Abstract
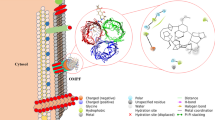
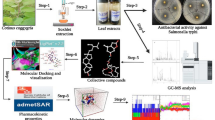
Melianone, the protolimonoid (24, 25-epoxyflindissone), was isolated from the medicinal tree species, Swietenia mahagoni (L.) JACQ (Meliaceae). The compound isolated from petroleum ether leaf extracts (5.39%) was quantified using high-performance thin-layer chromatography (HPTLC) method. In antimicrobial assays melianone inhibited Salmonella ser. Typhi with an MIC of 0.053 µM. Induced Fit Docking (IFD) of the ligand, melianone, with proteins involved in anaerobic virulence of the pathogen, revealed that it binds with FocA (a transport protein of formate ions) at its “periplasmic opening” with a glide energy of − 51.8576 kcal mol−1. Melianone altered the overall conformation of the protein (protomer A) by 0.347 Å RMSD. It induced a notable protein topology (Ω loop region) shift in the channel from an intermediate-open to a closed-state conformation and was supported by molecular dynamic simulations performed. FocA, a protein that contributes to its survival under anaerobic conditions, was further evaluated experimentally, after exposure of Salmonella ser. Typhi to melianone, resulting in the altered homeostasis of formate.








Similar content being viewed by others
References
Abdelgaleil SAM, Doe M, Morimoto Y, Nakatani M (2006) Rings B,D-secolimonoids from the leaves of Swietenia mahogani. Phytochemistry 67:452–458
Basak SP, Islam A, Chakraborthy DP (1970) Melianone from Swietenia mahagoni JACQ. Ind Chem Soc 47:501–503
Brenner FW, Villar RG, Angulo FJ, Tauxe R, Swaminathan B (2000) Salmonella nomenclature. J Clin Microbiol 38:2464–2467
Champagne DE, Koul O, Isman BM, Scudder GGE, Towers GHN (1992) Biological activity of limonoids from the Rutales. Phytochemistry 31:377–394
Chen YY, Wang XN, Fan CQ, Yin S, Yue JM (2007) Swiemahogins A and B, two novel limonoids from Swietenia mahogany. Tetrahedron Lett 48:7480–7484
Chiu SW, Pandit SA, Scott HL, Jakobsson E (2009) An improved united atom force field for simulation of mixed lipid bilayers. J Phys Chem B 113:2748–2763. https://doi.org/10.1021/jp807056c
Clinical and Laboratory Standards Institute (1999) Methods for determining bactericidal activity of antimicrobial agents. NCCLS, Wayne (PA: NCCLS document M26-A)
Clinical and Laboratory Standards Institute (2000) Performance standards for antimicrobial susceptibility testing; 18th informational supplement, vol 28, no 1: Document no. M100–S18. Clinical and Laboratory Standards Institute, Wayne
Das P, Lahiri A, Lahiri A, Chakravortty D (2009) Novel role of nitrite transporter NirC in Salmonella pathogenesis: SPI2-dependent suppression of inducible nitric oxide synthase in activated macrophages. Microbiology 255:2476–2489
Ekimoto H, Irie Y, Shigetoshi AYK, Kikuchi T (1991) Platelet aggregation inhibitors from the seeds of Swietenia mahagoni: inhibition of in-vitro and in-vivo platelet-activating factor-induced effects of tetranortriterpenoids related to swietenine and swietenolide. Planta Med 57:56–58
Feng Z, Hou T, Li Y (2012) Concerted movement in pH-dependent gating of FocA from molecular dynamic simulations. J Chem Inf Model 52:2119–2131. https://doi.org/10.1021/ci300250q
French GL (2006) Bactericidal agents in the treatment of MRSA infections—the potential role of daptomycin. J Antimicrob Chemother 58:1107–1117
Govindachari TR, Suresh S, Banumathy B, Masilimani S, Geetha G, Kumari GNK (1999) Antifungal activity of Some B,D-secolimonoids from two meliaceous plants. J Chem Ecol 5:923–933
Hapuarachchi SV, Cobbold SA, Shafik SH, Dennis ASM, McConville MJ, Martin RE, Kirk K, Lehane AM (2017) The malaria parasite’s lactate transporter PfFNT is the target of antiplasmodial compounds identified in whole cell phenotypic screens. PLoS Pathog 13:e1006180. https://doi.org/10.1371/journal.ppat.1006180
Haque M, Ullah MO, Nahar K (2009) In vitro antibacterial and cytotoxic activities of different parts of plant: Swietenia mahagoni. Pak J Biol Sci 12:599–602
Hultmark D, Engstrom A, Bennich H, Kapur R, Boman HG (1982) Insect immunity: isolation and structure of cecropin D and four minor antibacterial components from Cecropia pupae. Eur J Biochem 127:207–217
ICH Q14 (2018) ICH Q14: Analytical Procedure Development and Revision of Q2(R1) Analytical Validation. https://www.gmp-compliance.org/guidemgr/files/Q2(R1).pdf. Accessed November 2018
Kader A, Haque E, Khondkar P, Islam M, Rahman M (2009) Antibacterial and cytotoxic limonoids from the seeds of Swietenia mahagoni. Dhaka Univ J Pharma Sci 8:141–145
Laskowski RA (2001) PDBsum: summaries and analyses of PDB structures. Nucleic Acids Res 29:221–222
Laskowski RA, Swindells MB (2011) LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model 51:2778–2786
Lavie D, Jain MK, Kirson I (1967) Terpenoids-Part VI. The complete structure of melianone. J Chem Soc (C). https://doi.org/10.1039/J39670001347
Lu W, Du J, Schwarzer NJ, Gerbig-Smentek E, Einsle O, Andrade SLA (2012) The formate channel FocA exports the products of mixed-acid fermentation. PNAS 109:13254–13259. https://doi.org/10.1073/pnas.1204201109
Lu W, Du J, Schwarzer NJ, Wacker T, Andrade SLA, Einsle O (2013) The formate/ nitrate transporter family of anion channels. Biol Chem 394:715–727. https://doi.org/10.1515/hsz-2012-0339
Lu W, Du J, Wacker T, Gerbig-Smentek E, Andrade SLA, Einsle O (2011) pH-dependent gating in a FocAformate channel. Science 332:792. https://doi.org/10.1126/science.332.6031.792-b
Lv X, Liu H, Gong H (2013) Exploring the pH dependent substrate transport mechanism of FocA using molecular dynamics simulation. Biophys J 105:2714–2723. https://doi.org/10.1016/j.bpj.2013.11.006
Maeda T, Wood TK (2008) Formate detection by potassium permanganate for enhanced hydrogen production in Escherichia coli. Int J Hydrog Energ 33:2409–2412
Martins AP, Marrone A, Ciancetta A, Cobo AG, Echevarria M, Moura TF, Re N, Casini A, Soveral G (2012) Targeting aquaporin function: potent inhibition of aquaglyceroporin-3 by a gold-based compound. PLoS ONE 7:e37435. https://doi.org/10.1371/journal.pone.0037435
Microchem Laboratory (2015) Log and percent reductions in microbiology and antimicrobial testing. https://microchemlab.com/information/. Accessed March 2020.
