Abstract
Zinc oxide nanoparticles, especially those with a high aspect ratio (i. e., nanorods and nanowires), are of great interest for many applications as they are piezoelectric, photocatalytic and antimicrobial. In the present study, a plasma flight-thru synthesis method was developed that allows controlling the particle size and shape of the zinc oxide nanoparticles. In a direct current thermal plasma reactor operated at atmospheric pressure, zinc powder injected into the plasma jet was molten, vaporized and oxidized, which allowed growing zinc oxide nanoparticles. The particle spectrum ranged from small nanospheres to nanorods, nanowires and multipodic nanoparticles such as tetrapods. The influence of the oxygen rate and the plasma power (correlated to the discharge current) on the particle morphology was studied, and the feasibility of the nanowire-like particles as piezoelectric sensor material was investigated. Piezoelectric test sensors, equipped with the plasma-synthesized zinc oxide nanowires, successfully responded to mechanical stimulation after poling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to their various properties, zinc oxide nanoparticles in general and nanowires (ZnO NWs: linear particles with high aspect ratio) in particular are promising materials for a broad range of applications, e.g., piezoelectric sensors (Jeong et al. 2015) and piezoelectric energy harvesting (Lu et al. 2012; Wang 2008; Wang et al. 2015; Wang and Song 2006; Yang et al. 2009; Zhu et al. 2010), gas sensors (e.g., NOx, CO, O2) (Zhang et al. 2012), superhydrophobic surfaces (Mardosaitè et al. 2021), photocatalysis (Rackauskas et al. 2017), antibacterial activity (Sirelkhatim et al. 2015), spintronic devices, biosensors, etc.
ZnO is characterized by a wide band gap (3.37 eV) and a large exciton-binding energy (60 meV). Due to the wurtzite-type crystal structure (zincite), the asymmetric displacement of the ions Zn2+ and O2− upon pressure creates a piezoelectric current. Additionally, ZnO is photostable, temperature resistant and nontoxic. Further, it is a semiconductor of high potential for applications in optoelectronic devices, such as light-emitting diodes (LEDs), electrically pumped lasers, electron collection in photovoltaic cells and as transparent conducting oxide.
In order to explore the potential of zinc oxide nanoparticle-based technologies and to make them industrially viable, cost-efficient and scalable processes have to be developed, which allow tailoring the properties of the particles, such as their shape and size. Typical state-of-the-art processes to synthesize zinc oxide nanowires are wet-chemical methods (sol–gel processes, hydrothermal growth) (Arslan et al. 2022; Broitman et al. 2012; Hu et al. 2007; Maity et al. 2005; Wu et al. 2005; Xu et al. 2020; Zhang et al. 2003), laser ablation and pulsed laser deposition (Ghosh et al. 2013; Lynam et al. 2019; Rahm et al. 2006; Sun et al. 2004), electrochemical methods (e.g., potentiostatic, galvanostatic anodization) (Chang et al. 2002; Elias et al. 2011; Tello et al. 2021; Wang et al. 2002), physical and chemical vapour deposition (Falyouni et al. 2009; Huang et al. 2001; Khosravi-Nejad et al. 2019; Kong et al. 2001; Navarrete et al. 2022; Sharma et al. 2010), molecular beam epitaxy (Heo et al. 2003) and flame spray pyrolysis (Height et al. 2006).
Their main drawbacks are that they require a lot of energy, reaction time, seed layers, vacuum chambers, autoclaves, or catalysts. The hydrothermal growth, for example, allows oriented ZnO NW growth with a quantum dot seed layer at 65–95 °C for 3–7 h per growth step (Hu et al. 2007) (resulting in 2–8 μm long, vertically aligned ZnO NWs) or chaotic ZnO nanowire growth, which requires 12 h of autoclaving at 140 °C for 12 h, a cleaning procedure and several hours of drying (Wu et al. 2005) (resulting in up to 20 µm long nanowires). Treating large areas and generating large quantities of ZnO nanoparticles, especially nanowires, remain challenging tasks.
Plasma techniques, on the other hand, offer a broad bandwidth of different approaches to synthesize ZnO nanoparticles. E.g., it is possible to induce arcing in zinc swarf in oxygen-rich atmosphere with a magnetron (microwave air-plasma sputtering) to create ZnO nanoparticles of different length (Subannajui 2016). With electrodes emerged in an electrolyte with a zinc salt (e.g., ZnCl2), a solution plasma process allows forming ZnO nanoparticles with dopants when a salt containing the desired dopant (e.g. Fe) is added (Saqib et al. 2021). Similarly, an electrically conducting substrate, such as carbon fibers, may be coated with ZnO in a solution plasma process to form ZnO composite materials (Zhong et al. 2023). Even core–shell nanoparticles with ZnO shell can be synthesized from solid zinc sheets when they are emerged into a solution containing nanoparticles of a different kind and are treated with a plasma jet (Mohammed et al. 2022).
However, fast and cost-efficient process routes with high particle yield can be realized with atmospheric pressure plasma spraying (Primc et al. 2021). Generally, zinc-containing precursors (zinc nitrate, chloride, sulfate, acetate, …) or zinc powders are injected into a plasma jet, where they are converted into zinc oxide nanoparticles (flight-thru process). Primarily processes with zinc powder as starting material seem to be promising for scale-up, as they enable excellent ZnO nanoparticle production rates (24 g/min achieved by Peng et al. (2007), 20 g/min achieved by Ko et al. (2006)), limited mainly by the size of the plasma jet reactor, the zinc powder flow and the conversion rate. Depending on the reaction conditions, these nanoparticles may grow in different forms like spheres, rods, wires and tetrapods (Kim et al. 2008; Kim and Park 2009; Ko et al. 2006; Kumar et al. 2008; Lee et al. 2019; Lee et al. 2021; Liao 2006; Lin et al. 2005, 2009; Peng et al. 2007; Petzold et al. 2012). However, it is still challenging to control the growth of the ZnO nanoparticles and their morphology.
