Abstract
A comparative study on both wild type and mutant of Pediococcus pentosaceus for dextransucrase activity, its stability, dextran synthesizing activity, antibiotic sensitivity and carbohydrate utilization was performed. The wild type P. pentosaceus had specific activity of 0.58 U/mg whereas the mutant showed that of 1.0 U/mg with 72% enhancement. The antibiogram of 27 antibiotics tested against mutant showed significant differences with 9 antibiotics when compared to wild type. In carbohydrate fermentation profile, trehalose, galactose, maltose, lactose and fructose are metabolized by both the strains, but weakly in case of mutant. Stabilization of purified dextransucrase from wild type and mutant with various stabilizers was studied at 30 and 4 °C. Both enzymes were more stable at 4 °C. Among various stabilizers such as dextran (100 kDa, 10 μg/ml), glycerol (0.5%, v/v), PEG 8000 (10 μg/ml) and Tween 80 (0.5%, v/v), Tween 80 provided maximum stabilization at 4 and 30 °C. The mutant showed better stabilization than that of the wild type at both 30 and 4 °C. The loss of activity at 30 °C after 24 h in wild type and mutant in the presence of Tween 80 was only 34 and 32%, respectively, whereas the loss of activity in control of wild type and mutant was 76 and 59%, respectively. After 15 days at 4 °C, the loss of activity in control of wild type and mutant in the presence of Tween 80 was only 15 and 8%, respectively, whereas at 30 °C, the loss of activity in control of wild type and mutant was 49 and 42% respectively. Half-life of the enzyme with Tween 80 was 28.5 and 33.5 h for wild type and mutant, respectively, at 30 °C and 52.1 and 106.6 days for wild type and mutant respectively, at 4 °C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactic acid bacteria (LAB) have a long history of safe use by man for food production and preservation. The LAB are widely used as starter cultures for fermentation in the dairy, meat and other food industries (Mugula et al. 2003). LAB can be used as cell factories for the production of an array of food additives and aroma compounds. Certain strains of Lactococcus lactis through their surface physicochemical properties interact and retain aroma compounds in food (Ly et al.2008). Fermentation of lupin protein extracts using several LAB improve their aroma (Schindler et al. 2011). The LAB may also function as probiotics and contribute to the general health of the consumer (Sybesma et al. 2006). Moreover, the LAB are known to synthesize enzymes, vitamins, antioxidants, bioactive peptides and bacteriocins (Fernandes et al. 1987; Knorr 1998). Several non-starter LAB produce bioactive peptides, generate gamma-aminobutyric acid and inactivate antigenotoxins, thus implicated in cheese-making (Settanni and Moschetti 2010). Many strains of Lactobacillus produce antifungal compounds acetic and phenyllactic acids to inhibit bread mold spoilage (Gerez et al. 2009). Antibacterial bacteriocin producing Pediococcus pentosaceus have been isolated from fermented sausages (Abrams et al. 2011). Enterococcus faecium has been reported as a potential producer of pediocin-like bacteriocin with antiviral activity (Todorov et al. 2010). The LAB have attracted immense commercial interests, for their capacity to secrete a wide range of exo-polysaccharides having industrially useful physico-chemical properties (Sidebotham 1974; De Vuyst and Degeest 1999; Ricciardi and Clementi 2000). The genera of LAB that produce dextrans using dextransucrase enzyme include Streptococcus,Leuconostoc, Weissella and Lactobacillus (Kralj et al. 2004; Tieking et al. 2005; Majumder and Goyal 2008). Smitinont et al. (1999) had emphasized on dextran synthesizing ability of the Pediococcus genus. Patel et al. (2010) reported the dextran production ability of P. pentosaceus for the first time. Dextrans are employed as blood plasma substitutes, plasminogen activators, antithrombogenic agents, in treatment of iron deficiency anaemia and in the matrix preparation of chromatography columns (Naessens et al.2005; Purama and Goyal 2005). Dextrans have major use in food formulations as stabilizing, emulsifying, texturizing and gelling agent. Dextran can be used as a stabilising coating to protect metal nanoparticles from oxidation and improve biocompatibility of