Abstract
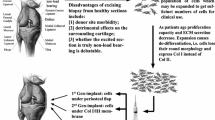
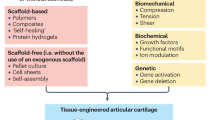
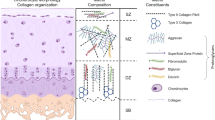
Lately, cellular-based cartilage joint therapies have gradually gained more attention, which leads to next generation bioengineering approaches in the development of cell-based medicinal products for human use in cartilage repair. The greatest hurdles of chondrocyte-based cartilage bioengineering are: (i) preferring the cell source; (ii) differentiation and expansion processes; (iii) the time necessary for chondrocyte expansion pre-implantation; and (iv) fixing the chondrocyte count in accordance with the lesion surface area of the patient in question. The chondrocyte presents itself to be the focal starting material for research and development of bioengineered cartilage-based medicinal products which promise the regeneration and restoration of non-orthopedic cartilage joint defects. Even though chondrocytes seem to be the first choice, inevitable complications related to proliferation, dedifferentation and redifferentiation are probable. Detailed studies are a necessity to fully investigate detailed culturing conditions, the chondrogenic strains of well-defined phenotypes and evaluation of the methods to be used in biomaterial production. Despite a majority of the current methods which aid amelioration of joint functionality, they are insufficient in fully restoring the natural structure and composition of the joint cartilage. Hence current studies have trended towards gene therapy, mesenchymal stem cells and tissue engineering practices. There are many studies addressing the outcomes of chondrocytes in the clinical scene, and many vital biomaterials have been developed for structuring the bioengineered cartilage. This study aims to convey to the audience the practical significance of chondrocyte-based clinical applications.


Similar content being viewed by others
References
Adkisson HD, Milliman C, Zhang X, Mauch K, Maziarz RT, Streeter PR (2010) Immune evasion by neocartilage-derived chondrocytes: implications for biologic repair of joint articular cartilage. Stem Cell Res 4(1):57–68. https://doi.org/10.1016/j.scr.2009.09.004
Amini AR, Laurencin CT, Nukavarapu SP (2012) Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 40(5):363–408. https://doi.org/10.1615/critrevbiomedeng.v40.i5.10
Angele P, Kujat R, Nerlich M, Yoo J, Goldberg V, Johnstone B (1999) Engineering of osteochondral tissue with bone marrow mesenchymal progenitor cells in a derivatized hyaluronan-gelatin composite sponge. Tissue Eng 5(6):545–554. https://doi.org/10.1089/ten.1999.5.545
Arakaki K, Kitamura N, Fujiki H, Kurokawa T, Iwamoto M, Ueno M, Kanaya F, Osada Y, Gong JP, Yasuda K (2010) Artificial cartilage made from a novel double-network hydrogel: in vivo effects on the normal cartilage and ex vivo evaluation of the friction property. J Biomed Mater Res A 93(3):1160–1168. https://doi.org/10.1002/jbm.a.32613
Archer CW, Francis-West P (2003) The chondrocyte. Int J Biochem Cell Biol 35(4):401–404. https://doi.org/10.1016/s1357-2725(02)00301-1
Atilla E, Kilic P, Gurman G (2018) Cellular therapies: day by day, all the way. Transfus Apher Sci. 57(2):187–196. https://doi.org/10.1016/j.transci.2018.04.019
Bartus K, James ND, Didangelos A, Bosch KD, Verhaagen J, Yáñez-Muñoz RJ, Rogers JH, Schneider BL, Muir EM, Bradbury EJ (2014) Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J Neurosci 34(14):4822–4836. https://doi.org/10.1523/JNEUROSCI.4369-13.2014
Baugé C, Boumédiene K (2015) Use of adult stem cells for cartilage tissue engineering: current status and future developments. Stem Cells Int. https://doi.org/10.1155/2015/438026
Bellavia D, Veronesi F, Carina V, Costa V, Raimondi L, De Luca A, Alessandro R, Fini M, Giavaresi G (2018) Gene therapy for chondral and osteochondral regeneration: is the future now? Cell Mol Life Sci 75(4):649–667. https://doi.org/10.1007/s00018-017-2637-3
Benya PD, Shaffer JD (1982) Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 30(1):215–224. https://doi.org/10.1016/0092-8674(82)90027-7