In this work, we develop a zinc oxide nanoparticle synthesis with a thermal atmospheric pressure plasma jet (APPJ) based on a direct current arc discharge. The water-cooled reactor wall allows collecting high yields of synthesized zinc oxide nanoparticles. Adopting the process parameters allows tailoring particle size and shape to form nanowires and nanorods as well as spherical particles and tetrapods. As proof of principle, we show that the synthesized ZnO nanowires have the potential to work as a piezoelectric material in flexible vibration sensors after poling when they are applied in a non-conductive matrix over electrodes.
Experimental
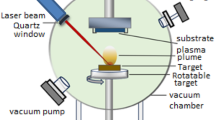
Zinc oxide nanoparticles (ZnO NPs) were synthesized from zinc powder (D50 = 5 µm, ECKART GmbH, Germany) and oxygen with an atmospheric pressure plasma jet reactor with water-cooled (~ 20 °C) outer wall (Fig. 1, scheme in a, picture in b). The commercial hot gas plasma jet (IC3 from INOCON Technologie GmbH, Austria) was operated with argon (5.0) as plasma gas (10 L/min) and mixtures of argon (5.0) and oxygen (5.0) as powder carrier gas (in sum always 10 L/min with 0–100 vol.-% O2). The powder was supplied from a powder feeder with an oscillating conveying channel (Medicoat AG, Switzerland) set to a flow rate of ~ 2 g/min.
A typical experiment is described as follows: with active cooling water, plasma gas and carrier gas flow, a direct current arc was ignited and maintained between the tip of the cathode and the plasma nozzle (hollow anode). Then, the operating discharge current was adjusted gradually (200–400 A). After 20 s of warm-up, the powder feeder was activated for 60 s. 10 s later, the plasma was deactivated, the temperature recorded (two positions, cf. Figure 1b), and the synthesized ZnO powder was collected from the main reactor wall in the area of the cooling coil.
The shape, length and thickness of the ZnO NPs were characterized using scanning electron microscopy (SEM). Due to the tiny diameters of the nanowires (reaching down to 10 nm), a TESCAN MIRA-3 microscope with a field-emission cathode was used. The Schottky field-emission cathode provides a narrow electron beam, which guarantees a superior image resolution at higher magnitudes (theoretically 1 nm) than a tungsten cathode SEM (Jiruše et al. 2014). For the analyses of the chemical elements, an OXFORD AZtecEnergy XT energy-dispersive X-ray spectroscopy (EDS) detector was used.
For the characterization by X-ray diffraction (XRD), the zincite/zinc nanoparticle powder samples were fixed on a glass specimen slide using a mixture of ethanol and glycerol (< 5%) for the dispersion of the particles. The height adjustment of the sample was carried out carefully to obtain a precision of about ± 0.25 mm. The characterization experiments by X-ray diffraction (XRD) were conducted on a D8 Discover diffractometer (Bruker, Germany) in parallel beam geometry (37 kV, 32 mA, Cu Kα radiation) to obtain phase composition, crystallite size and the lattice constants of the hexagonal zincite phase (ZnO). This device has a Sol-X energy-dispersive detector, an open Eulerian cradle and a polycapillary collimator. For each measurement, diffraction angles were scanned in the range of 20–100° 2θ with a corresponding step size of 0.05° and a counting time of 3.5 s/step. All experiments were conducted under air and laboratory conditions at 28 ± 1 °C.
The lattice cell parameters, the crystallite size and the phase contents were refined through the Rietveld technique; the TOPAS 5 Software by Bruker AXS, TOPAS V5 (1999–2014) was used. The crystallite size is here calculated assuming that each crystallite is composed of a set of columns along the scattering vector. The line broadening can be treated easily as a volume-weighted mean column length. No further assumptions in respect of shape and size are necessary. Furthermore, using the integral breadth (i. e., the width of a rectangle with the same height and area as the diffraction peak) and not the full width at half maximum of the diffraction peak, a Scherrer constant of 1 can be assumed (Laue 1926). The crystal structure data, taken from the Inorganic Crystal Structure Database (ICSD 2021) entries ICSD 26170 (hexagonal ZnO) and ICSD 247147 (elemental Zn), were used as starting values for the Rietveld refinement. Other crystal structures, e.g., atom position parameters or occupation factors, were not refined. A polycrystalline powder reference sample (LaB6 NIST 660a) and a solid polycrystalline corundum sample (Bruker) was measured using the identical diffractometer settings and taken as a reference for the correction of the instrumental influence regarding sample height error, peak width and shape.
For the piezoelectric sensors, 15 mg (labelled “ZnO NWs”) or 3.75 mg (labelled “¼ ZnO NWs”) of experimentally synthesized ZnO NWs were suspended into an acrylic resin (2.4 g Acrifix 2R 0190 (Röhm GmbH, Germany), 0.9 g methylmethacrylate (Sigma Aldrich, Merck KGaA, Germany) and 90 mg TPO-L (UV initiator from Sigma Aldrich, Merck KGaA, Germany)). To homogenize the ZnO nanowires, they were milled in a ball mill (Acrifix 2 R0190 with zirconium oxide beaker and milling balls: 500 rpm for 10 min in sequences of 10 s milling followed by 10 s break) before the other components were added. Further, a reference matrix was prepared without ZnO NWs.
For the piezoelectric experiments, the formulations were administered with a spatula onto dielectric substrates (self-adhesive Kapton tape (polyimide) on FR4 prepregs) with interdigit electrode structures (created with an OPTOMEC aerosol jet printer and PARU nano-silver ink in 350 µm line pitch and with 50 µm height). A poling potential (600 V and 700 V) was applied for 30 s or 60 s; after 15 s, a UV lamp was activated for 2 min to harden the acrylic resin. After curing, the piezoelectric currents of the sensors were recorded with a data acquisition system (DeweSoft Sirius, Slovenia).
