Abstract
Betulinic acid as a derivative of betulin is widely reported for its anti-HIV and antitumor activities. Betulin has three most significant positions, i.e., primary hydroxyl group at position C-28, secondary hydroxyl group at position C-3, and alkene moiety at position C-20, where chemical modifications were performed to yield pharmacologically more active derivatives. Bioconversion optimization was performed for the enhancement in the percentage of conversion using statistical approach by opting temperature, pH and betulin concentration as independent variables. Three hundred fifty isolates were screened from natural sources under selective medium containing up to 3 g/l of betulin for their tolerance and bioconversion efficiency. Isolate KD235 was found to grow in 3 g/l betulin with 23.34 ± 0.57 g/l biomass and 0.67 ± 0.06 g/l betulinic acid production. New isolate KD235 was characterized by molecular analysis and named as Bacillus megaterium KD235. Molecular characterization of a potentially active isolate for the transformation of betulin to betulinic acid was suggested as isolate Bacillus megaterium KD235. Maximum bioconversion (22 ± 1.5%) was found at optimized conditions, i.e., pH 6.5, temperature 30 °C and at 3 g/l betulin. Validations of experiments as ~11% more bioconversion i.e., 1 ± 0.1 g/l betulinic acid were obtained using 5 l lab fermenter as compared to shake flask.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the recent past 20 years, betulin (Fig. 1) and its derivatives as penta-cyclic triterpenes have attracted attention due to their diverse pharmacological activities. among which anti-HIV and anti-tumor activities are of prime concern (Aiken and Chen 2005; Alakurtti et al. 2006; Saxena et al. 2006; Csuk 2014). The bioactive molecules’ utilization from natural products offered exciting possibilities for the development of successful therapies. Terpenoids are group of phytochemical made up of squalene or related cyclic and acyclic 30-carbon precursors with already reported diverse biological activities (Domingues et al. 2014). Recent findings suggested that betulinic acid improves antioxidant system and reduces lipid peroxidation in the liver (Silva et al. 2016). This improved antioxidant system leads to hepato-protective effects of betulinic acid on alcohol-induced liver damage (Gonzalez-Burgos and Gomez-Serranillos 2012; Yi et al. 2014). Betulin is reported to show anti-fungal and anti-tumor activities as well (Alakurtti et al. 2006). A research group showed significant antifungal activities demonstrated by two Candida species, Candida krusei and Candida albican (Yogeeswari and Sriram 2005). Betulinic acid as a derivative of betulin has already been reported for its multiple pharmaceutical activities. Betulinic acid itself and with its derivatives has been considered for their specific cytotoxicity against various tumor cell lines and anti-HIV activity. Structurally betulin has three most significant positions where chemical modifications can be easily performed, namely primary hydroxyl group at position C-28, secondary hydroxyl group at position C-3, and alkene moiety at position C-20, as shown in (Fig. 1b). Earlier studies on chemo-synthetic pathway of betulinic acid mainly focus on the oxidation and reduction at position C-28 of betulin (Csuk et al. 2006). However, there are not enough literatures available on microbial transformations of betulin. Reports suggest that betulin can be transformed 3–5 metabolites (Chatterjee et al. 2000; Feng et al. 2013). The bio-chemical modifications at positions C-28 of the parent structure of betulin can produce betulinic acid (Fig. 1b) (Yogeeswari and Sriram 2005; Csuk et al. 2006; Baratto et al. 2013; Bache et al. 2014; Csuk 2014). Betulinic acid is commercially produced via chemical synthesis where betulin is used as raw material (Csuk et al. 2006). Biotransformation is preferred as an alternative route for chemical synthesis because biotransformation does not include large amount of costlier chemicals and leads to green synthesis of high-value fine chemicals (Schwab et al. 2013). Besides that, biotransformation methods are more stereo- and regio-selective. Including that few reactions that cannot be easily completed thorough chemical approaches are performed by microbial transformation in an easier way (Pervaiz et al. 2013; Bastos et al. 2007; Liu et al. 2011; Mao et al. 2012; Feng et al. 2013; Chen et al. 2008; Shao et al. 2016). Cunninghamella blakesleeana has also been investigated for biotransformation of betulin to betulinic acid analyzed by HPLC (Feng et al. 2013). The LC–MS characterization of this Cunninghamella blakesleeana catalyzed broth extract demonstrated five products among which betulinic acid was the most important (Feng et al. 2013). While in another report, under optimum conditions Rhodotorula mucilaginosa transformed 52.65% betulin to two products out of which one was detected as betulone (Mao et al. 2012). Another group of researchers investigated betulin transformation to betulinic acid using Armillaria luteovirens Sacc-QH with ~9.32% maximum productivity of betulinic acid under optimized environment (Liu et al. 2011). The growing cells of Rhodococcus rhodochrous IEGM 66 transformed 0.50 g/l betulin to betulone with 45% conversion rate within 240 h (Grishko et al. 2013). The present work is focused on bioprospection of an efficient biocatalyst for conversion of betulin to betulinic acid. Moreover, an improved bioprocess will also be developed through optimizing process variables for enhancement of bioconversion rate.