Boyd NL, Robbins KR, Dhara SK, West FD, Stice SL (2009) Human embryonic stem cell-derived mesodermlike epithelium transitions to mesenchymal progenitor cells. Tissue Eng Part A 15:1897. https://doi.org/10.1089/ten.tea.2008.0351
Brigham & Women’s Hospital Massachusetts, US (2007) Standard of care: autologous chondrocyte implantation (ACI), pp 1–8. https://www.brighamandwomens.org/assets/BWH/patients-and-families/rehabilitation-services/pdfs/knee-aci-bwh.pdf. Accessed 09 Jan 2020
Brittberg M (2008) Autologous chondrocyte implantation–technique and long-term follow-up. Injury 39(Suppl 1):40–49. https://doi.org/10.1016/j.injury.2008.01.040
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331(14):889–895. https://doi.org/10.1056/NEJM199410063311401
Brittberg M, Tallheden T, Sjögren-Jansson B, Lindahl A, Peterson L (2001) Autologous chondrocytes used for articular cartilage repair: an update. Clin Orthop Relat Res 391(Suppl):337–348. https://doi.org/10.1097/00003086-200110001-00031
Brittberg M, Peterson L, Sjögren-Jansson E, Tallheden T, Lindahl A (2003) Articular cartilage engineering with autologous chondrocyte transplantation. A review of recent developments. J Bone Jt Surg Am. 85-A(Suppl 3):109–115. https://doi.org/10.2106/00004623-200300003-00017
Brower-Toland BD, Saxer RA, Goodrich LR, Mi Z, Robbins PD, Evans CH, Nixon AJ (2001) Direct adenovirus-mediated insulin-like growth factor I gene transfer enhances transplant chondrocyte function. Hum Gene Ther 12(2):117–129. https://doi.org/10.1089/104303401750061186
Caron MM, Emans PJ, Coolsen MM, Voss L, Surtel DA, Cremers A, van Rhijn LW, Welting TJ (2012) Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthr Cartil 20(10):1170–1178. https://doi.org/10.1016/j.joca.2012.06.016
Chen FH, Rousche KT, Tuan RS (2006) Technology insight: adult stem cells in cartilage regeneration and tissue engineering. Nat Clin Pract Rheumatol. 2(7):373–382. https://doi.org/10.1038/ncprheum0216
Cheng A, Kapacee Z, Peng J, Lu S, Lucas RJ, Hardingham TE, Kimber SJ (2014) Cartilage repair using human embryonic stem cell-derived chondroprogenitors. Stem Cells Transl Med. 3(11):1287–1294. https://doi.org/10.5966/sctm.2014-0101
Cheng A, Cain SA, Tian P, Baldwin AK, Uppanan P, Kielty CM, Kimber SJ (2018) Recombinant extracellular matrix protein fragments support human embryonic stem cell chondrogenesis. Tissue Eng Part A 24:968–978. https://doi.org/10.1089/ten.TEA.2017.0285
Claus S, Aubert-Foucher E, Demoor M, Camuzeaux B, Paumier A, Piperno M, Damour O, Duterque-Coquillaud M, Galéra P, Mallein-Gerin F (2010) Chronic exposure of bone morphogenetic protein-2 favors chondrogenic expression in human articular chondrocytes amplified in monolayer cultures. J Cell Biochem 111(6):1642–1651. https://doi.org/10.1002/jcb.22897
Cooper KL, Oh S, Sung Y, Dasari RR, Kirschner MW, Tabin CJ (2013) Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature 495(7441):375–378. https://doi.org/10.1038/nature11940
Craft AM, Ahmed N, Rockel JS, Baht GS, Alman BA, Kandel RA, Grigoriadis AE, Keller GM (2013) Specification of chondrocytes and cartilage tissues from embryonic stem cells. Development 140:2597–2610. https://doi.org/10.1242/dev.087890
D’Andrea F, De Francesco F, Ferraro GA, Desiderio V, Tirino V, De Rosa A, Papaccio G (2008) Large-scale production of human adipose tissue from stem cells: a new tool for regenerative medicine and tissue banking. Tissue Eng Part C Methods 14(3):233–242. https://doi.org/10.1089/ten.tec.2008.0108
Danisovic L, Varga I, Zamborsky R, Böhmer D (2012) The tissue engineering of articular cartilage: cells, scaffolds and stimulating factors. Exp Biol Med (Maywood). 237(1):10–17. https://doi.org/10.1258/ebm.2011.011229
Davatchi F, Abdollahi BS, Mohyeddin M, Shahram F, Nikbin B (2011) Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 14(2):211–215. https://doi.org/10.1111/j.1756-185X.2011.01599.x
Davies RL, Kuiper NJ (2019) Regenerative medicine: a review of the evolution of autologous chondrocyte implantation (ACI) therapy. Bioengineering (Basel). https://doi.org/10.3390/bioengineering6010022
