Abstract
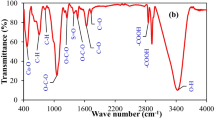
A composite of Fe3O4 nanoparticles and Eucalyptus camaldulensis (Ec) functionalized with ethylenediaminetetraacetic acid (EDTA) was characterized, and the efficacy of both adsorbents was investigated for the adsorption of lead ions (Pb2+) in batch studies. Fourier transform infrared spectra revealed successful modifications of the Fe3O4-Ec magnetic nanocomposite with EDTA and adsorption of Pb2+ ions on the surface of both adsorbents. A 15-min equilibrium contact time was estimated for optimal performance of both adsorbents due to the high adsorption capacity and the fast trend of removal efficiency upon immediate contact. Batch performance improved as the solution pH increased with the optimum value at 6.0 with maximum adsorption capacity and removal efficiency of 131 mg g−1 and 99.5%, respectively. Both adsorbents exhibited a decreasing trend in adsorption capacity reaching to 78 mg g−1 at 0.5 g, while the removal efficiency followed the opposite trend of a linear increase to almost 99% as the amount of adsorbent increased. The adsorption capacity increased as the initial Pb2+ concentration increased by 80 and 95 mg g−1 for Fe3O4-Ec and Fe3O4-Ec + EDTA, while the removal efficiency decreased by more than 50% for both adsorbents in the studied range of Pb2+ (20–100 mg L−1). The Langmuir model better fitted the experimental data using both adsorbents with high coefficient of determination (R2) values in addition to the Sips model, which proposes homogeneous as well as heterogeneous adsorption of Pb2+ on both adsorbents. Physical adsorption was proposed based on the estimated values of E (< 8 kJ mol−1) for both adsorbents, as estimated in the Dubinin–Radushkevich isotherm. A good fitting of the nonlinear pseudosecond-order model with R2 values of 0.86 and 0.89 supports chemisorption as the controlling mechanism of adsorption. The estimated maximum adsorption capacities of Fe3O4-Ec revealed its potential for efficient removal of Pb2+ and encourages further work on catalytic (functionalized with EDTA) abatement of inorganic contaminants.






Similar content being viewed by others
References
Aadil M, Zulfiqar S, Sabeeh H et al (2020) Enhanced electrochemical energy storage properties of carbon coated Co3O4 nanoparticles-reduced graphene oxide ternary nano-hybrids. Ceram Int 46:17836–17845. https://doi.org/10.1016/j.ceramint.2020.04.090
Abtahi M, Mesdaghinia A, Saeedi R, Nazmara S (2013) Biosorption of As(III) and As(V) from aqueous solutions by brown macroalga Colpomenia sinuosa biomass: kinetic and equilibrium studies. Desalin Water Treat 51:3224–3232. https://doi.org/10.1080/19443994.2012.749034
Abu-Dief AM, Abdel-Fatah SM (2018) Development and functionalization of magnetic nanoparticles as powerful and green catalysts for organic synthesis. Beni-Suef Univ J Basic Appl Sci 7:55–67. https://doi.org/10.1016/j.bjbas.2017.05.008
Ahmad W, Khan A, Ali N et al (2021) Photocatalytic degradation of crystal violet dye under sunlight by chitosan-encapsulated ternary metal selenide microspheres. Environ Sci Pollut Res 28:8074–8087. https://doi.org/10.1007/s11356-020-10898-7
Ali N, Ali F, Khurshid R et al (2020a) TiO2 nanoparticles and epoxy-TiO2 nanocomposites: a review of synthesis, modification strategies, and photocatalytic potentialities. J Inorg Organomet Polym 30:4829–4846. https://doi.org/10.1007/s10904-020-01668-6
Ali N, Ali F, Said A et al (2020b) Characterization and deployment of surface-engineered cobalt ferrite nanospheres as photocatalyst for highly efficient remediation of alizarin red S dye from aqueous solution. J Inorg Organomet Polym 30:5063–5073. https://doi.org/10.1007/s10904-020-01654-y