Paritala V, Chiruvellab KK, Thamminenic C, Ghant RG, Mohammed A (2015) Phytochemicals and antimicrobial potentials of mahogany family. RevistaBrasileira de Farmacognosia 25:61–83
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC et al (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 13:1605–1612
Polonsky J, Varon Z, Rabanal R, Jacquemin H (1977) 21, 20-Anhydromelianone and Melianone from Simaroubaamara(Simaroubaceae); carbon-13 NMR spectral analysis of Δ7-tirucallol-type triterpenes. Israel J Chem 16:16–19. https://doi.org/10.1002/ijch.197700006
Pronk S, Páll S, Schulz R, Larsson P, Bjelkmar P, Apostolov R, Shirts MR, Smith JC, Kasson PM, Spoel DVD, Hess B, Lindhal E (2013) GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. BMC Bioinform 29:845–854. https://doi.org/10.1093/bioinformatics/btt055
Raghuraman P, Sudan RJJ, Kumari JLJ, Sudandiradoss C (2017) Systematic prioritization of functional hotspot in RIG-1 domains using pattern based conventional molecular dynamic simulation. Life Sci 184:58–70. https://doi.org/10.1016/j.lfs.2017.07.011
Sawers G (2005) Formate and its role in hydrogen production in Escherichia coli. Biochem Soc Trans 33:42–46. https://doi.org/10.1042/BST0330042
Schüttelkopf AW, Aalten DMFV (2004) PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr B 60:1355–1363. https://doi.org/10.1107/S0907444904011679
Suppman B, Sawers G (1994) Isolation and characterizaion of hypophosphite-resistant mutants of Escherichia coli: identification of FocA protein, encoded by the pfl operon, as a putative formate transporter. Mol Microbiol 11:965–982
Szelest-Lewandowska A, Masiulanis M, Szymonowicz M, Pielka S, Paluch D (2007) Modified poly (carbonate urethane)-synthesis, properties and biological investigation in vitro. J Biomed Mater Res A 82:509–520
Szymonowicz M, Pielka S, Owczarek A, Haznar D, Pluta J (2007) Study on influence of gelatin–alginate matrixes on the coagulation system and morphotic blood elements. Macromol Symp 253:71–76
Waight BA, Love J, Wang DN (2010) Structure and mechanism of a pentamericformate channel. Nat Struct Mol Biol 17:31–37
Wang Y, Huang Y, Wang J, Cheng C, Huang W, Lu P, Xu Y, Wang P, Yan N, Shi Y (2009) Structure of the formate transporter FocA reveals a pentameric aquaporin-like channel. Nature 462:467–473. https://doi.org/10.1038/nature08610
Weichert M, Beitz E (2017) Mechanism of formate-nitrate transporters by dielectric shift of substrate acidity. EMBO J 36:949–958. https://doi.org/10.15252/embj.201695776
Xie PS, Sun S, Xu S, Guo L (2014) Value the unique merit of HPTLC Image analysis and extending its performance by digitalization for herbal medicines quality control. J Chromatogr Sep Tech 5:249
Acknowledgements
A. Veni is thankful to the management of Sri Ramachandra Institute of Higher Education and Research (Deemed-to-be University) for the award of SRU-Chancellor fellowship for this study. Plants were authenticated by Prof (Retd.) P. Jayaraman, Director, Institute of Herbal Botany, Plant Anatomy Research Centre, West Tambaram, Chennai-45 (Reg. No of the certificate: PARC/2014/2059). We sincerely acknowledge the contributions of other reviewers for their constructive criticisms. We record with gratitudes the invaluable suggestions from Prof. V Ganesh, Dept. of Human Genetics (SRIHER, DU) towards improvement of this manuscript.
Funding
(Information that explains whether and by whom the research was supported). A. Veni is thankful to the management of Sri Ramachandra Institute of Higher Education and Research (Deemed-to-be University) for the award of SRU-Chancellor fellowship for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Veni, A., Lokeswari, T.S., Krishna Kumari, G.N. et al. Bioactivity of melianone against Salmonella and in silico prediction of a membrane protein target. 3 Biotech 10, 460 (2020). https://doi.org/10.1007/s13205-020-02441-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-02441-9