Abstract
In the current work, the leaf extract of Bridelia retusa was used for the first time to synthesize zinc oxide nanoparticles (ZnONPs). A zinc nanoparticle-specific 364-nm peak was discerned via UV–Vis studies with a typical bandgap energy of 3.41 eV. FE-SEM micrographs revealed flower-shaped structure of the ZnONPs. EDS analysis corroborated the presence of zinc and oxygen. XRD spectrum established the wurtzite structure, sized at 11.06 nm. The mesoporous texture (4.89 nm) of the nanoparticles was deduced from BET analysis, proving a higher specific surface area than commercial ZnONPs. FTIR spectroscopy resulted in absorption bands typical for ZnONPs. Within a span of 165 min, under solar irradiation, the ZnONPs facilitated the photocatalytic degradation of Rhodamine B dye upto 94.74%. Exhibiting pseudo-first-order kinetics, the process had a degradation constant of 0.0109 min−1. It was concluded that numerous factors led to the high degradation efficiency. High values of bandgap energy and specific surface area, along with the mesoporous and crystalline nature of the ZnONPs led to the observed effect. The ZnONPs were also stabilized by the phytochemicals in the B. retusa leaves. The study is thus able to successfully demonstrate the huge potential in the field of environmental nanoremediation. The viability of using ZnONPs as solar photocatalysts for treating dye-laden industrial wastewater was thus attested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanomaterials have shown magnificent applications ranging from technology to biological science, to produce useful products. The nanoparticles have a large surface-to-volume ratio which makes them suitable for various sectors such as energy, medical, cosmetics, electronics, catalysis, wastewater treatment, food, and agriculture. One of the important metal oxide nanoparticles is zinc oxide nanoparticles (ZnONPs) which have distinct features such as sensing, UV blocking, thermal and chemical stability, and biocompatibility. Moreover, it has high bandgap energy with enormous binding energy and hence, utilized in different fields, including cosmetics, medicine, and environmental remediation (Kadam et al. 2020). Recently, green synthesis has grown to be a key motivating force for nanoparticle synthesis (Hasan 2021). The release of massive quantities of colorants in the water bodies is a major environmental crisis (Varadavenkatesan et al. 2020a, b). Different types of dyes are discharged from various industries which make undesirable impacts on living beings (Chakraborty et al. 2020). It is essential to remove these dyes from wastewater before entry into water bodies (Sharifpour et al. 2018). Among various dye-removal technologies, a popular technique is advanced oxidation process (AOP) that relies on highly reactive oxidants to destroy organic pollutants till their mineralization produces CO2 and H2O as end products.
The synthesis of ZnONPs is mostly achieved by expensive chemical and physical routes. Moreover, the chemical methods utilize toxic chemicals and the release of these chemicals in effluents is an environmental concern (Bandeira et al. 2020). To avoid such problems, there is a necessity to explore cheap and eco-friendly methods for the synthesis of nanoparticles. Plant extracts are more efficient due to their accessibility and the possession of several metabolites in them, helping to reduce Zn2+ to ZnONPs (Akbarian et al. (2020)). Moreover, the plant extracts are enriched with wide ranges of phytocompounds like polyphenols and flavonoids, deemed to be bio-reducing agents with no negative effects on the environment (Shayegan Mehr et al. 2018). Their use as reducing and capping agents has led to recognition as biologically benign materials for the formulation of eco-friendly and low-cost nanoparticles (Manjari et al. 2019; Krishnan et al. 2020). Rhodamine B (RhB) is a water-soluble dye, used as a coloring agent in many industries. The release of RhB-contaminated wastewaters may cause reproductive and nervous systems disorders and hence, it is essential to remove RhB dye from wastewater (Sonker et al. 2020). Photocatalysis is one AOP process, comprising the effective use of light energy to generate hydroxyl radicals for the degradation of dyes (Bishnoi et al. 2018; Sirdeshpande et al. 2018). Being cheap, non-toxic, and able to absorb a larger fraction of solar irradiation, the ZnONPs are preferred over TiO2 and Al2O3—the conventional photocatalysts (Ong et al. 2018). Besides, the ZnONPs have the same bandgap energy similar to TiO2.