De Windt TS, Saris DB, Slaper-Cortenbach IC, van Rijen MH, Gawlitta D, Creemers LB, de Weger RA, Dhert WJ, Vonk LA (2015) Direct cell-cell contact with chondrocytes is a key mechanism in multipotent mesenchymal stromal cell-mediated chondrogenesis. Tissue Eng Part A 21(19–20):2536–2547. https://doi.org/10.1089/ten.TEA.2014.0673
Demoor M, Ollitrault D, Gomez-Leduc T, Bouyoucef M, Hervieu M, Lafont J, Denoix JM, Audigié F, Mallein-Gerin F, Legendre F, Galera P (2014) Cartilage tissue engineering: molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochem Biophys Acta 1840:2414–2440. https://doi.org/10.1016/j.bbagen.2014.02.030
Deng Z, Jin J, Zhao J, Xu H (2016) Cartilage defect treatments: with or without cells? Mesenchymal stem cells or chondrocytes? Traditional or matrix-assisted? A systematic review and meta-analyses. Stem Cells Int. https://doi.org/10.1155/2016/9201492
Dewan AK, Gibson MA, Elisseeff JH, Trice ME (2014) Evolution of autologous chondrocyte repair and comparison to other cartilage repair techniques. Biomed Res Int 2014:272481. https://doi.org/10.1155/2014/272481
Diekman BO, Christoforou N, Willard VP, Sun H, Sanchez-Adams J, Leong KW, Guilak F (2012) Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc Natl Acad Sci USA 109:19172. https://doi.org/10.1073/pnas.1210422109/-/DCSupplemental
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Dj Prockop, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317. https://doi.org/10.1080/14653240600855905
Elder BD, Eleswarapu SV, Athanasiou KA (2009) Extraction techniques for the decellularization of tissue engineered articular cartilage constructs. Biomaterials 30(22):3749–3756. https://doi.org/10.1016/j.biomaterials.2009.03.050
Evans CH, Gouze JN, Gouze E, Robbins PD, Ghivizzani SC (2004) Osteoarthritis gene therapy. Gene Ther 11(4):379–389. https://doi.org/10.1038/sj.gt.3302196
Foss C, Merzari E, Migliaresi C, Motta A (2013) Silk fibroin/hyaluronic acid 3D matrices for cartilage tissue engineering. Biomacromolecules 14(1):38–47. https://doi.org/10.1021/bm301174x
Garcia J, Mennan C, McCarthy HS, Roberts S, Richardson JB, Wright KT (2016) Chondrogenic potency analyses of donor-matched chondrocytes and mesenchymal stem cells derived from bone marrow, infrapatellar fat pad, and subcutaneous fat. Stem Cells Int 2016:6969726. https://doi.org/10.1155/2016/6969726
Gille J, Behrens P, Schulz AP, Oheim R, Kienast B (2016) Matrix-associated autologous chondrocyte implantation: a clinical follow-up at 15 years. Cartilage. 7(4):309–315. https://doi.org/10.1177/1947603516638901
Glowacki J, Mizuno S (2008) Collegen scaffolds for tissue engineering. Biopolymers 89(5):338–344. https://doi.org/10.1002/bip.20871
Gosset M, Berenbaum F, Thirion S, Jacques C (2008) Primary culture and phenotyping of murine chondrocytes. Nat Protoc. https://doi.org/10.1038/nprot.2008.95
Grigolo B, Lisignoli G, Piacentini A, Fiorini M, Gobbi P, Mazzotti G, Duca M, Pavesio A, Facchini A (2002) Evidence for redifferentiation of human chondrocytes grown on a hyaluronan-based biomaterial (HYAff 11): molecular, immunohistochemical and ultrastructural analysis. Biomaterials 23(4):1187–1195. https://doi.org/10.1016/s0142-9612(01)00236-8
Grol MW, Lee BH (2018) Gene therapy for repair and regeneration of bone and cartilage. Curr Opin Pharmacol 40(1):59–66. https://doi.org/10.1016/j.coph.2018.03.005
Guilak F, Alexopoulos LG, Upton ML, Youn I, Choi JB (2006) The pericellular matrix as a trancducer of biomechanical and biochemical signals in articular cartilage. Ann N Y Acad Sci 1068:498–512. https://doi.org/10.1196/annals.1346.011
Haddo O, Mahroof S, Higgs D, David L, Pringle J, Bayliss M, Cannon SR, Briggs TW (2004) The use of chondrogide membrane in autologous chondrocyte implantation. Knee. 11(1):51–55. https://doi.org/10.1016/S0968-0160(03)00041-3
Hamahashi K, Sato M, Yamato M, Kokubo M, Mitani G, Ito S, Nagai T, Ebihara G, Kutsuna T, Okano T, Mochida J (2015) Studies of the humoral factors produced by layered chondrocyte sheets. J Tissue Eng Regener Med. 9(1):24–30. https://doi.org/10.1002/term.1610
Harimoto M, Yamato M, Hirose M, Takahashi C, Isoi Y, Kikuchi A, Okano T (2002) Novel approach for achieving double-layered cell sheets co-culture: overlaying endothelial cell sheets onto monolayer hepatocytes utilizing temperature-responsive culture dishes. J Biomed Mater Res 62(3):464–470. https://doi.org/10.1002/jbm.10228