Ali N, Hassan Riead MM, Bilal M et al (2021a) Adsorptive remediation of environmental pollutants using magnetic hybrid materials as platform adsorbents. Chemosphere 284:131279. https://doi.org/10.1016/j.chemosphere.2021.131279
Ali N, Hellen BJ, Duanmu C et al (2021b) Effective remediation of petrochemical originated pollutants using engineered materials with multifunctional entities. Chemosphere 278:130405. https://doi.org/10.1016/j.chemosphere.2021.130405
Altaf S, Zafar R, Zaman WQ et al (2021) Removal of levofloxacin from aqueous solution by green synthesized magnetite (Fe3O4) nanoparticles using Moringa olifera: Kinetics and reaction mechanism analysis. Ecotoxicol Environ Saf 226:112826. https://doi.org/10.1016/j.ecoenv.2021.112826
Amin MT, Alazba AA, Shafiq M (2021) Comparative removal of lead and nickel ions onto nanofibrous sheet of activated polyacrylonitrile in batch adsorption and application of conventional kinetic and isotherm models. Membranes 11:10. https://doi.org/10.3390/membranes11010010
Aramesh N, Bagheri AR, Bilal M (2021) Chitosan-based hybrid materials for adsorptive removal of dyes and underlying interaction mechanisms. Int J Biol Macromol 183:399–422. https://doi.org/10.1016/j.ijbiomac.2021.04.158
Aslam S, Ali A, Asgher M et al (2021) Fabrication and catalytic characterization of laccase-loaded calcium-alginate beads for enhanced degradation of dye-contaminated aqueous solutions. Catal Lett. https://doi.org/10.1007/s10562-021-03765-8
Ayranci E, Duman O (2010) Structural effects on the interactions of benzene and naphthalene sulfonates with activated carbon cloth during adsorption from aqueous solutions. Chem Eng J 156:70–76. https://doi.org/10.1016/j.cej.2009.09.038
Bayat M, Beyki MH, Shemirani F (2015) One-step and biogenic synthesis of magnetic Fe3O4–Fir sawdust composite: application for selective preconcentration and determination of gold ions. J Ind Eng Chem 21:912–919. https://doi.org/10.1016/j.jiec.2014.04.032
Chen J, Hao Y, Chen M (2014) Rapid and efficient removal of Ni(2+) from aqueous solution by the one-pot synthesized EDTA-modified magnetic nanoparticles. Environ Sci Pollut Res Int 21:1671–1679. https://doi.org/10.1007/s11356-013-2041-y
Cheng Z, Gao Z, Ma W et al (2012) Preparation of magnetic Fe3O4 particles modified sawdust as the adsorbent to remove strontium ions. Chem Eng J 209:451–457. https://doi.org/10.1016/j.cej.2012.07.078
Chowdhury S, Mishra R, Saha P, Kushwaha P (2011) Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination 265:159–168. https://doi.org/10.1016/j.desal.2010.07.047
Din MF, Ahmad I, Ahmad M et al (2014) Influence of Cd substitution on structural, electrical and magnetic properties of M-type barium hexaferrites co-precipitated nanomaterials. J Alloy Compd 584:646–651. https://doi.org/10.1016/j.jallcom.2013.09.043
Duman O, Özcan C, Gürkan Polat T, Tunç S (2019) Carbon nanotube-based magnetic and non-magnetic adsorbents for the high-efficiency removal of diquat dibromide herbicide from water: OMWCNT, OMWCNT-Fe3O4 and OMWCNT-κ-carrageenan-Fe3O4 nanocomposites. Environ Pollut 244:723–732. https://doi.org/10.1016/j.envpol.2018.10.071
Ghasemi E, Heydari A, Sillanpää M (2017) Superparamagnetic Fe3O4@EDTA nanoparticles as an efficient adsorbent for simultaneous removal of Ag(I), Hg(II), Mn(II), Zn(II), Pb(II) and Cd(II) from water and soil environmental samples. Microchem J 131:51–56. https://doi.org/10.1016/j.microc.2016.11.011
Gimbert F, Morin-Crini N, Renault F et al (2008) Adsorption isotherm models for dye removal by cationized starch-based material in a single component system: error analysis. J Hazard Mater 157:34–46. https://doi.org/10.1016/j.jhazmat.2007.12.072