Few recent reports on the synthesis of ZnONPs include the use of various plant extracts such as Azadirachta indica (Haque et al. 2020), Ilex paraguariensis (Bandeira 2020), Camellia sinensis L (Akbarian et al. 2020), Acacia caesia (Manjari et al. 2019), Jatropha gossypifolia (Krishnan et al. 2020), Lippia adoensis (Demissie et al. 2020), Justicia spicigera (Soto-Robles 1225), and Averrhoa carambola (Chakraborty et al. 2020). In addition, our research group also has published leaf extract-mediated ZnONPs using Calliandra haematocephala (Vinayagam et al. 2019), Cynometra ramiflora (Varadavenkatesan et al. 2019), and Peltophorum pterocarpum (Pai et al. 2019a). Already our research group has reported the potential of Bridelia retusa leaf extract to act as a reducing and capping agent for the synthesis of silver nanoparticles (Ramesh et al. 2017). There are reports available in the literature to utilize various plant extract-mediated ZnONPs as photocatalysts to degrade harmful dyes such as methylene blue (MB) (Haque et al. 2020; Vinayagam et al. 2019; Pai et al. 2019a), RhB (Varadavenkatesan et al. 2019), Basic Red 51 (Yashni et al. 2020), Congo red (Chakraborty et al. 2020), and other pollutants like p-nitrophenol (Kadam et al. 2020).
Taking into cognizance the current state of work, in the present study, we report the synthesis of ZnONPs using the B. retusa leaf extract. Post synthesis, we characterize the zinc oxide nanoparticles by various analytical methods. Moreover, we demonstrate the photocatalytic degradation of RhB dye under sunlight in the presence of the synthesized ZnONPs.
Materials and methods
Materials
Zinc acetate dihydrate (Merck, India) was used as the zinc precursor. RhB dye and sodium hydroxide (analytical-grade) were procured from Merck, India. Distilled water was used for all the experiments.
Preparation of Bridelia retusa leaf extract
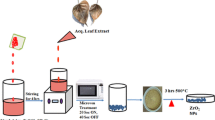
Healthy leaves of B. retusa were collected from Manipal, India, and rinsed with tap water initially, followed by distilled water-wash. 10 g of leaves were added to 200 mL of water and heated at 80 °C for 15 min to produce a pale-brown broth, labelled as BRLE.
Preparation of Bridelia retusa–facilitated ZnONPs
0.05 M zinc acetate, BRLE, and 1 N NaOH were mixed at a volume ratio of 2.5:1:1 yielding a creamy suspension. The resulting contents were heated for 1 h at 80 °C, forming an ivory precipitate and thus indicating the formation of ZnONPs. Later, the precipitate was removed by centrifugation with multiple water-washes and dried at 90 °C overnight, which resulted in a white powder, termed “BR–ZnONFs”.
Photodegradation of RhB dye under sunlight
The photodegradation ability of the BR–ZnONFs was examined by the degradation of RhB dye in the presence of sunlight. The experiments were performed by taking 100 mL of RhB dye (13 μM) in a conical flask with 40 mg BR–ZnONFs and magnetically mixed in dark for 1 h to obtain a homogenous suspension. Later, the flask was exposed to natural sunlight irradiation with constant stirring on a sunny day during October 2020. The absorbance spectra of the samples withdrawn at fixed time intervals were recorded and the concentrations were calculated using the absorbance data (λmax = 553 nm).
Characterization of BR–ZnONFs
The BR–ZnONFs were characterized by various techniques. The formation of ZnONPs and the concentration of RhB dye at regular intervals were examined by a UV–vis spectrometer (Shimadzu, UV-1800). The morphological characteristics and elemental compositions were analysed using a Field Emission Scanning Electron Microscope (FE-SEM) (Carl ZEISS, Sigma, Oxford Instruments) coupled with Energy Dispersive X-ray Spectroscopy (EDS). For the phase analysis and crystallinity, an X-ray diffractometer (Rigaku, Miniflex 600) was used. Fourier Transform Infrared (FTIR) spectrophotometer (Shimadzu, 8400S) was used for the analysis of functional groups present on the surface of BR–ZnONFs. The porous nature and the specific surface area of the BR–ZnONFs were examined by the Brunauer–Emmett–Teller (BET) apparatus (Smart Instruments, Mumbai).