Hellgren I, Drvota V, Pieper R, Enoksson S, Blomberg P, Islam KB, Sylvén C (2000) Highly efficient cell-mediated gene transfer using non-viral vectors and FuGene6: in vitro and in vivo studies. Cell Mol Life Sci 57(8–9):1326–1333. https://doi.org/10.1007/pl00000769
Hoch AI, Leach JK (2014) Concise review: optimizing expansion of bone marrow mesenchymal stem/stromal cells for clinical applications. Stem Cells Transl Med. 3(5):643–652. https://doi.org/10.5966/sctm.2013-0196
Hori Y, Winans AM, Irvine DJ (2009) Modular injectable matrices based on alginate solution/microsphere mixtures that gel in situ and co-deliver immunomodulatory factors. Acta Biomater 5(4):969–982. https://doi.org/10.1016/j.actbio.2008.11.019
Huang BJ, Hu JC, Athanasiou KA (2016) Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 98:1–22. https://doi.org/10.1016/j.biomaterials.2016.04.018
Hunter W (1995) Of the structure and disease of articulating cartilages. 1743. Clin Orthop Relat Res 317:3–6. https://doi.org/10.1098/rstl.1742.0079
Im GI, Lee JH (2010) Repair of osteochondral defects with adipose stem cells and a dual growth factor-releasing scaffold in rabbits. J Biomed Mater Res B Appl Biomater 92(2):552–560. https://doi.org/10.1002/jbm.b.31552
Ishibashi M, Hikita A, Fujihara Y, Takato T, Hoshi K (2017) Human auricular chondrocytes with high proliferation rate show high production of cartilage matrix. Regen Ther. 6:21–28. https://doi.org/10.1016/j.reth.2016.11.001
Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, Ra JC, Oh S, Yoon KS (2014) Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 32(5):1254–1266. https://doi.org/10.1002/stem.1634
Jones DG, Peterson L (2006) Autologous chondrocyte implantation. J Bone Jt Surg Am 88(11):2502–2520. https://doi.org/10.2106/00004623-200611000-00025
Jones IA, Wilson M, Togashi R, Han B, Mircheff AK, Thomas Vangsness Jr C (2018) A randomized, controlled study to evaluate the efficacy of intra-articular, autologous adipose tissue injections for the treatment of mild-to-moderate knee osteoarthritis compared to hyaluronic acid: a study protocol. BMC Musculoskelet Disord. 19(1):383. https://doi.org/10.1186/s12891-018-2300-7
Kafienah W, Jakob M, Démarteau O, Frazer A, Barker MD, Martin I, Hollander AP (2002) Three-dimensional tissue engineering of hyaline cartilage: comparison of adult nasal and articular chondrocytes. Tissue Eng 8(5):817–826. https://doi.org/10.1089/10763270260424178
Kafienah W, Al-Fayez F, Hollander AP, Barker MD (2003) Inhibition of cartilage degradation: a combined tissue engineering and gene therapy approach. Arthritis Rheum 48(3):709–718. https://doi.org/10.1002/art.10842
Kim MJ, Son MJ, Son MY, Seol B, Kim J, Park J, Kim JH, Kim YH, Park SA, Lee CH, Lee KS, Han YM, Chang JS, Cho YS (2011) Generation of human induced pluripotent stem cells from osteoarthritis patient-derived synovial cells. Arthritis Rheum 63(10):3010–3021. https://doi.org/10.1002/art.30488
Kim MK, Ha CW, In Y, Cho SD, Choi ES, Ha JK, Lee JH, Yoo JD, Bin SI, Choi CH, Kyung HS, Lee MC (2018) A multicenter, double-blind, phase III clinical trial to evaluate the efficacy and safety of a cell and gene therapy in knee osteoarthritis patients. Hum Gene Ther Clin Dev. 27(1):10. https://doi.org/10.1089/humc.2017.249
Koh YG, Choi YJ (2012) Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 19(6):902–907. https://doi.org/10.1016/j.knee.2012.04.001
Koh YG, Jo SB, Kwon OR, Suh DS, Lee SW, Park SH, Choi YJ (2013) Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy 29(4):748–755. https://doi.org/10.1016/j.arthro.2012.11.017
Kozhemyakina E, Lassar AB, Zelzer E (2015) A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation. Development. 142(5):817–883. https://doi.org/10.1242/dev.105536
Kushida A, Yamato M, Konno C, Kikuchi A, Sakurai Y, Okano T (2000) Temperature-responsive culture dishes allow nonenzymatic harvest of differentiated Madin–Darby canine kidney (MDCK) cell sheets. J Biomed Mater Res 51(2):216–223. https://doi.org/10.1002/(sici)1097-4636(200008)51:2%3c216:aid-jbm10%3e3.0.co;2-k