Results and discussion
Zinc oxide nanoparticle synthesis and characterization
For the zinc oxide nanoparticle synthesis development, a commercially available plasma spray system (IC3 from INOCON Technologie GmbH, Austria) was combined with the water-cooled chamber of an oil diffusion pump as a reactor tube (cf. Figure 1, b). The upper part of the tube surrounding the plasma was hermetically sealed with a flange adapter, which allowed excluding the hardly controllable oxidative effect of ambient air. Preliminary experiments with zinc powder and oxygen-free carrier gas (argon) confirmed that the reactor was airtight. Figure 2 shows the different powders obtained on the flange adapter of the coating nozzle when zinc powder was injected into the plasma jet reactor without (a) and with oxygen (b). Without oxygen addition, the blue-greyish starting material (5 µm grain size) turns into a black powder of spherical zinc nanoparticles with < 200 nm diameter. With oxygen, on the other hand, zinc converts into its white oxide.
The influence of the plasma discharge current (200–400 A) and the oxygen rate (0–100% in the carrier gas) on the zinc oxide particle shape and size were studied in detail. The aim was to find reaction conditions that favour the growth of zinc oxide nanowires (particles with ≤ 200 nm diameter and ≥ 0.5 µm length).
When solely oxygen was used as powder carrier gas, small, primarily spherical nanoparticles (< 10 nm) were grown with ≤ 300 A (Fig. 3). At higher currents, the particles turned into nanorods with similar diameters and a length of 20–40 nm.
When the current was fixed at 250 A and the oxygen rate of the carrier gas was reduced from 100 to 20% in argon, the spherical particles at 100% oxygen turned into nanoneedles, nanowires and particles with two or more branches (bipods, tripods, tetrapods) (Fig. 4). From 20 to 30% O2 the nanowire-like zinc oxide particles became nanorods, with 40% O2 they were already almost spherical nanoparticles. Overall, 20% oxygen in the carrier gas (10% oxygen in the plasma) enabled the best ZnO particle growth. Increasing the oxygen rate to 30% in the carrier gas (15% in the plasma) reduced the mean particle size from ~ 600 to ~ 300 nm (cf. Figure 4 and Fig. 9). Peng et al. (2007) described a similar observation for their plasma flight-thru process. They found that an increase in the oxygen rate supports the formation of ZnO nanowires up to a certain point, after which the particle size decreases. They argue that a higher number of ZnO nuclei is formed simultaneously with higher O2 flow rates, which increases the number of particles, but limits their growth. In their study, an oxygen rate of < 2.4% in the plasma resulted in decreased ZnO nanoparticles, when they investigated the effect of 0.5–30% O2 in the plasma.
To harden these findings, different currents were tested with a fixed oxygen rate of 20% in the carrier gas (Fig. 5). Nanoneedle- and nanowire-like particles were found from 250 to 350 A. Some of these particles were linear; others were partially branched (up to four branches). However, this confirms that, in our setup, 20% O2 in the carrier gas (10% in the plasma, respectively) is a sweet spot for the ZnO nanoneedle and nanowire synthesis.
The zinc and oxygen content in the nanoparticles were analysed with SEM/EDS. The atomic ratio of Zn:O in all samples was close to 1 when oxygen (or a mixture with argon) was injected as carrier gas (cf. Figure 6).
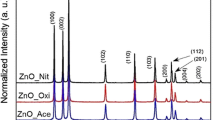
With XRD, the crystal structure of the ZnO nanoparticles was investigated. Typical XRD diffraction patterns of a Zn-rich sample (synthesized with 100% argon, 250 A: comparable to Fig. 2, a) and a ZnO-rich sample (synthesized with 30% O2, 250 A: comparable to Fig. 2, b) are shown in Fig. 7. There is a significant variation in the relative diffraction intensities corresponding to the phase composition or ZnO/Zn ratio. However, the changes in the diffraction angles indicating the lattice spacing of a specific maximum are only minor.
Typical XRD diffraction patterns of a zinc-rich ( +) and a zincite-rich (*) powder; the corresponding diffraction peaks according to the given Inorganic Crystal Structure Database (ICSD 2021) identification are indicated
With the Rietveld refinement, lattice constants could be determined in the approximate ranges of a = 0.324 to 0.326 nm to and c = 0.520 to 0.524 nm. The observed ratios a/c are close to 0.62 (c/a ~ 1.6), which fits well with the reference data for the hexagonal zinc oxide phase (wurtzite-type structure: zincite, chemical composition ZnO) (Klingshirn 2007). Processes with oxygen (≥ 20%) in the carrier gas resulted in greyish-white (occasionally yellowish) powders consisting of nearly 100% zincite (> 95%). Without oxygen, black powders with zincite fractions below 43% were obtained from the bluish-grey starting material (as in Fig. 2a). Thus, the zinc powder was partially oxidized, although the plasma was protected from the surrounding air (with the remaining oxygen in the reactor and the starting material).
Additionally, the zincite fractions in the oxygen-deficient powders slightly differed in the a/c ratios from the oxygen-rich samples, which can be seen in Fig. 8. In the diagrams, the lattice constants a and c of the APPJ samples (crosses) are plotted with the corresponding data of ~ 70 entries of that phase in the Inorganic Crystal Structure Database (circles) (ICSD 2021).
The plotted phases can be arranged in four discrete regions:
Region I comprehends the samples investigated by Sowa and collaborators under high-pressure conditions (ICSD 2021). These samples display a strongly compressed cell.
Region II corresponds to the results of the work of Farooqi and Srivastava (Sowa and Ahsbahs 2006). Their work describes the synthesis of ZnO nanoparticles from ZnS nanoparticles by solid-state reaction. These samples display a larger a/c ratio in the range of 0.629.
Region III refers to APPJ samples in the actual work prepared without oxygen addition. The ZnO sample described in Ekicibil (Hasan Farooqi and Srivastava 2017), obtained by solid-state reaction, displays similar lattice constants as our samples in III.