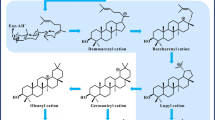
a Lupane type pentacyclic skeleton representing marked rings. b Structure of betulin representing different functional groups with position of carbon. c Betulin is biotransformed into betulinic acid by Bacillus megaterium KD235 and the modification is highlighted by red color circle (Fig. 1c)
Materials and methods
Chemicals and reagents
Standard betulin (98%) and betulinic acid (90% technical grade) were purchased from Sigma Aldrich Inc. Bangalore (India) and dissolved in dichloro-methane (also known as methylene di-chloride) 1 mg/ml as a stock solution for experiments. Acetonitrile, methanol, and milli-Q water (HPLC grade) were obtained from SRL Limited (Mumbai). Other components of media used in the study were of analytical grade, purchased from Hi-media, Merck and SRL Mumbai.
Collection, isolation, and acclimatization of isolated consortium
The microbial consortiums capable of growing under betulin stress were grown by enrichment culture in 250 ml flask similarly as followed by other researchers (Liu et al. 2011; Mao et al. 2012; Grishko et al. 2013). Initially isolated microbes were exposed for 05 weeks at 1 g/l betulin stress and then stable colonies as the resistant isolates were obtained after 6 weeks. These isolates were acclimatized for next generation with higher betulin concentration gradually. The pure cultures of isolates were identified according to “Bergey’s Manual of Determinative Bacteriology” (Vos et al. 2011).
Screening of most suitable biocatalyst for betulin biotransformation
Different isolated cultures from betulin-enriched medium were separately inoculated to 50 ml betulin screening medium (BSM) containing 2.5 g/l of dextrose, 0.25 g/l yeast extract, 1.25 g/l peptone, 1.25 g/l NaCl, 1.75 g/l K2HPO4, 0.50 g/l beef extract, and betulin 1.0 g/l, in 250 ml Erlenmeyer flask, at 37 °C, pH 6.5 for 48 h. Further 0.1 ml culture broth was inoculated to solid medium and cultivated for next 4–5 days for different isolates. The betulin-tolerant isolates were screened and maintained on modified nutrient agar and LB medium plates for auxiliary tests. Stock cultures of microbes were stored on slants of nutrient agar at 4 °C in refrigerator. The culture media used contained organic nitrogen sources (peptones, yeast extracts, tryptone, beef extracts, etc.) and carbon source (glucose, maltose, glycerin etc.) at a pH of 4.5–8.5, and the incubation temperature ranged from 25 to 45 °C. The pure cultures were maintained by transferring to fresh slants on every 15 days, whereas only fresh cultures were used for biotransformation experiments (Fig. 2).
Morphological and biochemical identification of suitable isolates for betulin biotransformation
The morphology of different isolates was observed under a camera-attached microscope (Labomed-Luxedo 4D). Various biochemical tests were performed for the identification of betulin-tolerant isolates (Vary 1994). The pure cultures were grown on nutrient agar or potato dextrose medium and transferred to Luria-Broth, Mac-conkey agar medium, EMB agar medium, and Mannitol salt agar medium for differentiating and identifying bacteria. The plates were incubated inverted at 28–37 °C in the incubator and growth were observed on every 24 h intervals for 05 days after inoculation.
Molecular characterization
One of the screened isolates (i.e., KD235) revealed similarity to Bacillus megaterium based on “Bergey’s Manual of Determinative Bacteriology” (Vos et al. 2011). Molecular characterization of isolate KD235 was outsourced by Xcelris Labs Limited, Ahmadabad (Gujarat State, India). The 16S rDNA analysis of reference stains with isolate KD235 shown 99% similarities to Bacillus megaterium. The constructed phylogenetic tree indicated isolate KD235 as Bacillus megaterium and further suggested a name as Bacillus megaterium KD235 during current study.