Results and discussions
Visual monitoring and UV–Vis studies
The formation of an ivory-colloidal suspension (Fig. 1c) was the indication of the incipient formation of ZnONPs, when the colourless 0.5 M zinc acetate solution (Fig. 1a), 1 M NaOH, and pale-brown BRLE (Fig. 1b) were mixed. Heating the contents at 80 °C for 1 h precipitated the nanoparticles (Fig. 1d) which were further purified and dried at 90 °C overnight. The white-powder (Fig. 1e) obtained was designated as BR–ZnONFs.
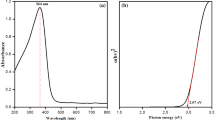
The UV–Vis spectra of the diluted BRLE and 0.1 wt% suspension of BR–ZnONFs are shown in Fig. 2. The BRLE (Fig. 2a) showed a broad peak at 276 nm which may be assigned to the phytocompounds present in the leaf extract (Groiss et al. 2017). This peak was not present in the spectrum of BR–ZnONFs (Fig. 2b) ascertaining the interaction between the phytocompounds and zinc acetate to form nanoparticles. The interaction was due to the reduction potential values of zinc and phytocompounds as explained in the literature (Vinayagam et al. 2019; Varadavenkatesan et al. 2019). Besides, Fig. 2b shows a peak at 364 nm—a characteristic feature of ZnONPs which may be due to the surface plasmon resonance or the transfer of electrons from the valence to the conduction band (Rambabu et al. 2020). The peak obtained in this study was in concordance with our earlier study for the green synthesis of ZnONPs using P. pterocarpum leaf extract wherein a peak at 365 nm was observed. Planck–Einstein equation was used to determine the bandgap energy and was calculated as 3.41 eV, similar to the Jatropha gum-mediated ZnONPs (Geetha et al. Sep. 2016).
FE-SEM–EDS analysis
FE-SEM images (Fig. 3a, b) revealed ‘flower-shaped’ zinc oxide nanoparticles. These asteroidal uniform nano-petals homogeneously exuded out from the center, being powerfully held to the spherical pedicle and providing an almost precise 3D alignment (Hasan 2021). The alkaline conditions used during the synthesis process formed flower-like ZnONPs. Flower-like structures are very common for ZnONPs synthesized using plant extracts as reported by many researchers such as the extracts of Carissa edulis (Fowsiya et al. 2016), Codonopsis lanceolate (Lu May 2019), Laurus nobilis L (Fakhari et al. 2019), Juglans regia L. (Darvishi et al. 2019), Calliandra haematocephala (Vinayagam et al. 2019), Cyanometra ramiflora (Varadavenkatesan et al. 2019), and Peltophorum pterocarpum (Pai et al. 2019a). The sheets had massive structural and surface defects making BR–ZnONFs porous materials. These defects play a significant role in the photocatalysis by acting as extra sites during photocatalytic degradation (Din et al. 2020). The agglomerations noticed in the images may be due to the interactions between the BR–ZnONFs and of the phytocompounds present in the leaf extract (Vinayagam et al. 2020).
The EDS image (Fig. 3c) showed strong signals for Zn (at 1 & 8.63 keV) and O (at 0.53 keV), confirming the existence of ZnO in the sample. As depicted in Fig. 3c, the weight % composition of Zn and O are 63.57% and 26.49%, respectively, which substantiated the presence of ZnO. The composition of the BR–ZnONFs matched well with the ZnONPs synthesized using the extracts of J. spicigera (Soto-Robles 1225). Also, a peak for C (at 0.27 keV) was witnessed which may be due to the contribution of phytocompounds during the formation of ZnONPs. Similar results for the EDS of green synthesized ZnONPs have been described in earlier work (Rambabu et al. 2020).