Region IV contains actual samples prepared under oxygen and many published structures found in the database with stoichiometric compositions. Albertsson’s work (1989) includes high-temperature investigations shifted towards higher lattice constants due to thermal expansion (Ekicibil 2012)
It is evident from Fig. 8 that the oxygen deficiency during the process shifts the lattice constants a and c in a region not typical for zincite. This shift is caused most likely by oxygen defects in the crystal lattice. Thus, a deviation from the stoichiometric composition (ZnO1-x) may be induced in the zincite. The data of the ZnO particles synthesized with oxygen, on the other hand, correspond well with the literature values. These results confirm that the ZnO nanoparticles synthesized within the plasma jet with oxygen are composed almost entirely of a ZnO phase with a zincite structure as intended.
Comparing the crystallite size (determined with XRD) and the particle size (determined with SEM) reveals that both correlate similarly with the varied process parameters (discharge current and oxygen rate in the carrier gas) (cf. Figure 9). With a constant discharge current of 250 A, the crystallite size increases, when the oxygen rate is reduced from 100 to 20% in the carrier gas (50–10% in the plasma). The particle size reaches its maximum at 20% oxygen (10% in the plasma) with 250 A. As discussed above, with increasing oxygen flow rate the number of zinc oxide nuclei formed increases which is likely to act as an antagonistic mechanism to the particle growth. Kim et al. (2009) studied the crystallite size of ZnO nanoparticles synthesized with an APPJ reactor at 750 Torr (Kim and Park 2009). During their experiments, they found that higher O2 flow rates favour the crystallite growth, which was beneficial for the photocatalytic activity of their ZnO powders. However, the oxygen rates they tested were 10–25% in the plasma, so it cannot be excluded that they found the optimum for the crystallite growth with their setup, meaning that a further increase of the O2 rate might have limited or reduced the growth. A direct comparison is hard because of the different plasma setup used.
When the oxygen rate, on the other hand, is kept constant at 20% in the carrier gas, the particle and crystallite size maxima are found around 250 A. This means that the oxygen rate of 20% and the discharge current of 250 A allow optimal growth. In the reactor, a specific amount of energy is necessary to melt and evaporate the zinc particles (which also depends on the zinc flow rate) and to atomize/ionize the oxygen molecules. The small and curved exhaust tube causes turbulences in the reactor which keep the zinc and oxygen atoms and ions ready for reaction long enough inside the hot zone around the plasma so that zinc oxide nanoparticles are formed. The cooled walls, on the other hand, work as cold traps. There, zinc oxide particles grown in the gas phase are caught. The harsh cooling most probably provides as a quenching effect, stopping the particle growth. This explains why the particle size increases with increasing current first, as the hot plasma zone is extended and an optimum amount of energetic reactive species is formed. However, not only the temperature and kinetic energy of the gases and particles inside the reactor increase with the current when they pass the plasma jet, the gas and particle velocities increase, too (which is in accordance with numerical plasma simulations supported by the plasma jet manufacturer INOCON). The increasing velocities may be responsible for the particle size reduction with increasing current, because the particles are trapped faster on the cooled walls and their residence time in the gas phase is reduced significantly. Regarding the influence of oxygen, as discussed above, with increasing oxygen flow rate increases the number of zinc oxide nuclei formed which is likely to act as an antagonistic mechanism to the particle growth. Controlling the zinc oxide nanoparticle growth with our setup means, therefore, to set the favoured current and oxygen rate for the particles with the shapes desired.
Follow-up experiments revealed good repeatability for receiving nanowire-like ZnO particles with 250–350 A and 20% oxygen in the carrier gas. Similar results were obtained when compressed air was used instead of a mixture of oxygen in argon. This is of great interest for a high-throughput process and scale-up, as replacing the argon/oxygen mixture with compressed air reduces the process costs significantly.
In order to investigate the process yield, batch processes with different powder-feeding intervals were tested (1–5 min, 250 A, 20% oxygen in argon as carrier gas). After each experiment, the ZnO powder was sampled from the inner reactor wall of the main tube, where the majority of the product accumulates. Powder from the coating nozzle, the bottom of the tube and the side tube was discarded, meaning that the effectively collectible sample amount is slightly higher than that taken for calculating the process yield.
No attempts were made to filter the exhaust gas during these experiments. Previous tests with different filter procedures and materials like water bubbling and filter tissue did not increase the yield significantly. The best filtering effect was achieved with water, which allowed accumulating appr. 10–20% more sample powder. One crucial factor limiting the filter capacity was the back pressure, which, when too high, either triggered the safety relief valve of the powder feeder or shifted the reaction conditions in a manner, in which indeed more powder was trapped inside the reactor, but in the form of unreacted black zinc.
The process yield for ZnO was calculated as the ratio of the ZnO powder collected from the main tube and the transported zinc powder under the premise that the oxidation rate was 100% (according to XRD analysis, the real oxidation rate was > 95%). With increasing powder-feeding period, the absolute ZnO amount increased almost linearly until 4 min. The typical yield was ~ 40%. A plateau seemed to be reached with more extended powder-feeding periods and the yield began to decrease slightly. Most probably, the capacity of the walls to catch and keep powder despite the hot gas flows was reached: they were saturated with product. In a series of more than 20 batch processes with 4 min powder-feeding time, the process yield could be specified at 47 ± 9%. Thus, approximately half of the powder left the reactor with the gas flow. As mentioned above, our attempts to filter the exhaust gas hardly increased the total yield. However, this shows there is enough potential for further development, regarding industrial applications maybe in combination with a cyclonic separator.
Evaluation of the potential for piezoelectric sensors
Test sensors were developed based on so-called interdigit electrode structures (Fig. 10) to evaluate the potential of the plasma-synthesized ZnO nanowires for piezoelectric sensors. The electrodes were printed with an OPTOMEC aerosol jet using PARU nano-silver ink on dielectric substrates (self-adhesive Kapton tape on FR4).
ZnO nanowires suspended in an acrylic resin were milled, mixed with methyl methacrylate and a UV initiator and applicated as a thick film (~ 200 µm) onto the electrodes, so that the space between the electrodes was filled with the nanowires containing matrix. To increase the piezoelectric effect of the nanowires, a poling voltage was applied (hysteresis poling) while the matrix was hardened under UV irradiation.