Validation of biotransformation by parameter testing and statistical optimization
Shake flask level (pH, temperature and betulin concentration)
Investigating the effect of pH on biotransformation in shake flask cultures, different pH were adjusted (4.5–9.0) using 1.0 M NaOH or HCl. The temperature changes (25–45 °C) were controlled in shaking incubator based on statistical design experiment (Design Expert free trial version 9.0). The biotransformation time was started to examine after 36 h of the addition of betulin as the substrate. The experiments at shake flask culture level were performed in a 250-ml Erlenmeyer flask containing 50 ml medium after inoculating with the Bacillus megaterium KD235 culture. The pH and shaking were controlled at 6.5 and 180, respectively, at 30 °C. After 36 h of inoculation, 0.15 ml of the already prepared betulin stock solution (1 mg/ml) was added to each flask and maintained under the same cultivation conditions for additional 120 h. Culture control was run with the inoculation of microorganism, while with the addition of the equal amount of dichloromethane instead of betulin. Consequently microorganism was growing while no substrate biotransformation took place in culture control. While substrate control was run without the inoculum that means betulin was present but betulin tolerant biocatalyst (i.e., Bacillus megaterium KD235) was absent keeping rest of the other culture conditions same with the biotransformation experiment. After 6 days biotransformation runs were stopped by harvesting the broth from shake flasks and a clear permeate obtained by filtering the broth using Whatman No. 1 filter paper.
Purification of biotransformed products by silica gel column chromatography and HPLC
The entire medium was used for further analysis for the presence of betulin and betulinic acid at the end of each experiment. The fermented broth were extracted for 2 h with different ratios using ethyl acetate (1:1, 1:1.5, 1:2, 1:2.5, 1:3, 1:3.5 and 1:4) and all the organic layers were taken individually, and combined together. Further, the extracted solutions were concentrated in vacuum evaporator at 60 °C (Hahn Shin, South Korea). The dried samples were further used for identification using thin layer chromatography and HPLC. The concentrated organic layer was subjected to column purification. The column was packed with silica gel and saturated with ethyl acetate before loading the sample. Gradient mobile phase consisting of ethyl acetate and hexane with 7:3 ratios was used for elution of betulinic acid (Chatterjee et al. 2000). The eluent was tested by HPLC at 1.0 ml/min flow rate and with 210 nm wavelength at room temperature (Liu et al. 2011; Feng et al. 2013). For getting crystals of betulinic acid, the elute was mixed twice the volume of elute with the mixture of ethyl acetate and methanol (9:1) and is kept at 4 ± 1 °C overnight. This crystallized sample was further analyzed for biophysical characterization of betulinic acid by NMR and ESI–MS (Carpenter et al. 1980; Chatterjee et al. 2000).
Experimental design and statistical analysis
Three factor temperature, pH and substrate concentration were selected according to statistical design and available literature for biotransformation of betulin (Dubey et al. 2008; Liu et al. 2011). The interaction effect in among these parameters (temperature 25–35 °C, pH 5.25–7.25, and betulin concentration 0.9–6 g/l) and their roles in transformation of betulin were determined by RSM. The model was validated at the predicted levels by running all the experiments in triplicate separately (Myers et al. 2016).
Model verification and time-course experiment at 5 l lab fermenter
The biotransformation process was performed by a procedure similar to normal transformation experiments using the optimized and non-optimized conditions at a 5-l lab fermenter. Lab-scale experiments were carried out in 5 l fermenter (Biostatplus, Sartorius, Germany) with 3 l working volume, where 6-bladed turbine type impeller of diameter 50 mm and the ring type sparger with 24 holes (size of hole 1.5 mm) were used. Bacillus megaterium KD235 (15% v/v) was inoculated in 3 l fermentation medium containing 4.5 g/l of dextrose, 0.25 g/l yeast extract, 1.25 g/l peptone, 1.25 g/l NaCl, 1.75 g/l K2HPO4, 0.50 g/l beef extract, and betulin 3.0 g/l, and fermentation initiated at 30 °C, pH 6.5, and with an agitation speed of 150 rpm. At the mid of log phase, approximately after 36 h of inoculation; betulin (3 g/l) was added to the fermentation medium. When the cell density reaches to 0.9 ± 1.1; observations started at a regular time interval of 8 h (such as 36, 44, 52, 60, 68, 76, 82, 90, up to 144 h). Sample of fermented broth was taken to evaluate the cell mass (Dry Cell Weight as g/l) and residual amount of betulin which corresponds to the yield of betulinic acid. The determination of cell mass was done by making a calibration curve between dry cell weigh and optical density at 600 nm using spectrophotometer (Lab India, 3000 plus). The dry cell weight was determined by measuring the filtrates of culture broth through a Whatman filter paper (0.2 µm) after 8 h of interval.
