Abstract
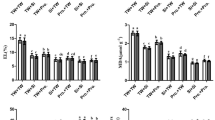
Purple corn (PC) is known to be rich in many bioactive and polyphenolic compounds and these compounds’ role in developing tolerance to unfavorable conditions is a matter of curiosity nowadays. We performed series of experiments to highlight the possible beneficial effects of Si priming [0, 0.75, 1.5, 3 and 6 mM] on PC’s A (photosynthesis rate), E (transpiration rate), WUE (water use efficiency), SPAD, LN (leaf number), SL (seedling length), SW (seedling weight), proline, MDA (malondialdehyde), TAC (total anthocyanin content), TAA (total antioxidant activity), TPC (total phenolic content), Chl A-B (chlorophyll A-B) and carotenoids both under deficit and well-watered conditions. There was a 1-week period between each trait sampling. Among all Si treatments, 1.5 mM (TAC, WUE, MDA) was the most effective under drought while effects of 0.75 mM (A, E, TPC, MDA) were mostly remarkable under well-watered conditions. 6 mM silicon priming triggered the mechanism of drought tolerance of PC by increasing proline and TAA levels while it decreased MDA concentrations. Maximum WUE was obtained from 1.5 mM Si, and the highest carotenoid level was obtained from 3 mM Si treatment under drought. Drought stress made the growth parameters decrease although Si primed seedlings were stronger than the non-primed ones (0.75, 3 mM). Silicon had enhancive effects on TAC, carotenoids, WUE and proline concentrations under well-watered conditions as well. According to our results, Si priming had remarkable effects on drought tolerance and also had beneficial effects under well-watered conditions; hence may be an alternative approach to prevent water waste in the possible PC breeding areas.


Similar content being viewed by others
References
Agarie S, Uchida H, Agata W, Kubota F, Kaufman PB (1998) Effects of silicon on transpiration and leaf conductance in rice plants (Oryza sativa L.). Plant Prod Sci 1:89–95
Ahmed M, Qadeer U, Ahmed ZI, Hassan F (2016) Improvement of wheat (Triticum aestivum) drought tolerance by seed priming with silicon. Arch Agron Soil Sci 62:299–315
Amin M, Ahmad R, Basra S, Murtaza G (2014) Silicon induced improvement in morpho-physiological traits of maize (Zea mays L.) under water deficit. Pak J Agric Sci 51(1):187–196. http://www.pakjas.com.pk/papers/2260.pdf
Anjum SA et al (2011) Gas exchange and chlorophyll synthesis of maize cultivars are enhanced by exogenously-applied glycinebetaine under drought conditions. Plant Soil Environ 57:326–331
Anjum SA, Tanveer M, Ashraf U, Hussain S, Shahzad B, Khan I, Wang L (2016) Effect of progressive drought stress on growth, leaf gas exchange, and antioxidant production in two maize cultivars. Environ Sci Pollut 23:17132–17141
Arabshahi M, Mobasser HR (2017) Effect of drought stress on carotenoid and chlorophyll contents and osmolyte accumulation. Med Chem Res 2:193–197
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beggs CJ, Wellmann E (1985) Analysis of light-controlled anthocyanin formation in coleoptiles of Zeu muys L.: B radiation. Photochem Photobiol 53:329–333
Biju S, Fuentes S, Gupta D (2017) Silicon improves seed germination and alleviates drought stress in lentil crops by regulating osmolytes, hydrolytic enzymes and antioxidant defense system. Plant Physiol Biochem 119:250–264
Bokhtiar SM, Huang HR, Li YR, Dalvi VA (2012) Effects of silicon on yield contributing parameters and its accumulation in abaxial epidermis of sugarcane leaf blades using energy dispersive X-ray analysis. J Plant Nutr 35:1255–1275
Boyer JS (1982) Plant productivity and environment. Science 218:443–448
Cervilla LM et al (2012) Parameters symptomatic for boron toxicity in leaves of tomato plants. J Bot 2012:1–17
Chalker-Scott L (1999) Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol 70:1–9
Chen D, Wang S, Yin L, Deng X (2018) How does silicon mediate plant water uptake and loss under water deficiency? Front Plant Sci 9:1–7
Cherif M, Asselin A, Bélanger RR (1994) Defense responses induced by soluble silicon in cucumber roots infected by Pythium spp. Phytopathology 84:236–242
Cia MC, Guimaraes ACR, Medici LO, Chabregas SM, Azevedo RA (2012) Antioxidant responses to water deficit by drought-tolerant and-sensitive sugarcane varieties. Ann Appl Biol 161:313–324