XRD studies
The XRD pattern of the BR–ZnONFs is exemplified in Fig. 4 which confirmed the crystalline nature and orientation of zinc oxide nanoflowers. The sharp peak indicated the smaller size of the formed BR–ZnONFs (Praveen et al. 2019). The peaks at 2θ magnitudes of 31.53°, 34.13°, 35.97°, 47.21°, 56.28°, 62.62°, 67.56°, and 76.82° were indexed to (100), (002), (101), (102), (110), (103), (112), and (202) planes which matched with JCPDS-36–1451 and confirmed the formation of a crystalline hexagonal assembly (Awwad et al. 2020). No additional signals were noticed signifying the purity of the BR–ZnONFs. The average crystallite size was calculated using Scherrer formula (Vinayagam et al. 2017) and was estimated as 11.06 nm, concordant with our previous study (Pai et al. 2019b). The lattice parameters were calculated conferring to the Bragg’s equation and reflecting the hexagonal structure of ZnONPs. The parameters ‘a’ and ‘c’ were estimated at 3.27 Å and 5.25 Å for samples, corroborating with the literature (Bandeira 2020).
BET studies
The BET specific surface area (SSA) of the BR–ZnONFs was estimated as 6.71 m2/g, almost two times higher than the commercial ZnONPs (3.23 m2/g) (Chakrabarti and Dutta 2004). Moreover, the SSA obtained in this study was comparatively higher than the ZnONPs synthesized by chemical method (5.178 m2/g) (Duo et al. 2016). Besides, the pore diameter was determined as 4.89 nm which confirmed the mesoscopic nature of the nanoparticles. Hence, the high surface area and mesoscopic nature of the BR–ZnONFs may play a pivotal part in various applications such as catalysis, photocatalysis, and wastewater treatment.
FTIR studies
FTIR analysis at room temperature was performed for the BR–ZnONFs to assess the functional groups present in it. The biomolecules present in the extract play an imperative role in reducing and stabilizing the nanoparticles which can be recognized by FTIR analysis (Anand et al. 2019). The FTIR spectrum (Fig. 5) shows strong bands (cm–1) at 3369.64, 2358.94, 1649.14, 1548.84, 1419.61, 1041.56, 723.31, 634.58, and 422.41. The bands between 400 and 800 cm–1 were designated to Zn and O bonding vibrations confirming the formation of ZnONPs (Bhuyan et al. 2015). The remaining bands endorsed the presence of various functional groups such as alcoholic, ketonic, alkanes, amines, and other carbonyl groups which belonged to the phytocompounds of BRLE. Table 1 summarizes the various FTIR bands and the functional groups to which they correspond. The presence of these functional groups corroborated the participation of the phytocompounds of BRLE to form BR–ZnONFs from zinc acetate.
Photodegradation of RhB dye under sunlight
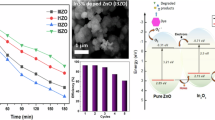
The photocatalytic degradation of RhB dye under sunlight irradiation was examined in the presence of BR–ZnONFs. The time-dependent absorption spectra of RhB dye is shown in Fig. 6. The fading-away of the RhB dye color can be witnessed from Fig. 6A confirming the dye degradation progress. Moreover, it can be visualized from the spectra that the intensity of the absorbance at 553 nm started declining as the sunlight exposure time increased. A degradation efficiency of 94.74% was observed within 165 min of sunlight exposure. This value was significantly higher than the values reported for the degradation of RhB dye by ZnONPs. For instance, only 38% and 71% degradation efficiencies for the RhB dye degradation under sunlight radiation were reported for the ZnONPs synthesized using algae (Vijai Anand et al. 2019) and chemical methods (Al-Bedairy and Alshamsi 2018) respectively. The enhanced degradation could be ascribed to the crystalline and mesoporous nature, high surface area, and good electron gaining characteristics of the BR–ZnONFs. Moreover, the improved photocatalytic activity may be due to the stabilization effect of the phytocompounds present in the BRLE. Similar conclusions were demonstrated for the solar light degradation of MB dye by neem leaf extract-mediated ZnONPs (Haque et al. 2020).
The kinetics of the photocatalytic degradation of RhB dye can be explained by pseudo-first-order kinetics: \(\mathrm{ln}\frac{{\mathrm{C}}_{\mathrm{o}}}{{\mathrm{C}}_{\mathrm{f}}}=\mathrm{kt}\)
where Co and Cf are the concentrations of the RhB dye at the initial and final time, ‘t’, and k is the degradation constant. A linear plot with a regression coefficient (R2) of 0.9631 was obtained by plotting ln (Cf/Co) vs ‘t’ which yielded a degradation constant (k) of 0.0109 min −1.