biomaterials (Sengupta et al. 2006). The increase in the use of exo-polysaccharides in food, pharmaceutical and cosmetics industries emphasizes the importance of exploration of the new species and characterization of their traits. It has been reported that the genetic alterations of LAB that occur during random mutagenesis may lead to strains with improved traits (Sybesma et al. 2006). Mutants of Leuconostoc strains NRRL B-512F (Kim and Robyt 1994), B-742 (Kim and Robyt 1995a, b), B-1299 (Kim and Robyt 1995a, b) and 512FMC (Kitaoka and Robyt 1998), are presently being used in the industry for their novel traits. Singh et al. (2009) conducted mutagenesis of Leuconostoc dextranicum NRRL B-1146 by UV irradiation and generated mutant strains with enhanced glucan production. Patel and Goyal (2010a) carried out UV mutagenesis on the natural isolate P. pentosaceus (Genbank Accession Number EU569832) and screened a novel mutant exhibiting higher dextransucrase activity. The wild type P. pentosaceus had an enzyme activity of 3.4 U/ml whereas the mutant showed 4.9 U/ml with 40% enhanced activity. The wild type P. pentosaceus had an enzyme activity of 3.4 U/ml whereas the mutant showed 4.9 U/ml with 40% enhanced activity. The wild type P. pentosaceus had specific activity of 0.58 U/mg whereas the mutant gave 1.0 U/mg showing 72% enhancement. The present study reports the comparative study of antibiotic resistance, carbohydrate fermentation, dextran synthesizing activity and stability of dextransucrase of wild type P. pentosaceus and its mutant.
Material and methods
Bacterial strains
The P. pentosaceus (PP) (Genbank Accession Number EU569832) isolate was screened from the soil sample collected from a sugarcane field of Assam (near Guwahati) (Patel and Goyal 2010a). Mutagenesis of P. pentosaceus was performed using UV irradiation. The colonies appeared on the UV-irradiated Petri plates were screened for their enzyme activity and specific activity. The mutant colony producing significantly higher dextransucrase than the wild type was selected for further study (Patel and Goyal 2010b). The stock cultures of wild type and mutant were maintained as MRS-S agar stab cultures at 4 °C and sub-cultured every 2 weeks (Goyal and Katiyar 1996).
Antibiotic sensitivity
The mutant of P. pentosaceus was tested for susceptibility to twenty seven antibiotics using agar disc diffusion test (Barry and Thornsberry 1980). The antibiotic tests were performed using commercially available antibiotic octodiscs containing Amoxyclav (Ac), Cephalexin (Cp), Ciprofloxacin (Cf), Clindamycin (Cd), Erythromycin (E), Ampicillin (A), Carbenicillin (Cb), Cephotaxime (Ce), Chloramphenicol (C), Co-Trimazine (Cm), Oxacillin (Ox), Amikacin (Ak), Amoxycillin (Am), Bacitracin (B), Cephalothin (Ch), Novobiocin (Nv), Oxytetracycline (O), Vancomycin (Va), Cephaloridine (Cr), Kanamycin (K), Lincomycin (L), Methicillin (M), Norfloxacin (Nx), Olaendomycin (Ol), PenicillinG (P), Tetracycline (T) and Gentamicin (G), from Hi-media Pvt. Ltd. India. MRS medium containing 2% glucose as carbohydrate source with 1.8% (w/v) agar and 0.8% (w/v) agar were used. The petri-plates were first prepared with MRS medium containing 1.8% (w/v) agar. The test strain was seeded in MRS-soft agar (0.8%, w/v) and overlaid in the Petri-plate having a bottom layer of above MRS agar (1.8%, w/v). The octodiscs were then gently placed over the surface of the seeded plate. The Petri plates were incubated in inverted position overnight in an incubator at 28 °C and were observed next day for zone of inhibition around the discs.
Carbohydrate fermentation
The wild type and mutant of P. pentosaceus were tested to 13 different carbohydrates for their ability to ferment using the method of (Kandler and Weiss 1986). From the overnight grown MRS broth containing 2% glucose as carbohydrate source, 50 μl was inoculated in 5 ml liquid MRS medium lacking glucose but containing phenol red (0.04 g/L) as pH indicator and other test carbohydrates to give a final inoculum to medium ratio of 1% (v/v). The test media were incubated at static condition, for 48 h at 28 °C (Purama et al. 2008). The acid production was observed between 24 and 48 h. The acid production as a result of carbohydrate fermentation was indicated by a change in colour of phenol red to yellow.