Dere Ş, Güneş T, Sıvacı R (1998) Spectrophotometric determination of chlorophyll-A, B and total carotenoid contents of some algae species using different solvents. Turk J Bot 22:13–18
Ding YF, Liang YC, Zhu J, Li ZJ (2007) Effects of silicon on plant growth, photosynthetic parameters and soluble sugar content in leaves of wheat under drought stress. Plant Nutr Fert Sci 13:471–478
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra S (2009) Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 29:185–212
Fei L, Gergory T, Giusti MM (2017) Health benefits of purple corn (Zea mays L.) phenolic compounds. Compr Rev Food Sci F 16:234–246
Gao X, Zou C, Wang L, Zhang F (2004) Silicon improves water use efficiency in maize plants. J Plant Nutr 27:1457–1470
Gao X, Zou C, Wang L, Zhang F (2006) Silicon decreases transpiration rate and conductance from stomata of maize plants. J Plant Nutr 29:1637–1647
Gong H, Zhu X, Chen K, Wang S, Zhang C (2005) Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci 169:313–321
Hamayun M, Sohn EY, Khan SA, Shinwari ZK, Khan AL, Lee IJ (2010) Silicon alleviates the adverse effects of salinity and drought stress on growth and endogenous plant growth hormones of soybean (Glycine max L.). Pak J Bot 42:1713–1722
Hameed A, Sheikh MA, Jamil A, Basra SMA (2013) Seed priming with sodium silicate enhances seed germination and seedling growth in wheat (Triticum aestivum L.) under water deficit stress induced by polyethylene glycol. Pak J Life Soc Sci 11:19–24
Hasan SA, Rabei SH, Nada RM, Abogadallah GM (2017) Water use efficiency in the drought-stressed sorghum and maize in relation to expression of aquaporin genes. Biol Plant 61:127–137
Hattori T et al (2007) Short term stomatal responses to light intensity changes and osmotic stress in sorghum seedlings raised with and without silicon. Environ Exp Bot 60:177–182
Kaliamoorthy S, Rao AS (1994) Effect of salinity on anthocyanin accumulation in the root of maize. Ind J Plant Physiol 37:169–170
Kaya C, Tuna L, Higgs D (2006) Effect of silicon on plant growth and mineral nutrition of maize grown under water stress conditions. J Plant Nutr 29:1469–1480
Khampas S, Lertrat K, Lomthaisong K, Suriharn B (2013) Variability in phytochemicals and antioxidant activity in corn at immaturity and physiological maturity stages. Int Food Res J 20:3149–3157
Kidd PS, Llugany M, Poschenrieder C, Gunse B, Barcelo J (2001) The role of root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.). J Exp Bot 52:1339–1352
Kim YH, Khan AL, Shinwari ZK, Kim DH, Waqas M, Kamran M, Lee IJ (2012) Silicon treatment to rice (Oryza sativa L. cv. ‘Gopumbyeo’) plants during different growth periods and its effects on growth and grain yield. Pak J Bot 44:891–897
Konrade D, Klava D (2017) Total content of phenolics and antioxidant activity in crispbreads with plant by-product addition. Rural Sustain Res 38:24–31
Latef AAA, Tran LSP (2016) Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Front Plant Sci 7:1–10
Lewin J, Reimann BEF (1969) Silicon and plant growth. Annu Rev Plant Physiol 20:289–304
Liang YC (1999) Effects of silicon on enzyme activity, and sodium, potassium and calcium concentration in barley under salt stress. Plant Soil 209:217–224
Liang YC, Si J, Römheld V (2005) Silicon uptake and transport is an active process in Cucumis sativus L. New Phytol 167:797–804
Liang Y, Hua H, Zhu YG, Zhang J, Cheng C, Romheld V (2006) Importance of plant species and external silicon concentration to active silicon uptake and transport. New Phytol 172:63–72
Liang Y, Sun W, Zhu YG, Christie P (2007) Mechanisms of silicon—mediated alleviation of abiotic stresses in higher plants: a review. Environ Pollut 147:422–428
Ma CC, Li QF, Gao YB, Xin TR (2004) Effects of silicon application on drought resistance of cucumber plants. Soil Sci Plant Nutr 50:623–632
Madhava RK, Sresty TVS (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci 157:113–128
Mafakheri A, Siosemardeh AF, Bahramnejad B, Struik PC, Sohrabi Y (2010) Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust J Crop Sci 4:580–585
Manivannan P, Jaleel CA, Sankar B, Kishorekumar A, Somasundaram R, Lakshmanan G, Panneerselvam R (2007) Growth, biochemical modifications and proline metabolism in Helianthus annuus L. as induced by drought stress. Colloids Surf B Biointerfaces 59:141–149