In most samples, the poling led to short circuits and arcing after some seconds, which irreversibly damaged the devices. The observed delay between poling start and short/arcing indicates that the nanoparticles started to align within the electric field until a conducting bridge between the electrodes was built. In a further test with acrylic resin without nanowires, a significantly increased dielectric strength and a significantly increased poling voltage up to arcing were observed. The alignment of the ZnO nanowires as chains during poling is thus likely to play a significant role in arcing. To avoid chain forming the concentration of the ZnO NWs in the matrix was reduced, and the poling voltage and time were varied.
Eventually, the successfully poled devices were tested.
The samples tested are listed in Table 1. ZnO nanopowders with nanowire-like structures were chosen for the piezoelectric experiments comparable to those in Fig. 4 and Fig. 5 that where synthesized with 250 A and 20% O2. Figure 11 shows the signals of the samples and the references (a) during bending, and a picture of the experimental setup (b) with a sample in front (yellow device). For these proof-of-concept experiments, the electrodes of the devices were contacted, and the electric charge was recorded as a function of time while the devices were stimulated mechanically. The films were bent by an operator using a ruler made of non-conductive material. Two thirds of the samples were fixed on a table, one third was free to be bent on the edge downwards. The reason for the different responses in Fig. 11 is that the sensors were not stimulated continuously. The pressure and pauses were varied by the operator on purpose, but were not quantified.
The "Dummy" and the "Matrix" sample show some responses to the mechanical stimulation, which have to be attributed to the electrostatic charging of the polymer stick with which the samples were bent. Similarly, the unpoled ZnO-loaded sample (“ZnO, 0 V”) did not respond to the mechanical stimulation. Only with poling, the ZnO-loaded samples react with signal peaks to the stimulation, which can be attributed to the piezoelectric effect. Because of the low particle loading and, thus, low charge displacements the piezoelectric effect ranges in the order of magnitude of the electrostatic charging. The values are, therefore, to be understood as semi-quantitative. From the poled ZnO-loaded samples, the sample “ZnO, 600 V, 30 s” shows the most significant charge peaks, which may be attributed to the four times higher ZnO concentration in the matrix. In terms of amplitude with ~ 100 pC, however, it is still orders of magnitude below the piezoelectric effect of polymer-based sensors (e.g., PyzoFlex® from JOANNEUM RESEARCH). One approach to improve the sensors would be to use a piezoelectric matrix material such as poly(vinylidene fluoride) (PVDF) or a PVDF co-polymer. Dodds and co-workers showed that the voltages generated can be increased with the ZnO content (Dodds et al. 2013), in their study up to a factor of 1.7 with 10–20% ZnO NPs. In a comparative study of PVDF composites with different nanoparticles (SiO2, TiO2 and ZnO), Arjun Hari and co-workers found the most improvement of the output voltage when PVDF composites with 3% ZnO nanoparticles were tested.
Furthermore, the inner polarizability of the ZnO nanowires was investigated. For this purpose, sample “ZnO, 0 V”, in which the matrix curing took place without applying a polarization voltage and in which the nanowires were thus immobilized without a preferred direction, was subjected to hysteresis polarization. In the case of polarizability, charge shifts and, thus, currents would have to be measurable in the course of repeated repolarization. Such charge shifts and currents could not be observed, not even with a gradual increase in the voltage amplitude up to the arcing at 1500 V.
To improve the polarizability of the ZnO nanoparticles and, thus, the piezoelectric responses, it could be beneficial to dope the nanoparticles during the plasma synthesis with shallow donors (group III/VII) or shallow acceptors (group I, V) (Albertsson et al. 1989). Lithium, for example, has proven to enhance the output voltage of piezoelectric devices based on ZnO nanowires after poling (Consonni and Lord 2021; Shin et al. 2014).
However, the fact that in the case of previously immobilized nanowires breakdown only occurs at ~ 1500 V, while in the case of an acrylate matrix that has not yet been cured arcing already occurs at 600–700 V, may be seen as a further strong indication that the ZnO nanowires themselves strongly favour the formation of short circuits as they orient themselves to chains.
In conclusion, although minor piezoelectric signals are discernible, the ZnO nanowires are not yet qualitatively sufficient to fabricate usable sensors. The signals are too low to allow quantitative measurements of the piezoelectric coefficients. Therefore, future research should focus on implementing dopants like Li in the plasma flight-thru synthesis (e.g., as aerosol) in combination with piezoelectric matrices based on PVDF.
Conclusion
In the present work, we demonstrated the successful development of an atmospheric pressure plasma jet reactor for the morphology-controlled zinc oxide nanoparticle synthesis with zinc powder and oxygen. The particle morphology was found to depend on the oxygen rate in the carrier gas and the plasma, the discharge current and the energy inside the reactor, respectively. Zinc oxide nanoparticles with a high aspect ratio (nanorods and nanowires) grew, when 20% O2 was present in the carrier gas (10% in the plasma) and discharge currents of 250–350 A were applied. Piezoelectric signals could be detected upon mechanical stimulation when these nanoparticles were dispersed in a matrix of acrylic resin and fixed between finger-electrodes while poling. The signals were significantly greater than the responses assigned to electrostatic charging of the untreated reference, a ZnO-free and a non-poled sample. Hence, the signals of the poled ZnO sample prove that the synthesized ZnO nanoparticles (grown in the wurtzite structure) have the potential to animate piezoelectric sensors and devices. However, a further improvement of the particle morphology control in combination with high-yield harvesting and homogenization techniques will be necessary to advance towards scale-up for industrial purposes. To improve the polarizability of the ZnO NPs and, thus, the piezoelectric responses, on the other hand, the plasma flight-thru process should be adopted to enable the incorporation of dopants like Li into the ZnO NPs.
Data availability
The data used in this study are available from the corresponding author (alexander.schwan@gmx.at) upon reasonable request.