The fundamental mechanism (Fig. 7) of the photocatalytic degradation of RhB by the BR–ZnONFs under solar radiation is due to the generation of electron–hole pairs when the solar light energy illuminates the surface of the BR–ZnONFs (Prasad et al. 2018). These electron–hole pairs move spontaneously to the BR–ZnONFs surface and begin redox reactions with molecular oxygen and hydroxide ions. Oxidation of water to reactive hydroxyl (·OH) and H+ occurs in the valence band due to the existence of holes. Electrons in the conduction band reduce surface oxygen molecules to form superoxide radicals (O2·−). The extra electrons generated stimulate the effects to yield hydroxyl radicals (Rana et al. 2016). The monodispersive nature fostered the high-level exciton splitting to create immense amounts of •OH and O2•− which readily degraded the RhB dye molecules to colorless products, resulting in high dye degradation efficiency of BR–ZnONFs (Rambabu et al. 2020).
Conclusions
ZnONPs were synthesized using the aqueous extract of B. retusa leaves and used as solar photocatalysts to efficiently degrade RhB dye. Initially, the formation of BR–ZnONFs was visually confirmed by the color changes of the reaction mixture. Later, the samples were characterized by various techniques to examine the elemental composition, surface morphology, crystalline nature, surface functional groups, surface area, and nature of pores. High degradation efficiency (94.74%) of RhB dye was observed under solar light in the presence of BR–ZnONFs. The degradation process was modeled according to pseudo-first-order kinetics. Hence, this study suggests that the BR–ZnONFs can be used as effective photocatalysts to remove dyes from wastewater.
References
Akbarian M, Mahjoub S, Mohammad S, Zabihi E (2020) Biointerfaces Green synthesis, formulation and biological evaluation of a novel ZnO nanocarrier loaded with paclitaxel as drug delivery system on MCF-7 cell line. Colloids Surf B 186(2019):110686. https://doi.org/10.1016/j.colsurfb.2019.110686
Al-Bedairy MA, Alshamsi HAH (2018) Environmentally friendly preparation of zinc oxide, study catalytic performance of photodegradation by sunlight for rhodamine B Dye. Anal Chem Eurasian J. https://doi.org/10.29333/ejac/101785
Anand GT, Renuka D, Ramesh R, Anandaraj L, Sundaram SJ (2019) Green synthesis of ZnO nanoparticle using Prunus dulcis (Almond Gum) for antimicrobial and supercapacitor applications. Surf Interfaces 17:100376. https://doi.org/10.1016/j.surfin.2019.100376
Awwad AM, Amer MW, Salem NM, Abdeen AO (2020) Green synthesis of zinc oxide nanoparticles ( ZnO-NPs ) using Ailanthus altissima fruit extracts and antibacterial activity. Chem Int 6(3):151–159
Bandeira M, Giovanela M, Roesch-Ely M, Devine DM, da Silva Crespo J (2020) Green synthesis of zinc oxide nanoparticles: a review of the synthesis methodology and mechanism of formation. Sustain Chem Pharm 15:100223. https://doi.org/10.1016/j.scp.2020.100223
Bandeira M et al (2020) Mechanism of formation, characterization and cytotoxicity of green synthesized zinc oxide nanoparticles obtained from Ilex paraguariensis leaves extract. Nano-Struct Nano-Objects 24:100532. https://doi.org/10.1016/j.nanoso.2020.100532
Bhuyan T, Mishra K, Khanuja M, Prasad R, Varma A (2015) Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater Sci Semicond Process 32:55–61. https://doi.org/10.1016/j.mssp.2014.12.053
Bishnoi S, Kumar A, Selvaraj R (2018) Facile synthesis of magnetic iron oxide nanoparticles using inedible Cynometra ramiflora fruit extract waste and their photocatalytic degradation of methylene blue dye. Mater Res Bull 97:121–127. https://doi.org/10.1016/j.materresbull.2017.08.040