Matoh T, Kairusmee P, Takahashi E (1986) Salt-induced damage to rice plants and alleviation effect of silicate. Soil Sci Plant Nutr 32:295–304
Moller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Mona FAD, Abdullah AMS, Tahjib-Ul-Arif MD, Arafat AHAL (2021) Hydrogen sulfide priming can enhance the tolerance of artichoke seedlings to individual and combined saline-alkaline and aniline stresses. Plant Physiol Biochem 159:347–362
Nair AS, Abraham TK, Jaya DS (2008) Studies on the changes in lipid peroxidation and antioxidants in drought stress induced cowpea (Vigna unguiculata L.) varieties. J Environ Boil 29:689–691
Parveen A, Liu W, Hussain S, Asghar J, Perveen S, Xiong Y (2019) Silicon priming regulates morpho-physiological growth and oxidative metabolism in maize under drought stress. Plants 8:1–14
Qian Q, Zai W, Zhu Z, Yu JQ (2006) Effects of exogenous silicon on active oxygen scavenging systems in chloroplasts of cucumber (Cucumis sativus L.) seedlings under salt stress. J Plant Physiol Mol Biol 32:107–112
Razzaq M, Akram NA, Ashraf M, Naz H, Al-Qurainy F (2017) Interactive effect of drought and nitrogen on growth, some key physiological attributes and oxidative defense system in carrot (Daucus carota L.) plants. Sci Hortic 225:373–379
Richmond KE, Sussman M (2003) Got silicon? The non-essential beneficial plant nutrient. Curr Opin Plant Biol 6:268–272
Romero-Aranda MR, Jurado O, Cuartero J (2006) Silicon alleviates the deleterious salt effect on tomato plant growth by improving plant water status. J Plant Physiol 163:847–855
Sacala E (2009) Role of silicon on plant resistance to water stress. J Elementol 14:629–630
Savant NK, Korndörfer GH, Datnoff LE, Snyder GH (1999) Silicon nutrition and sugarcane production: a review. J Plant Nutr 22:1853–1903
Sayed SA, Gadallah MAA (2014) Effects of silicon on Zea mays plants exposed to water and oxygen deficiency. Russian J Plant Physiol 61:493–499
Schmidhuber J, Tubiello FN (2007) Global food security under climate change. Proc Natl Acad Sci USA 104:19703–19708
Shen X, Zhou Y, Duan L, Li Z, Eneji AE, Li J (2010) Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. J Plant Physiol 167:1248–1252
Sirisuntornlak N, Ghafoori S, Datta A, Arirob W (2019) Seed priming and soil incorporation with silicon influence growth and yield of maize under water deficit stress. Arch Agron Soil Sci 65:197–207
Tian XZ, Paengkoum P, Paengkoum S, Chupawadee S, Ban C, Thongpea S (2018) Short communication: purple corn (Zea mays L.) stover silage with abundant anthocyanins transferring anthocyanins composition to the milk and increasing antioxidant status of lactating dairy goats. J Dairy Sci 102:413–418
Tuna AL, Kaya C, Higgs D, Murillo-Amador B, Aydemir S, Girgin AR (2008) Silicon improves salinity tolerance in wheat plants. Environ Exp Bot 62:10–16
Ullah H, Datta A, Shrestha S, Din SU (2017) The effects of cultivation methods and water regimes on root systems of drought-tolerant (RD6) and drought-sensitive (RD10) rice varieties of Thailand. Arch Agron Soil Sci 63:1198–1209
Wellmann E, Hrazdina G, Grisebach H (1976) Induction of anthocyanin formation and of enzymes related to its biosynthesis by UV light in cell cultures of Haplopappus grarilis. Phvtochemistrv 15:913–915
Yavaş İ, Ünay A (2017) The role of silicon under biotic and abiotic stress conditions. Turk J Agric Res 4:204–209
Yeo A (1999) Predicting the interaction between the effects of salinity and climate change on crop plants. Sci Hortic 78:159–174
Yoshida S, Ohnishi Y, Kitagishi K (1959) Role of silicon in rice nutrition. Plant Soil 5:127–133
Zargar SM, Mahajan R, Bhat JA, Nazir M, Deshmukh R (2019) Role of silicon in plant stress tolerance: opportunities to achive a sustainable cropping system. 3 Biotech 9:1–16
Zheng M et al (2016) Seed priming in dry direct-seeded rice: consequences for emergence, seedling growth and associated metabolic events under drought stress. Plant Growth Regul 78:167–178
Zhu ZJ, Wei GQ, Li J, Qian QQ, Yu JQ (2004) Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci 167:527–533
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Özdemir, E. Silicon stimulated bioactive and physiological metabolisms of purple corn (Zea mays indentata L.) under deficit and well-watered conditions. 3 Biotech 11, 319 (2021). https://doi.org/10.1007/s13205-021-02873-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-021-02873-x