Culture conditions for dextransucrase production
For the development of inoculum, a loopful of culture from modified MRS agar stab was transferred to 5 ml of enzyme production medium as described by Tsuchiya et al. 1952. This enzyme production medium consists of (%, w/v) sucrose, 2; yeast extract, 2; K2HPO4, 2; MgSO4·7H2O, 0.02; MnSO4·4H2O, 0.001; FeSO4·7H2O, 0.001; CaCl2, 0.001; NaCl, 0.001 and the pH was adjusted to 6.9 (Tsuchiya et al. 1952). The wild type and mutant of P. pentosaceus cultures were incubated at 25 °C at 180 rpm for 12 h.
Dextransucrase production from wild type and mutant of P. pentosaceus
One percent of the above 5 ml broth was again inoculated to 100 ml enzyme production medium contained in a 250 ml Erlenmeyer flask and incubated for 16 h at 25 °C under shaking at 180 rpm. One milliliter of broth sample was withdrawn and centrifuged at 10,000g for 10 min at 4 °C. The cell-free supernatant was analyzed for enzyme activity and protein concentration. All experiments were performed in duplicates for accuracy of results.
Enzyme activity and protein concentration assay
The assay of dextransucrase activity was carried out in 1.0 ml of a reaction mixture in 20 mM sodium acetate buffer, pH 5.4, containing 146 mM (5%, w/v) sucrose and 20 μl cell-free supernatant as the enzyme source. The reaction mixture was incubated at 30 °C for 15 min. Aliquots (0.1 ml), from the reaction mixture were analyzed for reducing sugar concentration. The enzyme activity was determined by estimating the liberated reducing sugar by Nelson–Somogyi method (Nelson 1944; Somogyi 1945). The absorbance was measured at 500 nm using a UV-visible spectrophotometer (Cary 100 Bio, Varian, Inc., USA) against a blank using D-fructose as standard. One unit (U) of dextransucrase activity is defined as the amount of enzyme that liberates 1 μmol of reducing sugar per min at 30 °C in 20 mM sodium acetate buffer, pH 5.4. The protein concentration of the cell-free supernatant and other purified protein samples were estimated by the method of Lowry et al. (1951) using BSA as standard.
Purification of dextransucrase with PEG fractionation
Dextransucrase used in the present study was purified by a single step fractionation method using polyethylene glycol (PEG) 400 (Purama and Goyal 2008). An ice cold PEG-400 solution was added to 100 ml cell-free extract at 4 °C, to get the final concentration 25% (v/v). The mixture was incubated at 4 °C for 12–16 h to allow dextransucrase to fractionate. The mixture was centrifuged at 13,000g at 4 °C for 30 min to separate the precipitated dextransucrase. The enzyme pellet was resuspended in 20 mM sodium acetate buffer (pH 5.4). The dextransucrase was subjected to dialysis using the same buffer and 5 kDa cutoff membrane to remove any trace of PEG-400. The purified dextransucrase obtained was analyzed for enzyme activity, protein concentration and purity by SDS-PAGE analysis.
In situ detection of dextransucrase activity
For the detection of dextransucrase activity, periodic acid staining (PAS) of sucrose incubated gel on 7.5% non-denaturing SDS-PAGE (Holt et al. 2001) was done. Non-denaturing SDS-polyacrylamide gel electrophoresis was performed with a vertical slab mini gel unit (BioRad) using 1.5-mm thick gels (Laemmli 1970). After the run, the gel was treated thrice for 20 min with 20 mM sodium acetate buffer, pH 5.4 containing 0.005% (w/v) CaCl2 and 0.1% (v/v) Triton X-100 to remove the SDS at room temperature. The gel was then incubated in 10% sucrose solution in 20 mM sodium acetate buffer pH 5.4 for 6–8 h at 30 °C. Following incubation, the gel was washed twice with 70% (v/v) ethanol for 20 min and incubated in a solution containing 0.7% (w/v) periodic acid and 5% (v/v) acetic acid for 60 min at room temperature. The gel was again washed thrice with a solution containing 0.2% (w/v) sodium metabisulphite and 5% (v/v) acetic acid and was stained finally with Schiff’s reagent (0.5%, w/v Fuchsin basic, 1%, sodium bisulphite and 0.1 N HCl) until the discrete magenta bands within the gel matrix appeared, which confirmed dextransucrase activity. The other gel was stained with Coomassie Brilliant Blue for location of activity bands. Molecular mass marker proteins (myosin from rabbit muscle 205,000, phosphorylase b 97,400, bovine serum albumin 66,000, ovalbumin 43,000, carbonic anhydrase 29,000 Da) purchased from Genei, India, were used as standard for SDS-PAGE.