Chakrabarti S, Dutta BK (2004) Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst. J Hazard Mater 112(3):269–278
Chakraborty S, Farida JJ, Simon R, Kasthuri S, Mary NL (2020) Averrhoe carrambola fruit extract assisted green synthesis of zno nanoparticles for the photodegradation of congo red dye. Surf Interfaces 19:100488. https://doi.org/10.1016/j.surfin.2020.100488
Darvishi E, Kahrizi D, Arkan E (2019) Comparison of different properties of zinc oxide nanoparticles synthesized by the green (using Juglans regia L. leaf extract) and chemical methods. J Mol Liq 286:110831. https://doi.org/10.1016/j.molliq.2019.04.108
Demissie MG, Sabir FK, Edossa GD, Gonfa BA (2020) Synthesis of Zinc Oxide Nanoparticles Using Leaf Extract of Lippia adoensis (Koseret) and Evaluation of Its Antibacterial Activity. J Chem 2020:7459042. https://doi.org/10.1155/2020/7459042
Din MI, Najeeb J, Hussain Z, Khalid R (2020) Biogenic scale up synthesis of ZnO nano-flowers with superior nano-photocatalytic performance. Inorg Nano-Metal Chem. https://doi.org/10.1080/24701556.2020.1723026
Duo S, Li Y, Zhang H, Liu T, Wu K, Li Z (2016) A facile salicylic acid assisted hydrothermal synthesis of different flower-like ZnO hierarchical architectures with optical and concentration-dependent photocatalytic properties. Mater Charact 114:185–196
Ekennia AC et al (2020) Green synthesis of biogenic zinc oxide nanoflower as dual agent for photodegradation of an organic dye and tyrosinase inhibitor. J Inorg Organomet Polym Mater. https://doi.org/10.1007/s10904-020-01729-w
Fakhari S, Jamzad M, Kabiri Fard H (2019) Green synthesis of zinc oxide nanoparticles: a comparison. Green Chem Lett Rev 12(1):19–24
Fowsiya J, Madhumitha G, Al-Dhabi NA, Arasu MV (2016) Photocatalytic degradation of Congo red using Carissa edulis extract capped zinc oxide nanoparticles. J Photochem Photobiol B Biol 162:395–401
Geetha MS, Nagabhushana H, Shivananjaiah HN (2016) Green mediated synthesis and characterization of ZnO nanoparticles using Euphorbia Jatropa latex as reducing agent. J Sci Adv Mater Devices 1(3):301–310. https://doi.org/10.1016/j.jsamd.2016.06.015
Groiss S, Selvaraj R, Varadavenkatesan T, Vinayagam R (2017) Structural characterization, antibacterial and catalytic effect of iron oxide nanoparticles synthesised using the leaf extract of Cynometra ramiflora. J Mol Struct 1128:572–578. https://doi.org/10.1016/j.molstruc.2016.09.031
Haque MJ, Bellah MM, Hassan MR, Rahman S (2020) Synthesis of ZnO nanoparticles by two different methods & comparison of their structural, antibacterial, photocatalytic and optical properties. Nano Express 1(1):10007
Hasan M et al (2021) Bioinspired synthesis of zinc oxide nano-flowers: a surface enhanced antibacterial and harvesting efficiency. Mater Sci Eng C 119:111280. https://doi.org/10.1016/j.msec.2020.111280
Kadam VV, Shanmugam SD, Ettiyappan JP, Balakrishnan RM (2020) Photocatalytic degradation of p-nitrophenol using biologically synthesized ZnO nanoparticles. Environ Sci Pollut. https://doi.org/10.1007/s11356-020-10833-w
Kaliraj L, Ahn JC, Rupa EJ, Abid S, Lu J, Yang DC (2019) Synthesis of panos extract mediated ZnO nano-flowers as photocatalyst for industrial dye degradation by UV illumination”. J. Photochem. Photobiol. B Biol 199:111588. https://doi.org/10.1016/j.jphotobiol.2019.111588
Krishnan BR, Selvakumar MRM, Sasikumar SKA, Geerthi DV (2020) A facile green approach of cone - like ZnO NSs synthesized via jatropha gossypifolia leaves extract for photocatalytic and biological activity. J Inorg Organomet Polym Mater. https://doi.org/10.1007/s10904-020-01576-9