Lai JH, Kajiyama G, Smith RL, Maloney W, Yang F (2013) Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Sci Rep. 3:3553. https://doi.org/10.1038/srep03553
Lee J, Smeriglio P, Chu CR, Bhutani N (2017) Human iPSC-derived chondrocytes mimic juvenile chondrocyte function for the dual advantage of increased proliferation and resistance to IL-1β. Stem Cell Res Ther. 8(1):244. https://doi.org/10.1186/s13287-017-0696-x
Li KC, Hu YC (2015) Cartilage tissue engineering: recent advances and perspectives from gene regulation/therapy. Adv Healthc Mater. 4(7):948–968. https://doi.org/10.1002/adhm.201400773
Lin Z, Willers C, Xu J, Zheng MH (2006) The chondrocyte: biology and clinical application. Tissue Eng 12(7):1971–1984. https://doi.org/10.1089/ten.2006.12.1971
Madry H, Cucchiarini M (2013) Advances and challenges in gene-based approaches for osteoarthritis. J Gene Med. 15(10):343–355. https://doi.org/10.1002/jgm.2741
Madry H, Zurakowski D, Trippel SB (2001) Overexpression of human insulin-like growth factor-I promotes new tissue formation in an ex vivo model of articular chondrocyte transplantation. Gene Ther 8(19):1443–1449. https://doi.org/10.1038/sj.gt.3301535
Madry H, Kaul G, Cucchiarini M, Stein U, Zurakowski D, Remberger K, Menger MD, Kohn D, Trippel SB (2005) Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor 1 (IGF-1). Gene Ther 12(15):1171–1179. https://doi.org/10.1038/sj.gt.3302515
Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA (2015) Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol 11(1):21–34. https://doi.org/10.1038/nrrheum.2014.157
Marcacci M, Kon E, Zaffagnini S (2007) Arthroscopic second generation autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc 15:610–619. https://doi.org/10.1007/s00167-006-0265-9
Martowicz M, Laska J (2010) Polymer biomaterials for skin regeneration: a review. Eng Biomater 13:2–9
McCarthy HS, Roberts S (2013) A histological comparison of the repair tissue formed when using either Chondrogide(®) or periosteum during autologous chondrocyte implantation. Osteoarthr Cartil 21(12):2048–2057. https://doi.org/10.1016/j.joca.2013.10.004
Mobasheri A, Kalamegam G, Musumeci G, Batt ME (2014) Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas. 78(3):188–198. https://doi.org/10.1016/j.maturitas.2014.04.017
Mumme M, Barbero A, Miot S, Wixmerten A, Feliciano S, Wolf F, Asnaghi AM, Baumhoer D, Bieri O, Kretzschmar M, Pagenstert G (2016) Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet. 388:1985–1994. https://doi.org/10.1016/S0140-6736(16)31658-0
Nagaya H, Ymagata T, Ymagata S, Iyoda K, Ito H, Hasegawa Y, Iwata H (1999) Examination of synovial fluid and serum hyaluronidase activity as a joint marker in rheumatoid arthritis and osteoarthritis patients (by zymography). Ann Rheum Dis 58(3):186–188. https://doi.org/10.1136/ard.58.3.186
Nakagawa T, Lee SY, Reddi AH (2009) Induction of chondrogenesis from human embryonic stem cells without embryoid body formation by bone morphogenetic protein 7 and transforming growth factor beta1. Arthritis Rheum 60:3686. https://doi.org/10.1002/art.27229
Naranda J, Gradišnik L, Gorenjak M, Vogrin M, Maver U (2017) Isolation and characterization of human articular chondrocytes from surgical waste after total knee arthroplasty (TKA). PeerJ. 21(5):e3079. https://doi.org/10.7717/peerj.3079
Niemeyer P, Pestka JM, Kreuz PC, Erggelet C, Schmal H, Suedkamp NP, Steinwachs M (2008) Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. Am J Sports Med 36(11):2091–2099. https://doi.org/10.1177/0363546508322131
Niemeyer P, Porichis S, Steinwachs M, Erggelet C, Kreuz PC, Schmal H, Uhl M, Ghanem N, Südkamp NP, Salzmann G (2014) Long-term outcomes after first-generation autologous chondrocyte implantation for cartilage defects of the knee. Am J Sports Med 42(1):150–157. https://doi.org/10.1177/0363546513506593
Nishida K, Yamato M, Hayashida Y, Watanabe K, Maeda N, Watanabe H, Yamamoto K, Nagai S, Kikuchi A, Tano Y, Okano T (2004) Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation 77(3):379–385. https://doi.org/10.1097/01.TP.0000110320.45678.30
Nukavarapu SP, Amini AR (2011) Optimal scaffold design and effective progenitor cell identification for the regeneration of vascularized bone. Conf Proc IEEE Eng Med Biol Soc. https://doi.org/10.1109/IEMBS.2011.6090684
