, Tsenov N, Feller U (2008) Antioxidative protection in wheat varieties under severe recoverable drought at seedling stage. Plant Soil Environ 54:529–536
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Sinha P, Singh VK, Suryanarayana V, Krishnamurthy L, Saxena RK, Varshney RK (2015) Evaluation and validation of housekeeping genes as reference for gene expression studies in pigeonpea (Cajanus cajan) under drought stress conditions. PLoS ONE 10:e0122847
Sofi PA, Baba ZA, Hamid B, Meena RS (2018) Harnessing Soil Rhizobacteria for improving drought resilience in legumes. In: Meena R, Das A, Yadav G, Lal R (eds) Legumes for soil health and sustainable management. Springer
Takahashi S, Murata N (2008) How do environmental stresses accelerate photoinhibition. Trends Plant Sci 13:178–182
Tanentzap FM, Stempel A, Ryser P (2015) Reliability of leaf relative water content (RWC) measurements after storage: consequences for in situ measurements. Botany 93:535–541
Teulat B, Zoumarou-Wallis N, Rotter B, Ben Salem M, Bahri H, This D (2003) QTL for relative water content in field-grown barley and their stability across Mediterranean environments. Theor Appl Genet 108:181–188. https://doi.org/10.1007/s00122-003-1417-7
Timmusk S, Wagner EGH (1999) The plant-growth-promoting rhizobacterium Paenibacillus polymyxa induces changes in Arabidopsis thaliana gene expression: a possible connection between biotic and abiotic stress responses. Mol Plant Microbe 12:951–959
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG (2012) Primer3 - new capabilities and interfaces. Nucleic Acids Res Spec Publ 40:e115
Wilhite DA, Sivakumar MV, Pulwarty R (2014) Managing drought risk in a changing climate: the role of national drought policy. Weather Clim Extremes 3:4–13
Acknowledgements
This work was supported by the Tamil Nadu Agricultural University (TNAU), India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest, no animals or humans are used in this study and all authors accepted the final version submitted to the journal.
Rights and permissions
About this article
Cite this article
Devi, N.S.A., Kumutha, K., Anandham, R. et al. Induction of moisture stress tolerance by Bacillus and Paenibacillus in pigeon pea (Cajanus cajan. L). 3 Biotech 11, 355 (2021). https://doi.org/10.1007/s13205-021-02901-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-021-02901-w