Results
Morphological, biochemical and molecular characterization of KD235 as efficient isolate for betulin biotransformation
Morphological and biochemical identification of isolate KD235
The characterizations of screened microbes were done on betulin screening medium (BSM) by isolating their pure cultures, further analyzed by plotting a graph between growth (Dry Cell Weight g/l) and optical density of culture at 600 nm. Due to these similarities and further sugar utilization these three isolates were grouped as Bacillus megaterium. From these three isolates only one (isolate KD235) showed the highest growth and maximum tolerance at 3 g/l concentration of betulin. This isolate, i.e., KD235, was further sent for molecular characterization to Xcelris Labs Limited, Ahmadabad (Gujarat state, India).
Molecular characterization of screened isolate, efficient for betulin transformation
Based on their morphological and biochemical characterization the isolate was assigned in genus Bacillus. Further, the isolate identification was confirmed by molecular characterization and comparing database with the available reference isolates. The molecular analysis of reference stains and KD235 was found similar to Bacillus megaterium (99%) consequently a name is suggested to this isolate as Bacillus megaterium KD235 (GenBank KR261097).
Statistical optimization of process variables for enhanced bioconversion
The microbial growth (Dry Cell Weight g/l) of Bacillus megaterium KD235 during the transformation of betulin was considered as response. The experimental design with three replicates, with different variables at center point, was used to verify the most significant factors affecting the betulin biotransformation (Liu et al. 2011; Feng et al. 2013). The RSM was employed to temperature, pH, and betulin concentration (as substrate) and dry cell weight (Dry Cell Weight g/l) for biomass of Bacillus megaterium KD235 as the response was analyzed on bioconversion. In order to fit the empirical second-order polynomial model, central composite designs (CCD) with three factors, at five coded levels, were performed. The biocatalyst was cultivated in shake flasks in above said culture medium with different combinations of parameters set by Design Expert (Trial Version 9.0). The maximum bioconversion (22 ± 1.5% as 0.67 ± 0.16 g/l betulinic acid) with biomass (23.34 ± 1.5 g/l Dry Cell Weight g/l) was obtained at pH 6.5, optimal temperature 30 °C, and substrate concentration at 3 g/l using shake flask method. Later on, results were transformed to 5 l lab-scale fermenter which showed better results in 84–90 h (1 ± 0.12 g/l betulinic acid and 28.14 g/l Dry Cell Weight (g/l).
Biophysical characterization of betulinic acid, as product of Bacillus megaterium KD235 catalyzed betulin bioconversion
Three hundred fifty isolates were grown on the BSM (Betulin Screening Media), and among them KD235 showed notable growth in the presence of betulin with high rate of bioconversion to betulinic acid. The data obtained after HPLC analysis was confirmed the presence of betulin and betulinic acid into fermented broth compared with chromatogram of standard chemicals (betulin and betulinic acid) and the retention time was reported as 3.4 min (Fig. 3a inset picture) and 12.8 min (Fig. 3b inset picture) for betulin and betulinic acid respectively. The biophysical characterization and structural elucidation of biotransformed products were confirmed by 13C NMR and spectra were recorded at 400 MHz on a Bruker Avance II 400 in DMSO (Table 1). Analysis of the spectra obtained by NMR spectroscopy was carried out to verify the Bacillus megaterium KD235 catalyzed transformed product of betulin (i.e., betulinic acid) and itself to identify the bonds between them. The shift was probably caused by the enzymes secreted by Bacillus megaterium KD235 in the medium creating the new carboxylic group (–COO) at C-28, which replaced alcohol group of betulin. ESI–MS was also done to verify the results which were similar to the available references. The differences shown in the NMR and ESI–MS peaks represent the biotransformation, respectively. Including HPLC analysis, the ESI–MS spectrum of exhibited a molecular peak at m/z [M + H]+ 443.3 which is representing betulin as the reference compound, while spectrum exhibited a molecular peak at m/z [M + H]+ 455.2 indicated betulinic acid as a isolate KD235 catalyzed biotransformed product of betulin used as the sole carbon source.