References
Albertsson J, Abrahams SC, Kvick A (1989) Atomic displacement, anharmonic thermal vibration, expansivity and pyroelectric coefficient thermal dependences in ZnO. Acta Crystallogr Sect B Struct Sci 45:34–40. https://doi.org/10.1107/S0108768188010109
Arjun Hari M, Karumuthil SC, Rajan L (2023) Optimization of PVDF nanocomposite based flexible piezoelectric tactile sensors: a comparative investigation. Sens Actuators A 353:114215. https://doi.org/10.1016/j.sna.2023.114215
Arslan HT, Arslan C, Baydogan N (2022) The effects of the curing parameters of the hydrothermal solution on the characteristic properties of ZnO nanowires. J Opt 51:79–88. https://doi.org/10.1007/s12596-021-00757-0
Bruker AXS, Topas V5 (1999–2014) General profile and structure analysis software for powder diffraction data. Karlsruhe, Germany
Broitman E, Bojorge C, Elhordoy F, Kent VR, Gadioli GZ, Marotti RE, Canepa HR, Dalchiele EA (2012) Comparative study on the properties of ZnO nanowires and nanocrystalline thin films. Surf Coat Technol 213:59–64. https://doi.org/10.1016/j.surfcoat.2012.10.015
Chang SS, Yoon SO, Park HJ, Sakai A (2002) Luminescence properties of Zn nanowires prepared by electrochemical etching. Mater Lett 53:432–436. https://doi.org/10.1016/S0167-577X(01)00521-3
Consonni V, Lord AM (2021) Polarity in ZnO nanowires: acritical issue for piezotronic and piezoelectric devices (Review). Nano Energy 83:105789. https://doi.org/10.1016/j.nanoen.2021.105789
Dodds JS, Meyers FN, Loh KJ (2013) Piezoelectric nanocomposite sensors assembled using zinc oxide nanoparticles and poly (vinylidene fluoride). Smart Struct Syst 12:55–71. https://doi.org/10.12989/sss.2013.12.1.055
Ekicibil A (2012) Structural and magnetic properties of the Zn0.8-4x Hox Oy (0.05 ≤ x ≤ 0.10) compounds prepared by solid state reactions. Solid State Sci 14:1486–1491. https://doi.org/10.1016/j.solidstatesciences.2012.08.028
Elias J, Michler J, Philippe L, Lin M-Y, Couteau C, Lerondel G, Levy-Clement C (2011) ZnO nanowires, nanotubes, and complex hierarchical structures obtained by electrochemical deposition. J Electron Mater 40:728–732. https://doi.org/10.1007/s11664-011-1530-3
Falyouni F, Benmamas L, Thiandoume C, Barjon J, Lusson A, Galtier P, Sallet V (2009) Metal organic chemical vapor deposition growth and luminescence of ZnO micro- and nanowires. J Vac Sci Technol, B 27:1662–1666. https://doi.org/10.1116/1.3137017
Ghosh P, Sharma AK (2013) Optical characterization and growth mechanism of combination of zinc oxide nanowires and nanorods at various substrate temperatures. J Nanomater 2013:480164. https://doi.org/10.1155/2013/480164
Hasan Farooqi MM, Srivastava RK (2017) Structural, optical and photoconductivity study of ZnO nanoparticles synthesized by annealing of ZnS nanoparticles. J Alloys Compd 691:275–286. https://doi.org/10.1016/j.jallcom.2016.08.245
Height MJ, Mädler L, Pratsinis SE, Krumeich F (2006) Nanorods of ZnO made by flame spray pyrolysis. Chem Mater 18:572–578. https://doi.org/10.1021/cm052163y
Heo YV, Kaufman M, Pruessner K, Norton DP, Ren F, Chisholm MF, Fleming PH (2003) Optical properties of Zn1-xMgxO nanorods using catalysis-driven molecular beam epitaxy. Solid State Electron 47:2269–2273. https://doi.org/10.1016/S0038-1101(03)00209-0
Hu H, Huang X, Deng C, Chen X, Qian Y (2007) Hydrothermal synthesis of ZnO nanowires and nanobelts on a large scale. Mater Chem Phys 106:58–62. https://doi.org/10.1016/j.matchemphys.2007.05.016
Huang MH, Wu YY, Feick H, Tran N, Weber E, Yang PD (2001) Catalytic growth of zinc oxide nanowires by vapor transport. Adv Mater 13:113–116. https://doi.org/10.1002/1521-4095(200101)13:2%3c113::AID-ADMA113%3e3.0.CO;2-H
ICSD Inorganic Crystal Structure Database (2021) ©2021 FIZ Karlsruhe GmbH
Jeong Y, Sim M, Shin JH, Choi J-W, Sohn JI, Cha SN, Choi H, Moon C, Jang JE (2015) Psychological tactile sensor structure based on piezoelectric nanowire cell arrays. RSC Adv 5:40363–40368. https://doi.org/10.1039/C5RA05744B
Jiruše J, Havelka M, Lopour F (2014) Novel field emission SEM column with beam deceleration technology. Ultramicroscopy 146:27–32. https://doi.org/10.1016/j.ultramic.2014.05.006
Khosravi-Nejad F, Teimouri M, Marandi SJ, Shariati M (2019) The highly crystalline tellurium doped ZnO nanowires photodetector. J Cryst Growth 522:214–220. https://doi.org/10.1016/j.jcrysgro.2019.06.020
Kim S-J, Park D-W (2009) Preparation of ZnO nanopowders by thermal plasma and characterization of photo-catalytic property. Appl Surf Sci 255:5363–5367. https://doi.org/10.1016/j.apsusc.2008.10.028
Kim JH, Kumar V, Chernomordik B, Sunkara MK (2008) Design of an efficient microwave plasma reactor for bulk production of inorganic nanowires. Informacije MIDEM 38:237–243
Klingshirn C (2007) ZnO: material, physics and applications. Chem Phys Chem 8:782–803. https://doi.org/10.1002/cphc.200700002
Ko TS, Yang S, Hsu HC, Chu CP, Lin HF, Liao SC, Lu TC, Kuo HC, Hsieh WF, Wang SC (2006) ZnO nanopowders fabricated by dc thermal plasma synthesis. Mater Sci Eng, B 134:54–58. https://doi.org/10.1016/j.mseb.2006.07.019