Godwin PM, Pan Y, Xiao H, Afzal MT (2019) Progress in preparation and application of modified biochar for improving heavy metal ion removal from wastewater. J Bioresour Bioprod 4:31–42. https://doi.org/10.21967/jbb.v4i1.180
Günay A, Arslankaya E, Tosun İ (2007) Lead removal from aqueous solution by natural and pretreated clinoptilolite: adsorption equilibrium and kinetics. J Hazard Mater 146:362–371. https://doi.org/10.1016/j.jhazmat.2006.12.034
Gunay A (2007) Application of nonlinear regression analysis for ammonium exchange by natural (Bigadiç) clinoptilolite. J Hazard Mater 148:708–713. https://doi.org/10.1016/j.jhazmat.2007.03.041
Hameed BH, El-Khaiary MI (2008) Batch removal of malachite green from aqueous solutions by adsorption on oil palm trunk fibre: equilibrium isotherms and kinetic studies. J Hazard Mater 154:237–244. https://doi.org/10.1016/j.jhazmat.2007.10.017
Hassan A, Azhar Khan M, Shahid M et al (2015) Nanocrystalline Zn1−x Co0.5xNi0.5x Fe2O4 ferrites: fabrication via co-precipitation route with enhanced magnetic and electrical properties. J Magn Magn Mater 393:56–61. https://doi.org/10.1016/j.jmmm.2015.05.033
Kalaivani SS, Vidhyadevi T, Murugesan A et al (2014) The use of new modified poly(acrylamide) chelating resin with pendent benzothiazole groups containing donor atoms in the removal of heavy metal ions from aqueous solutions. Water Resour Ind 5:21–35. https://doi.org/10.1016/j.wri.2014.04.001
Kannan RRR, Stirk WA, Van Staden J (2013) Synthesis of silver nanoparticles using the seaweed Codium capitatum P.C. Silva (Chlorophyceae). S Afr J Bot 86:1–4. https://doi.org/10.1016/j.sajb.2013.01.003
Karapinar N, Donat R (2009) Adsorption behaviour of Cu2+ and Cd2+ onto natural bentonite. Desalination 249:123–129. https://doi.org/10.1016/j.desal.2008.12.046
Kataria N, Garg VK (2018) Green synthesis of Fe3O4 nanoparticles loaded sawdust carbon for cadmium (II) removal from water: regeneration and mechanism. Chemosphere 208:818–828. https://doi.org/10.1016/j.chemosphere.2018.06.022
Khan A, Malik S, Ali N et al (2021a) Biopolymer-based sorbents for emerging pollutants. Sorbents materials for controlling environmental pollution: current state and trends. Elsevier, Amsterdam, pp 463–491
Khan M, Khan A, Khan H et al (2021b) Development and characterization of regenerable chitosan-coated nickel selenide nano-photocatalytic system for decontamination of toxic azo dyes. Int J Biol Macromol 182:866–878. https://doi.org/10.1016/j.ijbiomac.2021.03.192
Khan S, Khan A, Ali N et al (2021c) Degradation of Congo red dye using ternary metal selenide-chitosan microspheres as robust and reusable catalysts. Environ Technol Innov 22:101402. https://doi.org/10.1016/j.eti.2021.101402
Khanniri E, Yousefi M, Mortazavian AM et al (2021) Effective removal of lead (II) using chitosan and microbial adsorbents: Response surface methodology (RSM). Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2021.02.065
Komkiene J, Baltrenaite E (2016) Biochar as adsorbent for removal of heavy metal ions [Cadmium(II), Copper(II), Lead(II), Zinc(II)] from aqueous phase. Int J Environ Sci Technol 13:471–482. https://doi.org/10.1007/s13762-015-0873-3
Kumar PS, Ramakrishnan K, Gayathri R (2010) Removal of Nickel(II) from Aqueous Solutions by Ceralite IR 120 Cationic Exchange Resins. J Eng Sci Technol 5:232–243
Liu Y, Chen M, Yongmei H (2013) Study on the adsorption of Cu(II) by EDTA functionalized Fe3O4 magnetic nano-particles. Chem Eng J 218:46–54. https://doi.org/10.1016/j.cej.2012.12.027
Liu Y, Fu R, Sun Y et al (2016) Multifunctional nanocomposites Fe3O4@SiO2-EDTA for Pb(II) and Cu(II) removal from aqueous solutions. Appl Surf Sci 369:267–276. https://doi.org/10.1016/j.apsusc.2016.02.043