a Identification (inset image showing HPLC peaks) and characterization of betulin ESI–MS characterization of betulin (443.31 is molecular weight of betulin characterized by ESI–MS). b Identification (showing HPLC peaks in inset image) and characterization of betulin and betulinic acid as products of biotransformation; b ESI–MS characterization of betulin (441.28) and betulinic acid (455.27) characterized by ESI–MS, respectively
Effect of temperature and pH on enhancement of biotransformation
To determine the maximum growth and betulin transformation on suitable temperature for Bacillus megaterium KD235 the experiments were performed at different temperatures ranging from 25 to 45 °C. The maximum microbial growth 22.72 ± 0.8 g/l is evident from Fig. 4a, which is suggested to be directly proportional to betulin biotransformation. Accordingly, maximum betulinic acid production (32.17 ± 0.46 µg/l) was found at 30 °C and as the temperature goes on increasing the biotransformation decreases (7.9 ± 1.3 µg/l) as evidenced by previous findings on biotransformation (Dubey et al. 2008; Grishko et al. 2013). This decrease in biotransformation is because of heat stress and the oxidation of betulin to betulinic acid with reduced enzyme activity of the bacterium (Vary 1994). To validate the suitable pH of biotransformation, the experiments were performed according to designed combination of parameters at varying pH from 4.5 to 8.5. Due to increase in pH from 5.25 to 6.5 (Fig. 4b) the rate of biotransformation increases (16.46 ± 0.6–33.24 ± 1.6 µg/l) and gives the maximum growth (11.32 ± 1.2–23.34 ± 1.8 g/l on pH 6.5. At pH higher than 7.5, reduction in biotransformation was observed (10.47 ± 0.8 µg/l) and it was assumed that this reduction was due to the production of lesser biomass (9.87 ± 1.4 g/l). It is also demonstrated in Fig. 4b that betulin biotransformation is prone to pH change towards alkalinity. This decrease in biotransformation is because of stress caused on microbial cells due higher alkalinity (Vary 1994; Grishko et al. 2013).
Effect of betulin concentration on the rate of bioconversion
To find the effect of the substrate concentrations, experiments were performed in the 250-ml flask with the increasing concentration of betulin ranges from 0.9 to 6.0 g/l. It is shown in Fig. 4c that 3 g/l conc. of betulin was found to be the most suitable for maximum growth (22.38 ± 2.7 g/l which is directly proportional to bioconversion of supplied substrate as maximum production of betulinic acid (32.89 ± 1.4 µg/l). But as soon the betulin concentration rises above 5.0 g/l the biomass generation is inhibited (2.69 ± 0.3 g/l) due to toxicity of betulin towards Bacillus megaterium KD235 (Dubey et al. 2008; Grishko et al. 2013). Maximum microbial transformation by Bacillus megaterium KD235 as 0.67 ± 0.16 g/l betulinic acid (22 ± 1.5% bioconversion) and 23.34 ± 1.2 g/l growth were observed. While in case of the whole cell mass of cultured Armillaria luteovirens Sacc ZJUQH100-6 the overall productivity was observed to be only 9.63% after 3 days of incubation (Liu et al. 2011). In this work betulin tolerant isolate was characterized and named as Bacillus megaterium KD235 (NCBI GenBank reference no. KR261097). It was observed that more than 3.0 g/l concentration of betulin hinders the growth of Bacillus megaterium KD235. At 3.0 g/l betulin concentration, maximum biomass ~23 (~23.34 ± 1.2 g/l) and 0.67 ± 0.16 g/l betulinic acid were found using 250 ml shake flask method. These optimized conditions have shown improved results in 5- l lab-scale fermenter with the production of 1.01 ± 0.12 g/l betulinic acid and 28.14 ± 1.7 g/l biomass (Table 2). The findings suggested that the production of betulinic acid is directly related to tolerance of betulin to the isolate and its ability to utilize betulin with other available nutrients. With respect to dry cell weight, the productivity of betulinic acid was found to be 0.029 g/g in shake flask and 0.036 g/g in 5 l lab scale fermenter, respectively. Present study indicates that optimized parameters for Bacillus megaterium KD235-catalyzed transformation of betulin into betulinic acid are 3 g/l betulin, incubation temperature 30 °C and pH 6.5.