Nukavarapu SP, Dorcemus DL (2013) Osteochondral tissue engineering: current strategies and challenges. Biotechnol Adv 31(5):706–721. https://doi.org/10.1016/j.biotechadv.2012.11.004
O’Brien FJ, Harley BA, Yannas IV, Gibson LJ (2005) The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 26(4):433–441
Ochi M, Uchio Y, Tobita M, Kuriwaka M (2001) Current concepts in tissue engineering technique for repair of cartilage defect. Artif Organs 25:172–179. https://doi.org/10.1046/j.1525-1594.2001.025003172.x
Ogura T, Mosier BA, Bryant T, Minas T (2017) A 20-year follow-up after first-generation autologous chondrocyte implantation. Am J Sports Med 45(12):2751–2761. https://doi.org/10.1177/0363546517716631
Oh SH, Kim TH, Im GI, Lee JH (2010) Investigation of pore size effect on chondrogenic differentiation of adipose stem cells using pore size gradient scaffold. Biomacromolecules 11(8):1948–1955. https://doi.org/10.1021/bm100199m
Okano T, Yamada N, Sakai H, Sakurai Y (1993) A novel recovery-system for cultured-cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J Biomed Mater Res 27:1243–1251. https://doi.org/10.1002/jbm.820271005
Oldershaw RA, Baxter MA, Lowe ET, Bates N, Grady LM, Soncin F, Brison DR, Hardingham TE, Kimber SJ (2010) Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol 28(11):1187–1194. https://doi.org/10.1038/nbt.1683
Olee T, Grogan SP, Lotz MK, Colwell CW Jr, D’Lima DD, Snyder EY (2014) Repair of cartilage defects in arthritic tissue with differentiated human embryonic stem cells. Tissue Eng Part A 20(3–4):683–692. https://doi.org/10.1089/ten.TEA.2012.0751
Onofrillo C, Duchi S, O’Connell CD, Blanchard R, O’Connor AJ, Scott M, Wallace GG, Choong PFM, Di Bella C (2018) Biofabrication of human articular cartilage: a path towards the development of a clinical treatment. Biofabrication. 10(4):045006. https://doi.org/10.1088/1758-5090/aad8d9
Oseni AO, Butler PE, Seifalian AM (2013) Optimization of chondrocyte isolation and characterization for large-scale cartilage tissue engineering. J Surg Res 181(1):41–48. https://doi.org/10.1016/j.jss.2012.05.087
Owida HA, De Las Heras Ruiz T, Dhillon A, Yang Y, Kuiper NJ (2017) Co-culture of chondrons and mesenchymal stromal cells reduces the loss of collagen VI and improves extracellular matrix production. Histochem Cell Biol 148(6):625–638. https://doi.org/10.1007/s00418-017-1602-4
Pascher A, Palmer GD, Steinert A, Oligino T, Gouze E, Gouze JN, Betz O, Spector M, Robbins PD, Evans CH, Ghivizzani SC (2004) Gene delivery to cartilage defects using coagulated bone marrow aspirate. Gene Ther 11(2):133–141. https://doi.org/10.1038/sj.gt.3302155
Pei M, He F (2012) Extracellular matrix deposited by synovium-derived stem cells delays replicative senescent chondrocyte dedifferentiation and enhances redifferentiation. J Cell Physiol 227(5):2163–2174. https://doi.org/10.1002/jcp.22950
Pei M, He F, Kish VL, Vunjak-Novakovic G (2008) Engineering of functional cartilage tissue using stem cells from synovial lining: a preliminary study. Clin Orthop Relat Res 466(8):1880–1889. https://doi.org/10.1007/s11999-008-0316-2
Pei M, He F, Boyce BM, Kish VL (2009) Repair of full-thickness femoral condyle cartilage defects using allogeneic synovial cell-engineered tissue constructs. Osteoarthr Cartil 17(6):714–722. https://doi.org/10.1016/j.joca.2008.11.017
Phull AR, Eo SH, Abbas Q, Ahmed M, Kim SJ (2016) Applications of chondrocyte-based cartilage engineering: an overview. Biomed Res Int 2016:1879837. https://doi.org/10.1155/2016/1879837
Sakaguchi Y, Sekiya I, Yagishita K, Muneta T (2005) Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum 52(8):2521–2529. https://doi.org/10.1002/art.21212
Saxer RA, Bent SJ, Brower-Toland BD, Mi Z, Robbins PD, Evans CH, Nixon AJ (2001) Gene mediated insulin-like growth factor-I delivery to the synovium. J Orthop Res 19(5):759–767. https://doi.org/10.1016/S0736-0266(00)00077-2
Shafa M, Sjonnesen K, Yamashita A, Liu S, Michalak M, Kallos MS, Rancourt DE (2012) Expansion and long-term maintenance of induced pluripotent stem cells in stirred suspension bioreactors. J Tissue Eng Regen Med. 6:462–472. https://doi.org/10.1002/term.450