Łuszczek-Trojnar E, Drąg-Kozak E, Popek W (2013) Lead accumulation and elimination in tissues of Prussian carp, Carassius gibelio (Bloch, 1782), after long-term dietary exposure, and depuration periods. Environ Sci Pollut Res Int 20:3122–3132. https://doi.org/10.1007/s11356-012-1210-8
Mahdavi M, Namvar F, Ahmad MB, Mohamad R (2013) Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules 18:5954–5964. https://doi.org/10.3390/molecules18055954
Malik PK (2003) Use of activated carbons prepared from sawdust and rice-husk for adsorption of acid dyes: a case study of Acid Yellow 36. Dyes Pigm 56:239–249. https://doi.org/10.1016/S0143-7208(02)00159-6
Manohar DM, Noeline BF, Anirudhan TS (2006) Adsorption performance of Al-pillared bentonite clay for the removal of cobalt(II) from aqueous phase. Appl Clay Sci 31:194–206. https://doi.org/10.1016/j.clay.2005.08.008
Martínez M, Miralles N, Hidalgo S et al (2006) Removal of lead(II) and cadmium(II) from aqueous solutions using grape stalk waste. J Hazard Mater 133:203–211. https://doi.org/10.1016/j.jhazmat.2005.10.030
Mireles S, Parsons J, Trad T et al (2019) Lead removal from aqueous solutions using biochars derived from corn stover, orange peel, and pistachio shell. Int J Environ Sci Technol 16:5817–5826. https://doi.org/10.1007/s13762-018-02191-5
Muhammad Z, Ali F, Sajjad M et al (2021) Zirconium-doped chromium IV oxide nanocomposites: synthesis, characterization, and photocatalysis towards the degradation of organic dyes. Catalysts 11:117. https://doi.org/10.3390/catal11010117
Naddafi K, Rastkari N, Nabizadeh R et al (2016) Adsorption of 2,4,6-trichlorophenol from aqueous solutions by a surfactant-modified zeolitic tuff: batch and continuous studies. Desalin Water Treat 57:5789–5799. https://doi.org/10.1080/19443994.2015.1005693
Natarajan S, Anitha V, Gajula GP, Thiagarajan V (2020) Synthesis and characterization of magnetic superadsorbent Fe3O4-PEG-Mg-Al-LDH nanocomposites for ultrahigh removal of organic dyes. ACS Omega 5:3181–3193. https://doi.org/10.1021/acsomega.9b03153
Peralta ME, Ocampo S, Funes IG et al (2020) Nanomaterials with tailored magnetic properties as adsorbents of organic pollutants from wastewaters. Inorganics 8:24. https://doi.org/10.3390/inorganics8040024
Rahman MS, Islam MR (2009) Effects of pH on isotherms modeling for Cu(II) ions adsorption using maple wood sawdust. Chem Eng J 149:273–280. https://doi.org/10.1016/j.cej.2008.11.029
Rahman A, Aadil M, Akhtar M et al (2020) Magnetically recyclable Ni1-xCdxCeyFe2-yO4-rGO nanocomposite photocatalyst for visible light driven photocatalysis. Ceram Int 46:13517–13526. https://doi.org/10.1016/j.ceramint.2020.02.136
Rahmanian O, Dinari M, Neamati S (2018) Synthesis and characterization of citrate intercalated layered double hydroxide as a green adsorbent for Ni2+ and Pb2+ removal. Environ Sci Pollut Res 25:36267–36277. https://doi.org/10.1007/s11356-018-3584-8
Raji F, Pakizeh M (2013) Study of Hg(II) species removal from aqueous solution using hybrid ZnCl2-MCM-41 adsorbent. Appl Surf Sci 282:415–424. https://doi.org/10.1016/j.apsusc.2013.05.145
Rasheed T, Bilal M, Nabeel F et al (2018) Fluorescent sensor based models for the detection of environmentally-related toxic heavy metals. Sci Total Environ 615:476–485. https://doi.org/10.1016/j.scitotenv.2017.09.126
Rasheed T, Ahmad N, Ali J et al (2021) Nano and micro architectured cues as smart materials to mitigate recalcitrant pharmaceutical pollutants from wastewater. Chemosphere 274:129785. https://doi.org/10.1016/j.chemosphere.2021.129785
Salman M, Athar M, Farooq U et al (2014) A new approach to modification of an agro-based raw material for Pb(II) adsorption. Korean J Chem Eng 31:467–474. https://doi.org/10.1007/s11814-013-0264-8