Effect of stabilizers on stability of dextransucrase
Effect of stabilizers on stability of dextansucrase was studied by incubating the dextransucrase at different temperatures (30 and 4 °C). Aqueous solutions of dextran (100 kDa), PEG-8000, glycerol, Tween-80 were added to dextansucrase solution of wild type and mutant (0.24 mg/ml, 18 U/mg specific activity and 0.3 mg/ml, 18.2 U/mg) in sodium acetate buffer, pH 5.4 to obtain the final concentrations of dextran (100 kDa, 10 μg/ml), glycerol (0.5%, v/v), PEG 8000 (10 μg/ml) and Tween 80 (0.5%, v/v). The enzyme with or without any stabilizers was incubated at 30 °C for 24 h and 4 °C for 15 days (Purama et al. 2010). The aliquots (20 μl) were taken at indicated time intervals for the enzyme assay.
Results and discussion
Antibiotic susceptibility
A standardized filter-paper disc-agar diffusion assay allows a rapid determination of the efficacy of the drug by measuring the diameter of the zone of inhibition. The mutant of P. pentosaceus was tested for susceptibility to 27 antibiotics that represent the major antibiotic groups. Out of 27 antibiotics tested, the mutant displayed the significant differences in sensitivity and susceptibility to 9 antibiotics when compared with wild type as reported earlier (Patel and Goyal 2010b). In contrast to wild type, the mutant showed high or moderate sensitivity towards clindamycin, cephotaxime, amikacin, bacitracin, cephalothin, novobiocin, oxacillin and resistance against cephalexin, methicillin (Table 1).
Carbohydrate fermentation
The mutant was tested for its ability to ferment 13 carbohydrates and compared with wild type as reported earlier (Patel and Goyal 2010b). The critical nature of the fermentation and the activity of the indicator make it essential that all cultures should be observed within 48 h. Extended incubation may mask acid producing reactions by production of alkali because of enzymatic action on substrates other than the carbohydrate (Purama et al. 2008). The carbohydrate fermentation profile of both the wild type and mutant of P. pentosaceus was 62% similar. In carbohydrate fermentation profiling the mutant metabolized trehalose, galactose, maltose, lactose, and fructose with reduced efficiency as compared to wild type (Table 2).
In situ detection of dextransucrase activity
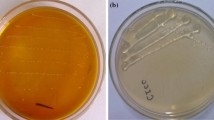
Non-denaturing SDS-PAGE was used for in situ detection of enzyme activity to characterize dextransucrase production by wild type and mutant of P. pentosaceus. This study, however, was carried out to see if both the wild type and mutant of P. pentosaceus produce a similar or different dextran pattern that could be used to distinguish among the dextransucrase producing species (Purama et al. 2008). The results showed the presence of single protein band of approximately 180 kDa molecular size from both the wild type and mutant. The white bands were observed on the gels incubated in sucrose after 6–8 h. These white bands turn to magenta color after PAS staining, which confirmed the presence of dextran formed on polyacrylamide gels (Fig. 1b). The PAS staining of the sucrose incubated gels showed that the activity bands corresponded to the native and active form of the purified enzyme of approximately 180 kDa molecular size appearing on the denaturing gels stained with Coomassie brilliant blue (Fig. 1a).