Discussion
Recent reports highlighted that Armillaria luteovirens Sacc ZJUQH100-6 and Cunninghamella blakesleeana may perform the modification (oxidation and reduction) at specific positions of betulin (Liu et al. 2011). Earlier findings for cytochrome P450 ability (CYP450) suggested to perform hydroxylation, oxidation, reduction and demethylation through fungal metabolism (Patten et al. 1993; Nebert and Russell 2002). Mono-oxygenases are the foremost enzymes involved in the microbial metabolism of many of such substrates. Although CYP hydroxylation of cholesterol and testosterone is common in higher eukaryotic systems, the specific CYP monooxygenases that are associated with conversion of steroids are still unknown in microbial system. Thus, it may be assumed that bioconversion of betulin involves the participation of P450, such as monooxygenases and reductase. A previous study reported that action of dehydrogenase can be improved by increasing the solubility of substrate and product through the addition of a surfactant to the reaction medium. The past reports advocated that betulin and betulinic acid are strongly nonpolar compounds leading to lower hydrophilicity and pose a great problem for pharmaceutical application in vivo. The addition of nonionic hydrophilic surfactant seems to be useful to improve solubility of betulin and overcome mass transfer problem also. Previous study regarding the concentration of precursor highlighted that the concentration of precursor not only affects the biotransformation efficiency but also the procedural cost. When high concentration of betulin was used, the yield of betulinic acid decreased, which may be because of the toxicity of betulin on cells resulting from the high concentration. In a betulin transformation study with Chaetomium longirostre IFO 9873 hydroxylation, oxidative ring cleavage and decarboxylation products were found to be the potent inhibitors of tumor promotion (Akihisa et al. 2002). Another report observed that betulinic acid can be glucosylated and oxidized by the transformation of cultured fungi cells (Bastos et al. 2007). In one study, a nonionic surfactant, span 80 was reported as the best surfactant for enhancing the hydrolysis of castor oil by Candida rugosa. In another study Tween 80 as a surfactant considerably improved the biotransformation process of betulin to betulinic acid by Armillaria luteovirens Sacc ZJUQH100-6. The catalytic activity of microbial cells is affected by several factors, such as temperature, cell concentrations, substrate concentrations and pH values of the buffer solution (Rong et al. 2016). The present study gives a comparative result for the production of biomass at 9.38 ± 0.4, 13.19 ± 1.1, and 22.72 ± 1.3 g/l and sharp reduction of biomass to 11.27 ± 1.4 g/l with biocatalytic production of betulinic acid at 8.41 ± 1.2, 15.61 ± 1.4, 32.17 ± 2.6 µg/l and 12.63 ± 0.6 µg/l with respect to a temperature of 20, 25, 30 and 35 °C. The microbial transformation of betulin to betulinic acid is a one-step regioselective oxidation of the primary C-28 hydroxyl group of betulin and is most likely catalyzed by alcohol dehydrogenase. It is well known that pH plays a crucial role in the dehydrogenase catalyzed reaction. The present study strongly advocates that pH played a significant role for this feasible bioconversion where production of 13.44 ± 1.3, 16.46 ± 1.1, 19.38 ± 0.7 and 33.24 ± 2.3 µg/l of betulinic acid was obtained at pH 4.5, 5.5, 6.25 and 6.5, respectively. On the other hand as the pH rose to 7 ± 0.2 the production of betulinic acid decreased to approximately 15.5 ± 0.7 µg/l. A correlation between cell concentration and conversion rate is typical for many biocatalytic processes. The betulinic acid production was increased with respect to biomass (Dry Cell Weight as g/l) as 11.63 ± 0.6, 14.13 ± 0.5 and 32.89 ± 1.8 µg/l of betulinic acid was obtained at 10.82 ± 0.7, 11.73 ± 0.3, and 22.38 ± 1.4 g/l biomass, respectively (Fig. 4c). Further cell concentration up to 21.31 ± 1.7 g/l changed this trend, leading to a drastic decrease (17.28 ± 0.9 µg/l) in betulinic acid production corresponding to betulin biotransformation. This effect may be explained by mass transfer limitations or restrictions of substrate transport caused by the higher cell concentration. Later experiments on higher concentration of substrate have shown that betulin concentrations ranging from 0.9 to 6.0 g/l impacted the bacterial respiration where the oxygen uptake reached its maximum within the first 36 h. This period was associated with the highest catalytic activity toward betulin to betulinic acid production (Fig. 4c). A subsequent study found that the respiration rate and the catalytic activity of Bacillus megaterium KD235 decreased and stabilized. Maximal betulin transformation (32.89 ± 1.7 µg/l) to betulinic acid was achieved after 84 h at a betulin concentration of 3.0 g/l. In an earlier study betulone production by Dothideomycete HQ 316564 and Rhodotorula mucilaginosa (Mao et al. 2012) did not exceed 52.65 and 43.4%, respectively, although far lower substrate concentrations were used (0.57 and 1 g/l, respectively).
In an ESI–MS analysis of the transformed product which exhibited the [M + H]+ peaks at m/z 455.27 with 99.83% relative abundance (Fig. 3b) the molecular formula of transformed product was determined to be C30H48O3. The 13C NMR and 1H NMR data (Table 1) indicated that the acid group (–COO) is located at the C-28 position. Therefore, the product was identified as betulinic acid (Fig. 1) with the spectroscopic data identical to those reported in the literature (Carpenter et al. 1980). The most reasonable explanation is that the transformation of betulin by Bacillus megaterium KD235 added one oxygen atom in the parent betulin. Microbial transformation strategies are very remarkable for transforming betulin like hydrophobic molecule. Reactive oxygen species (ROS) are highly toxic intermediates generated inside the body as a result of metabolism which promotes aging and different diseases in humans including cancer, neurodegenerative diseases, inflammation, and cardiovascular disease. The present work developed an improved process for getting higher conversion of betulin to their respective derivative, i.e., betulinic acid, which is widely reported to possess significant antioxidant potential using Bacillus megaterium KD235. Betulinic acid is a more potent, relatively non-toxic, more hydrophilic derivative of betulin which has already been reported to possess a wide spectrum of biological activities such as anti-diabetic, anti-cancer, and precursor for anti-HIV drug bevirimat. The present work highlighted a simple, cost-effective process for the biotransformation of betulin to betulinic acid, which is the precursor molecule for synthesis of anti-HIV drugs like DSB, using Bacillus megaterium KD235. Further work on scale up and molecular basis of reaction with enzymatic modulations is needed to be done. The metabolic process is still unknown and the metabolic flux is under further investigation. Indisputably, main enzymes or these reactions involved will need to be investigated in a further study.