Shafiq M, Alazba AA, Amin MT (2018) Removal of heavy metals from wastewater using date palm as a biosorbent: a comparative review. Sains Malaysiana 47:35–49. https://doi.org/10.17576/jsm-2018-4701-05
Shafiq M, Alazba AA, Amin MT (2019) Lead and copper scavenging from aqueous solutions using Eucalyptus camaldulensis derived activated carbon: equilibrium, kinetics and sorption mechanism. DWT 158:187–198. https://doi.org/10.5004/dwt.2019.24262
Shakir I, Shahid M, Rana UA, Warsi MF (2014) In situ hydrogenation of molybdenum oxide nanowires for enhanced supercapacitors. RSC Adv 4:8741–8745. https://doi.org/10.1039/C3RA44837A
Taamneh Y, Sharadqah S (2017) The removal of heavy metals from aqueous solution using natural Jordanian zeolite. Appl Water Sci 7:2021–2028. https://doi.org/10.1007/s13201-016-0382-7
Tan L, Xu J, Xue X et al (2014) Multifunctional nanocomposite Fe3O4@SiO2–mPD/SP for selective removal of Pb(II) and Cr(VI) from aqueous solutions. RSC Adv 4:45920–45929. https://doi.org/10.1039/C4RA08040H
Tang H, Zhou W, Zhang L (2012) Adsorption isotherms and kinetics studies of malachite green on chitin hydrogels. J Hazard Mater 209–210:218–225. https://doi.org/10.1016/j.jhazmat.2012.01.010
Taty-Costodes VC, Fauduet H, Porte C, Delacroix A (2003) Removal of Cd(II) and Pb(II) ions, from aqueous solutions, by adsorption onto sawdust of Pinus sylvestris. J Hazard Mater 105:121–142. https://doi.org/10.1016/j.jhazmat.2003.07.009
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metals toxicity and the environment. EXS 101:133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Venkatraman Y, Priya AK (2021) Removal of heavy metal ion concentrations from the wastewater using tobacco leaves coated with iron oxide nanoparticles. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-021-03202-8
Verma R, Vijayalakshmy K, Chaudhiry V (2018) Detrimental impacts of heavy metals on animal reproduction: a review. J Entomol Zool Stud 6:27–30
Wang H, Gao B, Wang S et al (2015) Removal of Pb(II), Cu(II), and Cd(II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood. Biores Technol 197:356–362. https://doi.org/10.1016/j.biortech.2015.08.132
Yang Y, Ali N, Khan A et al (2021) Chitosan-capped ternary metal selenide nanocatalysts for efficient degradation of Congo red dye in sunlight irradiation. Int J Biol Macromol 167:169–181. https://doi.org/10.1016/j.ijbiomac.2020.11.167
Yu X, Tong S, Ge M et al (2012) One-step synthesis of magnetic composites of cellulose@iron oxide nanoparticles for arsenic removal. J Mater Chem A 1:959–965. https://doi.org/10.1039/C2TA00315E
Yu J-X, Wang L-Y, Chi R-A et al (2013) Competitive adsorption of Pb2+ and Cd2+ on magnetic modified sugarcane bagasse prepared by two simple steps. Appl Surf Sci 268:163–170. https://doi.org/10.1016/j.apsusc.2012.12.047
Zaghouane-Boudiaf H, Boutahala M, Arab L (2012) Removal of methyl orange from aqueous solution by uncalcined and calcined MgNiAl layered double hydroxides (LDHs). Chem Eng J 187:142–149. https://doi.org/10.1016/j.cej.2012.01.112
Zeb S, Ali N, Ali Z et al (2020) Silica-based nanomaterials as designer adsorbents to mitigate emerging organic contaminants from water matrices. J Water Process Eng 38:101675. https://doi.org/10.1016/j.jwpe.2020.101675
Funding
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (12-WAT2623-02).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shafiq, M., Alazba, A.A. & Amin, M.T. Synthesis of a novel EDTA-functionalized nanocomposite of Fe3O4-Eucalyptus camaldulensis green carbon fiber for selective separation of lead ions from synthetic wastewater: isotherm and kinetic studies. Appl Nanosci 12, 3607–3620 (2022). https://doi.org/10.1007/s13204-022-02420-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-022-02420-x