Ko SH, Lee D, Kang HW, Nam KH, Yeo JY, Hong SJ, Grigoropoulos CP, Sung HJ (2011) Nanoforest of hydrothermally grown hierarchical ZnO nanowires for a high efficiency dye-sensitized solar cell. Nano Lett 11:666–671. https://doi.org/10.1021/nl1037962
Kong YC, Yu DP, Zhang B, Fang W, Feng SQ (2001) Ultraviolet-emitting ZnO nanowires synthesized by a physical vapor deposition approach. Appl Phys Lett 78(1):1342050. https://doi.org/10.1063/1.1342050
Kumar V, Kim JH, Pendyala C, Chernomordik B, Sunkara MK (2008) Gas-phase, bulk production of metal oxide nanowires and nanoparticles using microwave plasma jet reactor. J Phys Chem C 112:17750–17754. https://doi.org/10.1021/jp8078315
Laue M (1926) Lorentzfaktor und Intensitätsverteilung in Debye-Scherrer Ringen. Z Krist 64:115–142. https://doi.org/10.1524/zkri.1926.64.1.115
Lee B-J, Jo S-I, Jeong G-H (2019) Synthesis of ZnO nanomaterials using low-cost compressed air as microwave plasma gas at atmospheric pressure. Nanomaterials 9:942. https://doi.org/10.3390/nano9070942
Lee B-J, Jo S-I, Heo S-G, Lee W-Y, Jeong G-H (2021) Structure-controllable synthesis of ZnO nanowires using water vapor in an atmospheric pressure plasma system. Curr Appl Phys 28:52–58. https://doi.org/10.1016/j.cap.2021.05.004
Liao S-C (2006) dc thermal plasma synthesis and properties of zinc oxide nanorods. J Vac Sci Technol B 24:1322. https://doi.org/10.1116/1.2197513
Lin H-F, Liao S-C, Hung S-W (2005) The DC thermal plasma synthesis of ZnO nanoparticles for visible-light photocatalyst. J Photochem Photobiol A 174:82–87. https://doi.org/10.1016/j.jphotochem.2005.02.015
Lin H-F, Liao S-C, Hu C-T (2009) A new approach to synthesize ZnO tetrapod-like nanoparticles with DC thermal plasma technique. J Cryst Growth 311:1378–1384. https://doi.org/10.1016/j.jcrysgro.2008.12.033
Lu M-P, Lu M-Y, Chen L-J (2012) p.-Type ZnO nanowires: from synthesis to nanoenergy. Nano Energy 1:247–258. https://doi.org/10.1016/j.nanoen.2011.12.004
Lynam MF, Ke N-J, Bradley SJ, Nann T, Neiman A, Reeves RJ, Downard AJ, Golovko VB, Allen MW (2019) Size-controlled, high optical quality ZnO nanowires grown using colloidal Au nanoparticles and ultra-small cluster catalysts. APL Mater 7:022518. https://doi.org/10.1063/1.5054355
Maity R, Das S, Mitra MK, Chattopadhyay KK (2005) Synthesis and characterization of ZnO nano/microfibers thin films by catalyst free solution route. Physica E 25:605–612. https://doi.org/10.1016/j.physe.2004.09.002
Mardosaite R, Jurkeviciute A, Rackauskas S (2021) Superhydrophobic ZnO nanowires: wettability mechanisms and functional applications. Cryst Growth Des 21:4765–4779. https://doi.org/10.1021/acs.cgd.1c00449
Mohammed RS, Aadim KA, Ahmed KA (2022) Estimation on in vivo toxicity of MgO/ZnO core/shell nanoparticles synthesized by eco-frriendly non-thermal plasma technology. Appl Nanosci 12:3783–3795. https://doi.org/10.1007/s13204-022-02608-1
Navarrete E, Guell F, Martinez-Alanis PR, Llobet E (2022) Chemical vapour deposited ZnO nanowires for detecting ethanol and NO2. J Alloys Compd 890:161923. https://doi.org/10.1016/j.jallcom.2021.161923
Peng H, Fangli Y, Liuyang B, Jinlin L, Yunfa C (2007) Plasma synthesis of large quantities of zinc oxide nanorods. J Phys Chem C 111:194–200. https://doi.org/10.1021/JP065390B
Petzold FG, Jasinski J, Clark EL, Kim JH, Absher J, Toufar H, Sunkara MK (2012) Nickel supported on zinc oxide nanowires as advanced hydrodesulfurization catalysts. Catal Today 198:219–227. https://doi.org/10.1016/j.cattod.2012.05.030
Primc G, Brenčič K, Mozetič M, Gorjanc M (2021) Recent advances in the plasma-assisted synthesis of zinc oxide nanoparticles. Nanomater 11:1191. https://doi.org/10.3390/nano11051191
Rackauskas S, Barbero N, Barolo C, Viscardi G (2017) ZnO nanowire application in chemoresistive sensing: a review. Nanomater 7:381. https://doi.org/10.3390/nano7110381
Rahm A, Kaidashev EM, Schmidt H, Diaconu M, Poppl A, Bottcher R, Meinecke C, Butz T, Lorenz M, Grundmann M (2006) Growth and characterization of Mn- and Co-doped ZnO nanowires. Microchim Acta 156:21–25. https://doi.org/10.1007/s00604-006-0602-1
Saqib ANS, Huong NTT, Kim S-W, Jung M-H, Lee YH (2021) Structural and magnetic properties of highly Fe-doped ZnO nanoparticles synthesized by one-step solution plasma process. J Alloys Compd 853:157153. https://doi.org/10.1016/j.allcom.2020.157153
Sharma SP, Ting JM, Chang ZK (2010) Growth of ZnO nanowires on carbon fibers by RF magnetron sputtering technique. Adv Sci Lett 3:74–79. https://doi.org/10.1166/ASL.2010.1088
Shin S-H, Kim Y-H, Lee MH, Jung J-Y, Seol JH, Nah J (2014) Lithium-doped zinc oxide nanowires-polymer composite for high performance flexible piezoelectric nanogenerator. ACS Nano 8:10844–10850. https://doi.org/10.1021/nn5046568
Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM, Hasan H, Mohamad D (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett 7:219–242. https://doi.org/10.1007/s40820-015-0040-x