Lu J et al (2019) The assessment of photocatalytic activity of zinc oxide nanoparticles from the roots of Codonopsis lanceolata synthesized by one-pot green synthesis method. Optik (Stuttg) 184:82–89. https://doi.org/10.1016/j.ijleo.2019.03.050
Manjari G, Saran S, Radhakrishanan S, Rameshkumar P, Pandikumar A, Devipriya SP (2020) Facile green synthesis of Ag–Cu decorated ZnO nanocomposite for effective removal of toxic organic compounds and an efficient detection of nitrite ions. J Environ Manage 262(2019):110282. https://doi.org/10.1016/j.jenvman.2020.110282
Ong CB, Ng LY, Mohammad AW (2018) A review of ZnO nanoparticles as solar photocatalysts: synthesis, mechanisms and applications. Renew Sustain Energy Rev 81:536–551
Padalia H, Chanda S (2017) Characterization, antifungal and cytotoxic evaluation of green synthesized zinc oxide nanoparticles using Ziziphus nummularia leaf extract. Artif Cells Nanomed Biotechnol 45(8):1751–1761. https://doi.org/10.1080/21691401.2017.1282868
Pai S, S. H, T. Varadavenkatesan, R. Vinayagam, and R. Selvaraj, (2019) Photocatalytic zinc oxide nanoparticles synthesis using Peltophorum pterocarpum leaf extract and their characterization. Optik (Stuttg) 185:248–255. https://doi.org/10.1016/j.ijleo.2019.03.101
Pai S, Sridevi H, Varadavenkatesan T, Vinayagam R, Selvaraj R (2019) Photocatalytic zinc oxide nanoparticles synthesis using Peltophorum pterocarpum leaf extract and their characterization. Optik (Stuttg) 185:248–255. https://doi.org/10.1016/j.ijleo.2019.03.101
Prasad AR, Rugmini Ammal P, Joseph A (2018) Effective photocatalytic removal of different dye stuffs using green synthesized zinc oxide nanogranules. Mater Res Bull 102:116–121. https://doi.org/10.1016/j.materresbull.2018.02.022
Praveen B, Arthanareeswari M, Sridharan SDM, Arockia J, Pushpa T (2019) Green synthesis of zinc oxide nanoparticles using typha latifolia. L leaf extract for photocatalytic applications. Mater Today Proc 14:332–337. https://doi.org/10.1016/j.matpr.2019.04.155
Rambabu K, Bharath G, Banat F, Show PL (2021) Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment. J Hazard Mater 402:123560. https://doi.org/10.1016/j.jhazmat.2020.123560
Ramesh V, Thivaharan V, Raja S (2017) Synthesis, structural characterization and catalytic activity of Bridelia retusa leaf extract stabilized silver nanoparticles. Synth Green Process. https://doi.org/10.1515/gps20160236
Rana N, Chand S, Gathania AK (2016) Green synthesis of zinc oxide nano-sized spherical particles using Terminalia chebula fruits extract for their photocatalytic applications. Int Nano Lett 6(2):91–98. https://doi.org/10.1007/s40089-015-0171-6
Selvaraj H, Raja; Yadav, P. Manjunath, (2019) Antibacterial and dye degradation potential of zero-valent silver nanoparticles synthesised using the leaf extract of Spondias dulcis. IET Nanobiotechnology 13(1):84–89
Sharifpour E, Ghaedi M, Nasiri Azad F, Dashtian K, Hadadi H, Purkait MK (2018) Zinc oxide nanorod-loaded activated carbon for ultrasound-assisted adsorption of safranin O: central composite design and genetic algorithm optimization. Appl Organomet Chem 32(2):1–11. https://doi.org/10.1002/aoc.4099
Sharma SC (2016) ZnO nano-flowers from Carica papaya milk: degradation of Alizarin Red- S dye and antibacterial activity against Pseudomonas aeruginosa and Staphylococcus aureus. Opt Int J Light Electron Opt 16:6498–6512. https://doi.org/10.1016/j.ijleo.2016.04.036
Shayegan Mehr E, Sorbiun M, Ramazani A, Taghavi Fardood S (2018) Plant-mediated synthesis of zinc oxide and copper oxide nanoparticles by using ferulago angulata (schlecht) boiss extract and comparison of their photocatalytic degradation of Rhodamine B (RhB) under visible light irradiation. J Mater Sci Mater Electron 29(2):1333–1340. https://doi.org/10.1007/s10854-017-8039-3