Conclusions
In present study the bioconversion of betulin into betulinic acid was achieved through the structural modification of betulin at carbon 28 position. The bioconversion optimization using statistical approach by opting temperature, pH and betulin concentration as independent variables was done by isolate KD235. Isolate KD235 was characterized through biochemical as well as molecular analysis and named as Bacillus megaterium KD235. In the present study the isolated strain of Bacillus megaterium KD235 was supplemented with 3 g/l betulin which produced 23.34 ± 1.2 g/l biomass and 0.67 ± 0.16 g/l betulinic acid. In these optimized conditions (pH 6.5, temperature 30 °C, and 3 g/l betulin) highest bioconversion (22.16 ± 1.5%) of betulin into betulinic acid was obtained. Including this, maintenance of culture conditions for Bacillus megaterium KD235 in 5-l bioreactor caused ~11% more bioconversion as compared with shake flask (Table 2).
The present work highlighted the process development for the biotransformation of betulin to betulinic acid, which is a precursor molecule for synthesis of novel and more effective anti-HIV drugs using Bacillus megaterium KD235. Further work on scale up and more focus with molecular basis of reaction with enzymatic modulations are needed to be done.
References
Aiken C, Chen CH (2005) Betulinic acid derivatives as HIV-1 antivirals. Trends Mol Med 11:31–36
Akihisa T, Takamine Y, Yoshizumi K, Tokuda H, Kimura Y, Ukiya M, Nakahara T, Yokochi T, Ichiishi E, Nishino H (2002) Microbial transformations of two lupane-type triterpenes and anti-tumor-promoting effects of the transformation products. J Nat Prod 65:278–282
Alakurtti S, Mäkelä T, Koskimies S, Yli-Kauhaluoma J (2006) Pharmacological properties of the ubiquitous natural product betulin. Eur J Pharm Sci 29:1–13
Bache M, Bernhardt S, Passin S, Wichmann H, Hein A, Zschornak MP, Kappler M, Taubert H, Paschke R, Vordermark D (2014) Betulinic acid derivatives NVX-207 and B10 for treatment of glioblastoma-an in vitro study of cytotoxicity and radiosensitization. Int J Mol Sci 15:19777–19790
Baratto LC, Porsani MV, Pimentel IC, Netto ABP, Paschke R, Oliveira BH (2013) Preparation of betulinic acid derivatives by chemical and biotransformation methods and determination of cytotoxicity against selected cancer cell lines. Eur J Med Chem 68:121–131
Bastos DZ, Pimentel IC, de Jesus DA, de Oliveira BH (2007) Biotransformation of betulinic and betulonic acids by fungi. Phytochemistry 68:834–839
Carpenter RC, Sotheeswaran S, Sultanbawa MUS, Ternai B (1980) 13C NMR studies of some lupane and taraxerane triterpenes. Org Magn Reson 14:462–465
Chatterjee P, Kouzi SA, Pezzuto JM, Hamann MT (2000) Biotransformation of the antimelanoma agent betulinic acid by Bacillus megaterium ATCC 13368. Appl Environ Microbiol 66:3850–3855
Chen J, Zheng YG, Shen YC (2008) Biotransformation of p-methoxy-phenyl acetonitrile into p-methoxy-phenyl-acetic acid by resting cells of Bacillus subtilis. Biotechnol Appl Biochem 50:147–153
Csuk R (2014) Betulinic acid and its derivatives: a patent review (2008–2013). Expert Opin Ther Pat 24:913–923
Csuk R, Schmuck K, Schäfer R (2006) A practical synthesis of betulinic acid. Tetrahedron Lett 47:8769–8770
Domingues MA, Guerra RR, Duarte A, Freire MSR, Neto CP, Silva CMS, Silvestre JD (2014) Bioactive triterpenic acids: From agroforestry biomass residues to promising therapeutic tools. Mini-Rev Org Chem 11:382–399
Dubey KK, Ray A, Behera B (2008) Production of demethylated colchicine through microbial transformation and scale-up process development. Process Biochem 43:251–257
Feng Y, Li M, Liu J, Xu TY, Fang RS, Chen QH, He GQ (2013) A novel one-step microbial transformation of betulin to betulinic acid catalysed by Cunninghamella blakesleeana. Food Chem 136:73–79