Identification of PEG purified dextransucrase from wild type and mutant of P. pentosaceus. a Lane M-Protein molecular weight marker (myosin from rabbit muscle 200 kDa, phosphorylase b 97.4 kDa, bovine serum albumin 66 kDa, ovalbumin 43 kDa, carbonic anhydrase 29 kDa); Lane pp-denaturing SDS-PAGE with Coomassie Brilliant Blue staining from wild type; Lane ppm-denaturing SDS-PAGE with Coomassie Brilliant Blue staining from mutant. b Lane pp- nondenaturing SDS-PAGE with Periodic Acid Schiff staining of the dextransucrase from wild type; Lane ppm-nondenaturing SDS-PAGE with Periodic Acid Schiff staining of the dextransucrase from mutant
Effect of stabilizers on stability of dextransucrase
The effects of various stabilizers on the stability of dextransucrase from the wild type and mutant of P. pentosaceus were studied at 30 and 4 °C. Both enzymes were more stable at 4 °C (Fig. 3). The residual activity of dextransucrase with Tween 80, PEG 8000, dextran (100 kDa), glycerol and without any stabilizer at 30 °C at 24 h was 66, 24, 26, 19 and 26% for wild type and 68, 28, 45, 38 and 41% for mutant of P. pentosaceus, respectively (Fig. 2). The residual activity of dextransucrase with Tween 80, PEG 8000, dextran (100 kDa), glycerol and without any stabilizer at 4 °C on 15th day was 85, 47, 58, 40 and 50% and 92, 48, 60, 53 and 57% for wild type and mutant of P. pentosaceus respectively (Fig. 3). The other stabilizers dextran (100 kDa), glycerol and PEG-8000 did not show any significant effect at both the temperatures on the enzyme. The data for glycerol and PEG-8000 are not shown in Figs. 2 and 3. It has been reported earlier that dextransucrase is stable at lower temperatures (10–30 °C) and loses rapidly the enzyme activity at temperatures higher than 30 °C (Purama et al. 2010). Our results are similar to those reported earlier for L. mesenteroides NRRL B-640 (Purama et al. 2010). The results clearly indicate that the mutant enzyme is more stable than the wild type enzyme and Tween 80 was the best stabilizer for dextransucrase of both the wild type and mutant of P. pentosaceus.
Half-life of stabilizer treated dextransucrase
The residual activity of dextransucrase was measured at various temperatures with respect to time with and without stabilizers. The enzyme deactivation followed first-order rate kinetics. The half-life (t1/2) of dextransucrase and stabilizers treated dextransucrase was calculated by assuming that the decay followed first-order kinetics (Purama et al. 2010). Amongst all the stabilizers Tween 80 displayed maximum stabilization of dextransucrase with t1/2 of 28.5 and 33.5 h for wild type and mutant, respectively, at 30 °C and t1/2 of 52.1 and 106.6 days for wild type and mutant respectively, at 4 °C. The addition of Tween 80 to dextransucrase, incubated at both the temperatures (30 and 4 °C) resulted in higher t1/2 than that of with no Tween 80 (Table 3). The t1/2 of mutant enzyme was 49% higher and 24% higher than that of wild type at 30 and 4 °C, respectively. The t1/2 of dextransucrase from mutant with Tween was 17.5% higher and 104.6% higher than that of wild type at 30 and 4 °C, respectively. Taken together all these results, it can be summarized that Tween 80 provided the maximum stabilization at 30 and 4 °C and the mutant showed better stabilization than that of the wild type at both the temperatures.
Conclusion
The comparison of antibiotic resistance, carbohydrate utilization pattern, dextransucrase activity and dextransucrase stabilization of wild type and mutant of P. pentosaceus was reported. The results of antibiotic resistance, carbohydrate utilization pattern, dextransucrase activity and dextransucrase stabilization will enhance understanding of these industrially significant species and will aid in distinguishing between physiologically similar species. The data will be useful for industrial applications where the strains are required with higher enzyme stability. Both dextransucrase of wild type and mutant were more stable at 4 °C than at 30 °C. Amongst various stabilizers Tween 80 provided the maximum stabilization to dextransucrase against activity loss at 30 and 4 °C. The addition of Tween 80 to dextransucrase at 30 and 4 °C resulted in higher t1/2 than that of without Tween 80. The residual activity and t1/2 were higher for mutant enzyme than that of wild type. The results suggested that dextransucrase from the mutant showed better stabilization than that of the wild type and therefore have greater importance.