Sirdeshpande KD, Sridhar A, Cholkar KM, Selvaraj R (2018) Structural characterization of mesoporous magnetite nanoparticles synthesized using the leaf extract of Calliandra haematocephala and their photocatalytic degradation of malachite green dye. Appl Nanosci 8(4):675–683. https://doi.org/10.1007/s13204-018-0698-8
Sonker RK, Hitkari G, Sabhajeet SR, Sikarwar S, Rahul, and S. Singh, (2020) Green synthesis of TiO2 nanosheet by chemical method for the removal of Rhodamin B from industrial waste. Mater Sci Eng B 258:114577. https://doi.org/10.1016/j.mseb.2020.114577
Soto-Robles CA et al (2021) Biosynthesis, characterization and photocatalytic activity of ZnO nanoparticles using extracts of Justicia spicigera for the degradation of methylene blue. J Mol Struct. https://doi.org/10.1016/j.molstruc.2020.129101
Ullah S, Ri H, Khan AU (2019) Green synthesis of catalytic Zinc Oxide nano—flowers and their bacterial infection therapy. Appl Organomet Chem 32(1):1–11. https://doi.org/10.1002/aoc.5298
Varadavenkatesan T, Lyubchik E, Pai S, Pugazhendhi A, Vinayagam R, Selvaraj R (2019) Photocatalytic degradation of Rhodamine B by zinc oxide nanoparticles synthesized using the leaf extract of Cyanometra ramiflora. J Photochem Photobiol B Biol 199:111621. https://doi.org/10.1016/j.jphotobiol.2019.111621
Varadavenkatesan T, Selvaraj R, Vinayagam R (2020) Green synthesis of silver nanoparticles using Thunbergia grandiflora flower extract and its catalytic action in reduction of Congo red dye. Mater Today Proc 23:39–42. https://doi.org/10.1016/j.matpr.2019.05.441
Varadavenkatesan T, Vinayagam R, Selvaraj R (2020) Green synthesis and structural characterization of silver nanoparticles synthesized using the pod extract of Clitoria ternatea and its application towards dye degradation. Mater Today Proc 23:27–29. https://doi.org/10.1016/j.matpr.2019.04.216
Vijai Anand K et al (2019) Photocatalytic degradation of rhodamine B dye using biogenic hybrid ZnO-MgO nanocomposites under visible light. ChemistrySelect 4(17):5178–5184
Vinayagam R, Varadavenkatesan T, Selvaraj R (2017) Evaluation of the anticoagulant and catalytic activities of the bridelia retusa fruit extract-functionalized silver nanoparticles. J Clust Sci 28(5):2919–2932. https://doi.org/10.1007/s10876-017-1270-5
Vinayagam R, Selvaraj R, Arivalagan P, Varadavenkatesan T (2019) Synthesis, characterization and photocatalytic dye degradation capability of Calliandra haematocephala-mediated zinc oxide nanoflowers. J Photochem Photobiol B 203:111760. https://doi.org/10.1016/j.jphotobiol.2019.111760
Vinayagam R, Pai S, Varadavenkatesan T (2020) Structural characterization of green synthesized α -Fe 2 O 3 nanoparticles using the leaf extract of Spondias dulcis. Surfaces and Interfaces 20:1–9. https://doi.org/10.1016/j.surfin.2020.100618
Yashni G, Al-Gheethi A, Mohamed R, Arifin NH, Salleh NA (2020) Green ZnO Nanoparticles photocatalyst for efficient BR51 degradation: Kinetics and mechanism study. Environ Prog Sustain Energy 2020:13559
Acknowledgements
The authors are grateful to the Department of Chemical Engineering, Manipal Institute of Technology (MIT), Manipal Academy of Higher Education (MAHE) for providing lab facilities and equipment to perform this study. The authors thank DST PURSE Laboratory, Mangalore University, Mangalagangotri for providing the FE-SEM and EDS facilities.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vinayagam, R., Pai, S., Varadavenkatesan, T. et al. Characterization and photocatalytic activity of ZnO nanoflowers synthesized using Bridelia retusa leaf extract. Appl Nanosci 13, 493–502 (2023). https://doi.org/10.1007/s13204-021-01816-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-021-01816-5