Shafiee A, Kabiri M, Langroudi L, Soleimani M, Ai J (2016) Evaluation and comparison of the in vitro characteristics and chondrogenic capacity of four adult stem/progenitor cells for cartilage-based repair. J Biomed Mater Res A. 104(3):600–610. https://doi.org/10.1002/jbm.a.35603
Shen Y, Fu Y, Wang J, Li G, Zhang X, Xu Y, Lin Y (2014) Biomaterial and mesenchymal stem cells for articular cartilage reconstruction. Curr Stem Cell Res Ther 9(3):254–267. https://doi.org/10.2174/1574888x09666140213202700
Shi S, Mercer S, Trippel SB (2010) Effect of transfection strategy on growth factor overexpression by articular chondrocytes. J Orthop Res 28(1):103–109. https://doi.org/10.1002/jor.20945
Shimizu T, Sekine H, Yang J, Isoi Y, Yamato M, Kikuchi A, Kobayashi E, Okano T (2006) Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 20(6):708–710. https://doi.org/10.1096/fj.05-4715fje
Shui W, Yin L, Luo J, Li R, Zhang W, Zhang J, Huang W, Hu N, Liang X, Deng ZL, Hu Z, Shi LL, Luu HH, Haydon RC, He TC, Ho SH (2013) Characterization of chondrocyte scaffold carriers for cell-based gene therapy in articular cartilage repair. J Biomed Mater Res A 101(12):3542–3550. https://doi.org/10.1002/jbm.a.34661
Somoza RA, Welter JF, Correa D, Caplan AI (2014) Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng Part B Rev 20(6):596–608. https://doi.org/10.1089/ten.TEB.2013.0771
Song Y, Du H, Dai C, Zhang L, Li S, Hunter DJ, Lu L, Bao C (2018) Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen Med 13(3):295–307. https://doi.org/10.2217/rme-2017-0152
Sopena Juncosa JJ, Carillo Poveda JM, Rubio Zaragoza M, Redondo Garcia JI, Serra Aguado I, Soler Canet C (2000) Estructura y función del cartílago articular. Portada (Armas Frente a la Patología Articular) 1:24–26. https://doi.org/10.1016/S1286-935X(05)43399-7
Stoop R (2008) Smart biomaterials for tissue engineering of cartilage. Injury 39(Suppl 1):77–87. https://doi.org/10.1016/j.injury.2008.01.036
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 25 126(4):663–676. https://doi.org/10.1016/j.cell.2006.07.024
Tavassoli A, Mahdavi-Shahri N, Matin MM, Fereidoni M, Shahabipour F (2014) Bovine articular cartilage decellularized matrix as a scaffold for use in cartilage tissue engineering. Iran J Vet Sci Technol 4(1):1–8. https://doi.org/10.22067/veterinary.v4i1.17878
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145–1147. https://doi.org/10.1126/science.282.5391.1145
Toh WS, Lee EH, Guo XM, Chan JK, Yeow CH, Choo AB, Cao T (2010) Cartilage repair using hyaluronan hydrogel-encapsulated human embryonic stem cell-derived chondrogenic cells. Biomaterials 31:6968–6980. https://doi.org/10.1016/j.biomaterials.2010.05.064
Trzeciak T, Augustyniak E, Ritcher M, Kaczmarczyk J, Suchorska WM (2014) Induced pluripotent and mesenchymal stem cells as a promising tool for articular cartilage regeneration. J Cell Sci Ther. https://doi.org/10.4172/2157-7013.1000172
Vedicherla S, Buckley CT (2017) Rapid chondrocyte isolation for tissue engineering applications: the effect of enzyme concentration and temporal exposure on the matrix forming capacity of nasal derived chondrocytes. Biomed Res Int. https://doi.org/10.1155/2017/2395138
Vega A, Martín-Ferrero MA, Del Canto F, Alberca M, García V, Munar A, Orozco L, Soler R, Fuertes JJ, Huguet M, Sánchez A, García-Sancho J (2015) Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation 99(8):1681–1690. https://doi.org/10.1097/TP.0000000000000678
Venkatesan JK, Frisch J, Rey-Rico A, Schmitt G, Madry H, Cucchiarini M (2017) Impact of mechanical stimulation on the chondrogenic processes in human bone marrow aspirates modified to overexpress sox9 via rAAV vectors. J Exp Orthop 4(1):22. https://doi.org/10.1186/s40634-017-0097-1
Vinatier C, Mrugala D, Jorgensen C, Guicheux J, Noël D (2009) Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends Biotechnol 27(5):307–314. https://doi.org/10.1016/j.tibtech.2009.02.005
Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM (1994) Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Jt Surg Am 76(4):579–592. https://doi.org/10.2106/00004623-199404000-00013