References
Abrams D, Barbosa J, Albano H, Silva J, Gibbs PA, Teixeira P (2011) Characterization of bacPPK34 a bacteriocin produced by Pediococcus pentosaceus strain K34 isolated from “Alheira”. Food Control 22:940–946
Barry AL, Thornsberry C (1980) Susceptibility testing: diffusion procedures. In: Lennette EH, Balows A, Hausler WJ Jr, Truant JP (eds) Manual of clinical microbiology. Am Soc Microbiol, Washington, D.C., pp 463–474
De Vuyst L, Degeest B (1999) Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol Rev 23:153–177
Fernandes CF, Shahani KM, Amer MA (1987) Therapeutic role of dietary lactobacilli and lactobacillic fermentated dairy products. FEMS Microbiol Rev 46:343–356
Gerez CL, Torino MI, Rollán G, de Valdez GF (2009) Prevention of bread mould spoilage by using lactic acid bacteria with antifungal properties. Food Control 20:144–148
Goyal A, Katiyar SS (1996) Regulation of dextransucrase productivity of Leuconostoc mesenteroides B-512F by the maintenance media. J Gen Appl Microbiol 42:81–85
Holt SM, Al-Sheikh H, Shin KJ (2001) Characterization of dextran-producing Leuconostoc strains. Lett Appl Microbiol 32:185–189
Kandler O, Weiss N (1986) Regular, nonsporing gram-positive rods. In: Sneath PHA, Mair NS, Sharpe ME, Holt JG (eds) Bergey’s manual of systematic bacteriology. Williams & Wilkins, Baltimore, pp 1208–1219
Kim D, Robyt JF (1994) Production and selection of mutants of Leuconostoc mesenteroides constitutive for glucansucrases. Enzym Microb Technol 16:659–654
Kim D, Robyt JF (1995a) Production, selection, and characteristics of mutants of Leuconostoc mesenteroides B-742 constitutive for dextransucrases. Enzym Microb Technol 17:689–695
Kim D, Robyt JF (1995b) Dextransucrase constitutive mutants of Leuconostoc mesenteroides B-1299. Enzym Microb Technol 17:1050–1056
Kitaoka M, Robyt JF (1998) Large-scale preparation of highly purified dextransucrase from a high-producing constitutive mutant of Leuconostoc mesenteroides B-512FMC. Enzym Microbiol Technol 23:386–391
Knorr D (1998) Technology aspects related to microorganisms in functional foods. Trends Food Sci Technol 9:295–306
Kralj S, Van Geel-Schutten GH, Dondroff MG, Kirsanovs S, Van Der Maarel MJEC, Dijkhuizen L (2004) Glucan synthesis in the genus Lactobacillus: isolation and characterization of glucansucrase genes, enzymes and glucan products from six different strains. Microbiology 150:3681–3690
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Ly MH, Covarrubias-Cervantes M, Dury-Brun C, Bordet S, Voilley A, Le TM, Belin JM, Waché Y (2008) Retention of aroma compounds by lactic acid bacteria in model food media. Food Hydrocoll 22:211–217
Majumder A, Goyal A (2008) Enhanced production of exocellular glucansucrase from Leuconostoc dextranicum NRRL B-1146 using response surface method. Bioresour Technol 99:3685–3691
Mugula JK, Narvhus JA, Sorhaug T (2003) Use of starter cultures of lactic acid bacteria and yeasts in the preparation of togwa, a Tanzanian fermented food. Int J Food Microbiol 3:307–318
Naessens M, Cerdobbel A, Soetaert W, Vandamme EJ (2005) Leuconostoc dextransucrase and dextran: production, properties and applications. J Chem Technol Biotechnol 80:845–860
Nelson N (1944) A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 153:375–380
Patel S, Kasoju N, Bora U, Goyal A (2010) Structural analysis and biomedical applications of dextran produced by a new isolate Pediococcus pentosaceus screened from biodiversity hot spot Assam. Bioresour Technol 101(17):6852–6855
Patel S, Goyal A (2010a) 16S rRNA based identification and phylogenetic analysis of a novel dextran producing Pediococcus pentosaceus isolated from north-east Indian microbial diversity. Curr Trends Biotechnol Pharm 4:746–754