Gonzalez-Burgos E, Gomez-Serranillos MP (2012) Terpene compounds in nature: a review of their potential antioxidant activity. Curr Med Chem 19:5319–5341
Grishko VV, Tarasova EV, Ivshina IB (2013) Biotransformation of betulin to betulone by growing and resting cells of the actinobacterium Rhodococcus rhodochrous IEGM 66. Process Biochem 48:1640–1644
Liu J, Fu M, Chen Q (2011) Biotransformation optimization of betulin into betulinic acid production catalysed by cultured Armillaria luteovirens Sacc ZJUQH100-6 cells. J Appl Microbiol 110:90–97
Mao DB, Feng YQ, Bai YH, Xu CP (2012) Novel biotransformation of betulin to produce betulone by Rhodotorula mucilaginosa. J Taiwan Inst Chem Eng 43:825–829
Myers RH, Montgomery DC, Anderson-Cook CM (2016) Response surface methodology: process and product optimization using designed experiments. Wiley, New York
Nebert DW, Russell DW (2002) Clinical importance of the cytochrome P450. Lancet 360:1155–1162
Patten CJ, Thomas PE, Guy RL, Lee M, Gonzalez FJ, Guengerich FP, Yang CS (1993) Cytochrome P450 enzymes involved in acetaminophen activation by rat and human liver microsomes and their kinetics. Chem Res Toxicol 6:511–518
Pervaiz I, Ahmad S, Madni M, Ahmad H, Khaliq F (2013) Microbial biotransformation: a tool for drug designing. Appl Biochem Microbiol 49:437–450
Rong S, Wang J, Li Q, Guan S, Cai B, Zhang S, Hu J (2016) The enhanced production of 11α-hydroxyandrosta-1,4-diene-3,17-dione based on the application of organic silica hollow spheres in the biotransformation of β-sitosterol. J Chem Technol Biotechnol. doi:10.1002/jctb.4983
Saxena BB, Zhu L, Hao M, Kisilis E, Katdare M, Oktem O, Bomshteyn A, Rathnam P (2006) Boc-lysinated-betulonic acid: a potent, anti-prostate cancer agent. Bioorg Med Chem 14:6349–6358
Schwab W, Fuchs C, Huang FC (2013) Transformation of terpenes into fine chemicals. Eur J Lipid Sci Technol 115:3–8
Shao M, Zhang X, Rao Z, Xu M, Yang T, Li H, Xu Z, Yang S (2016) A mutant form of 3-ketosteroid-Δ1-dehydrogenase gives altered androst-1,4-diene-3,17-dione/androst-4-ene-3, 17-dione molar ratios in steroid biotransformations by Mycobacterium neoaurum ST-095. J Ind Microbiol Biotechnol 43:691–701
Silva FS, Oliveira PJ, Duarte MF (2016) Oleanolic, ursolic, and betulinic acids as food supplements or pharmaceutical agents for type 2 diabetes: promise or illusion? J Agric Food Chem 64:2991–3008
Vary PS (1994) Prime time for Bacillus megaterium. Microbiology 140:1001–1013
Vos P, Garrity G, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH, Whitman W (2011) Bergey’s manual of systematic bacteriology 3. Springer Science & Business, Berlin
Yi J, Xia W, Wu J, Yuan L, Wu J, Tu D, Fang J, Tan Z (2014) Betulinic acid prevents alcohol-induced liver damage by improving the antioxidant system in mice. J Vet Sci 15:141–148
Yogeeswari P, Sriram D (2005) Betulinic acid and its derivatives: a review on their biological properties. Curr Med Chem 12:657–666
Acknowledgements
The authors sincerely acknowledge University Grant Commission, New Delhi, India, for providing the financial support (F. No. 40-119/2011, SR), Maharshi Dayanand University Rohtak, Haryana, India, for providing the necessary lab facilities for this research work. They also wish to thank Xcelris Labs Limited, Ahmadabad (Gujarat state, India), for molecular analysis, AIRF JNU Delhi and SAIF Panjab University, Chandigarh for providing technical support for ESI MS and NMR analysis, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kumar, D., Dubey, K.K. An efficient process for the transformation of betulin to betulinic acid by a strain of Bacillus megaterium . 3 Biotech 7, 157 (2017). https://doi.org/10.1007/s13205-017-0759-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0759-9