Wakitani S, Kawaguchi A, Tokuhara Y, Takaoka K (2008) Present status and future direction for articular cartilage. J Bone Miner Metab 26:115–122. https://doi.org/10.1007/s00774-007-0802-8
Wakitani S, Okabe T, Horibe S, Mitsuoka T, Saito M, Koyama T, Nawata M, Tensho K, Kato H, Uematsu K, Kuroda R, Kurosaka M, Yoshiya S, Hattori K, Ohgushi H (2011) Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med. 5(2):146–150. https://doi.org/10.1002/term.299
Wang X, Wenk E, Zhang X, Meinel L, Vunjak-Novakovic G, Kaplan DL (2009) Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J Control Release 134(2):81–90. https://doi.org/10.1016/j.jconrel.2008.10.021
Waters NP, Stoker AM, Carson WL, Pfeiffer FM, Cook JL (2014) Biomarkers affected by impact velocity and maximum strain of cartilage during injury. J Biomech 47(12):3185–3195. https://doi.org/10.1016/j.jbiomech.2014.06.015
Weisleder N, Takizawa N, Lin P, Wang X, Cao C, Zhang Y, Tan T, Ferrante C, Zhu H, Chen PJ, Yan R, Sterling M, Zhao X, Hwang M, Takeshima M, Cai C, Cheng H, Takeshima H, Xiao RP, Ma J (2012) Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Sci Transl Med. 4(139):139ra85. https://doi.org/10.1126/scitranslmed.3003921
Yu P, Wang X, Fu YX (2006) Enhanced local delivery with reduced systemic toxicity: delivery, delivery, and delivery. Gene Ther 13(15):1131–1132. https://doi.org/10.1038/sj.gt.3302760
Zhang W, Ouyang H, Dass CR (2016) Current research on pharmacologic and regenerative therapies for osteoarthritis. Xu J Bone Res 4:15040. https://doi.org/10.1038/boneres.2015.40
Zhou C, Zheng H, Seol D, Yu Y, Martin JA (2014) Gene expression profiles reveal that chondrogenic progenitor cells and synovial cells are closely related. J Orthop Res 32(8):981–988. https://doi.org/10.1002/jor.22641
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sectors. Pelin Kilic, Damla Alkaya and Cansu Grucan performed the literature search and data analysis, and all authors had the idea for the article and thereafter drafted and critically revised the work.
Appendix 1
Appendix 1
A list of some cell-based, tissue engineered autologous chondrocyte therapies which are either on the market, were reluctant to reach the market or were withdrawn (Huang et al. 2016; Atilla et al. 2018)
Product proprietary name | Active composition | Current regulatory status |
|---|---|---|
Biocart II | Autologous chondrocytes (passage number unknown) | No progress after Phase II clinical studies |
Bioseed-C | Autologous chondrocytes (passage number unknown) | Open to access only in some European countries |
Cartipatch | Autologous chondrocytes (up to passage 3) | Phase III clinical studies ceased |
Chondrosphere | Autologous chondrocytes (passage number unknown) | Phase III clinical studies expected to finish in 2020 |
Hyalograft C | Autologous chondrocytes (up to passage 3) | Withdrawn from the market |
MACI | Autologous chondrocytes (passages 1-3) | Too high price margin, manufacturing plant shut down due to reluctancy in the cover of expenditures |
NeoCart | Autologous chondrocytes (passage number unknown) | Phase III clinical studies completed in 2017 |
NOVOCART 3D | Autologous chondrocytes (passage 1) | Phase III clinical studies expected to finish in 2019 |
RevaFlex | Allogeneic, juvenile chondrocytes (passage number unknown) | Phase III clinical studies expected to finish in 2019 |
CaReS | Autologous chondrocytes (primary culture) | Delivered to Turkey, Iran and China but no access due to the high price margin |
INSTRUCT | Autologous chondrocytes (primary culture) + bone marrow cells | Phase II clinical studies completed in 2014 |
ChondroCelect | Autologous chondrocytes containing specific marker proteins | Withdrawn from the market upon request of the authorization holder |
Spherox | Autologous chondrocyte spheroids | On the market |
Rights and permissions
About this article
Cite this article
Alkaya, D., Gurcan, C., Kilic, P. et al. Where is human-based cellular pharmaceutical R&D taking us in cartilage regeneration?. 3 Biotech 10, 161 (2020). https://doi.org/10.1007/s13205-020-2134-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-2134-5