Patel S, Goyal A (2010b) Isolation, characterization and mutagenesis of exopolysaccharide synthesizing new strains of lactic acid bacteria. Internet J Microbiol 8(1):3–4
Purama RK, Agrawal M, Majumder A, Ahmed S, Goyal A (2008) Antibiotic sensitivity, carbohydrate fermentation characteristics and plasmid profiles of glucansucrase producing four Leuconostoc strains. J Pure Appl Microbiol 2:139–146
Purama RK, Goyal A (2005) Dextransucrase production by Leuconostoc mesenteroides. Ind J Microbiol 2:89–101
Purama RK, Goyal A (2008) Identification, effective purification and functional characterization of dextransucrase from Leuconostoc mesenteroides NRRL B-640. Bioresour Technol 99:3635–3642
Purama RK, Agrawal M, Goyal A (2010) Stabilization of dextransucrase from Leuconostoc mesenteroides NRRL B-640. Ind J Microbiol 50:57–61
Ricciardi A, Clementi F (2000) Exopolysaccharide from lactic acid bacteria: structure, production and technological applications. Ital J Food Sci 12:23–45
Schindler S, Wittig M, Zelena K, Krings U, Bez J, Eisner P, Berger RG (2011) Lactic fermentation to improve the aroma of protein extracts of sweet lupin (Lupinus angustifolius). Food Chem 128:330–337
Sengupta A, Wang S, Link E, Anderson EH, Hofmann C, Lewandowski J, Kottke-Marchant K, Marchant RE (2006) Glycocalyx-mimetic dextran modified poly (vinyl amine) surfactant coating reduces platelet adhesion on medical-grade polycarbonate surface. Biomaterials 27:3084–3095
Settanni L, Moschetti G (2010) Non-starter lactic acid bacteria used to improve cheese quality and provide health benefits. Food Microbiol 27:691–697
Sidebotham RL (1974) Dextrans. Adv Carbohydr Chem Biochem 30:371–444
Singh A, Majumder A, Goyal A (2009) Mutagenesis of Leuconostoc dextranicum NRRL B-1146 for higher glucan production. Inter J Microbiol 7(1):1–7
Smitinont T, Tansakul C, Tanasupawat S, Keeratipibul S, Navarini L, Bosco M, Cescutti P (1999) Exopolysaccharide-producing lactic acid bacteria strains from traditional Thai fermented foods: isolation, identification and exopolysaccharide characterization. Int J Food Microbiol 51:105–111
Somogyi M (1945) A new reagent for the determination of sugars. J Biol Chem 160:61–68
Sybesma W, Hugenholtz J, de Vos Willem M, Smid EJ (2006) Safe use of genetically modified lactic acid bacteria in food: bridging the gap between consumers, green groups, and industry. Electron J Biotechnol 9((4 ISSN)):0717–3458
Tieking M, Kaditzky S, Valcheva R, Korakli M, Vogel RF, Ganzle MG (2005) Extracellular homopolysaccharides and oligosaccharides from intestinal Lactobacilli. J Appl Microbiol 99:692–702
Todorov SD, Wachsman M, Tomé E, Dousset X, Destro MT, Dicks LMT, de Melo Franco BDG, Vaz-Velho M, Drider D (2010) Characterisation of an antiviral pediocin-like bacteriocin produced by Enterococcus faecium. Food Microbiol 27:869–879
Tsuchiya HM, Koepsell HJ, Corman J, Bryant G, Bogard MO, Feger VH, Jackson RW (1952) The effect of certain cultural factors on production of dextransucrase by Leuconostoc mesenteroides. J Bact 64:521–526
Acknowledgments
The research work was supported by a project grant from Department of Biotechnology, Ministry of Science and Technology, New Delhi, India to AG.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kothari, D., Tyagi, A., Patel, S. et al. Dextransucrase from the mutant of Pediococcus pentosaceus (PPm) is more stable than the wild type. 3 Biotech 1, 199–205 (2011). https://doi.org/10.1007/s13205-011-0018-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13205-011-0018-4