Sowa H, Ahsbahs H (2006) High-pressure X-ray investigation of zincite ZnO single crystals using diamond anvils with an improved shape. J Appl Cryst 39:169–175. https://doi.org/10.1107/S0021889805042457
Subannajui K (2016) Super-fast synthesis of ZnO nanowires by microwave air-plasma. Chem Commun 52:3195. https://doi.org/10.1039/c5cc09051b
Sun Y, Fuge GM, Ashfold MNR (2004) Growth of aligned ZnO nanorod arrays by catalyst-free pulsed laser deposition methods. Chem Phys Lett 396:21–26. https://doi.org/10.1016/j.cplett.2004.07.110
Tello A, Boulett A, Sanchez J, Pizarro GdC, Soto C, Perez OEL, Sanhueza R, Oyarzun DP (2021) An unexplored strategy for synthesis of ZnO nanowire films by electrochemical anodization using an organic-based electrolyte Morphological and optical properties characterization. Chem Phys Lett 778:138825. https://doi.org/10.1016/j.cplett.2021.138825
Wang ZL (2008) Towards self-powered nanosystems: from nanogenerators to nanopiezotronics. Adv Funct Mater 18:3553–3567. https://doi.org/10.1002/adfm.200800541
Wang ZL, Song JH (2006) Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Sci 312:242–246. https://doi.org/10.1126/science.1124005
Wang YC, Leu IC, Hon MH (2002) Preparation of nanosized ZnO arrays by electrophoretic deposition. Electrochem Solid-State Lett 5:C53–C55. https://doi.org/10.1149/1.1454547
Wang Z, Pan X, He Y, Hu Y, Gu H, Wang Y (2015) Piezoelectric nanowires in energy harvesting applications. Adv Mater Sci Eng 2015:165631. https://doi.org/10.1155/2015/165631
Wu GS, Xie T, Yuan XY, Li Y, Yang L, Xiao YH, Zhang LD (2005) Controlled synthesis of ZnO nanowires or nanotubes via sol-gel template process. Solid State Commun 134:485–489. https://doi.org/10.1016/j.ssc.2005.02.015
Xu Q, Guo Y, Li C, Zhang BH, Wang X, Zhang H (2020) High dose gamma-ray effects of solution-growth vertical ZnO nanowires. Radiat Phys Chem 176:109027. https://doi.org/10.1016/j.radphyschem.2020.109027
Yang RS, Qin Y, Dai LM, Wang ZL (2009) Power generation with laterally packaged piezoelectric fine wires. Nat Nanotechnol 4:34–39. https://doi.org/10.1038/nnano.2008.314
Zhang H, Ma XY, Xu J, Niu JJ, Yang DR (2003) Arrays of ZnO nanowires fabricated by a simple chemical solution route. Nanotechnol 14:423–426. https://doi.org/10.1088/0957-4484/14/4/303
Zhang Y, Manoj KR, Stefanakos EK, Goswami DY (2012) Synthesis, characterization, and applications of ZnO nanowires. J Nanomater. https://doi.org/10.1155/2012/624520
Zhong Z, Wang C, Han R, Gao M, Huang Y, Ramakrishna S (2023) Synthesis of zinc oxide/carbon fiber composites with improved piezoelectric response by plasma-liquid interaction. Compos Commun 38:101495. https://doi.org/10.1016/j.coco.2023.101495
Zhu G, Yang R, Wang S, Wang ZL (2010) Flexible highoutput nanogenerator based on lateral ZnO nanowire array. Nano Lett 10:3151–3155. https://doi.org/10.1021/nl101973h
Acknowledgements
The research for this article would not have been possible without funding. We are grateful to thank the Austrian Research Promotion Agency (FFG) with the funding programmes “BRIDGE” and “Produktion der Zukunft” promoted by the Federal Ministry of Labour and Economy and the Federal Ministry for Transport, Innovation and Technology, as well as the European Union’s Horizon 2020 research and innovation programme.
Funding
Open access funding provided by JOANNEUM RESEARCH Forschungsgesellschaft mbH. The research was funded partially by the Austrian Research Promotion Agency (FFG) with the funding programmes “BRIDGE” (project number 855751) and “Produktion der Zukunft” (project number 871461) promoted by the Federal Ministry of Labour and Economy and the Federal Ministry for Transport, Innovation and Technology, as well as the European Union’s Horizon 2020 research and innovation programme (grant agreement number 101006952).
Author information
Authors and Affiliations
Contributions
Conceptualization: JML, AMS, WW. Methodology/study design: JML, AMS, MT, MZ. ZnO NW process development SC, CH AH, RK, DK, JML, MS, AMS. Piezoelectric sensor development MT, MZ. SEM/EDS characterization: SA, DH. XRD characterization PA, BF. Writing—original draft: AMS. Writing—review and editing PA, SA, SC, JML, MS, MT, MZ, RK. Visualization: AMS. Supervision WW. Project administration RK, JML, AMS. Funding acquisition: RK, JML, WW.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest. As mentioned above, the work was funded (all companies/institutes the authors are affiliated to have received funding of at least one of the acknowledged funding instruments).
Ethical statement
This work involves no live subjects (human or animal).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schwan, A.M., Chwatal, S., Hendler, C. et al. Morphology-controlled atmospheric pressure plasma synthesis of zinc oxide nanoparticles for piezoelectric sensors. Appl Nanosci 13, 6421–6432 (2023). https://doi.org/10.1007/s13204-023-02936-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-023-02936-w