Abstract
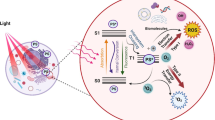
Nanosized reduced graphene oxide (rGO) is found in active microcarbon used in popular face cream from the manufacturers like Ponds, Nevia, and Garnier which, under visible light exposure, gets activated by aerial oxygen to generate reactive oxygen species (ROS) harmful to skin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Activated carbon powder has long been in use in the purification of water (Shannon et al. 2008), sugar (Harris 1942), and other food materials (Roy 1994). It has also been in use as air filter and in adsorbing several adverse species like, color, odor, organic dyes, pesticides, herbicides, metals ions, and microbes of varied origin to the benefit of human. However, its use in facial cream formulations is a recent development with blitz campaign that is shrouded with the lack of awareness of any ill effect (Gillbro and Olsson 2011; Khanna and Datta Gupta 2002) on its use.
In recent times, several multinational companies introduced newer products related to facial application mainly for male population. The level of advertisement to introduce such products is eye catching involving popular matinee idols and sport celebrities in the electronic media. The base slogan of such advertisement is to combat dark spot, acne, oily skin, and environmentally inflicted damages on the exposed face outdoor. The introduction of such a cream to get away with all these problems is credited with the addition of active microsized carbon with the additional advantage of getting fairer skin day by day.
On application of such face cream may result quick effect where the known adsorbing property of microcarbon acts. However, its use over a period of time could be disastrous. The common side effect of any face cream is itching, allergy, dry skin, pimples, or photosensitivity (Bhushan 2012; Ellison 2014; Palmer et al. 2000; Gamboni et al. 2013; Hamed et al. 2012; Idrus and Hymans 2014; McRill et al. 2000; Monheit and Dreher 2013; Rodgers and Chabrol 2009; Sin and Tsang 2003; Spiewak and Dutkiewicz 2002; Szayna and Kuhn 1998; Tlacuilo-Parra et al. 2001; Trattner et al. 2009; Tzanck 1952; Weldon et al. 2000). However, the use of microcarbon in facial cream poses extra problem as these contain a fair proportion of reduced graphene oxide in the nanodomain. The general protocol to synthesize microcarbon is by carbonization process of wood or dry plant waste under limited supply of air by burning or simply by pyrolyzing. The milled product formed as powder generally thought with the upper size cap as only microcarbon in size. Industries use fine carbon powder for its diverse application and they never intended to use any nano sized carbon powder. The product normally used is called active charcoal in microsize form and such carbon retains some adsorbed oxygen. Followed by the discovery of nanoforms of carbon and their identification with state-of-the-art microscopic and spectroscopic techniques, the physico-chemical properties of such forms of carbon have been shown to vary drastically under ambient conditions leading to deleterious effects to human once applied directly especially under visible light and air to induce diseases caused by the generation of reactive oxygen species (ROS) structure.
Experimental
Materials
We investigated the status of alleged microcarbon used in four popular brand face cream like Ponds pure white deep cleaning facial foam (P; a product of Unilever, UK), Nivea Men Oil Control Face Wash (N1); Nivea Men All-in-1 (N2) (products of Beiersdorf, Hamburg, Germany) and Garnier Men Power White Double Action(G) (a products of L’Oréal, France). The black component as carbon from each of these P, N1, N2, and G face creams is routinely separated and washed out and subjected to spectroscopic and microscopic analyses.
Isolation of carbon
Ponds pure white deep cleaning facial foam (P): 10 gm of P was taken in 100 ml beaker containing 80 ml 1:1 dichloromethane:ethanol mixture. It was sonicated for 5 min and centrifuged for 3 min at 4500 rpm. The solid residue left was washed with ethanol two times after 5 min sonication each time. The black carbon left out was dried in air. The amount of carbon isolated was 4.7 mg.
Nivea Men Oil Control Face Wash (N1), Nivea Men All-in-1 (N2) and Garnier Men Power White Double Action(G): For all these, the same technique was followed and a typical process is described below. 10 gm of a sample was taken in 40 ml petroleum ether in a 100 ml beaker. It was sonicated for 5 min, and then, the petroleum ether was decanted off. 80 ml 2:1 ethanol and water mixture was added into the left out solid. After 5 min sonication, the whole part was centrifuged for 3 min at 4500 rpm. The solid residue was washed with 1.1 ethanol and water mixture two times and then finally washed with ethanol two times. This part was further washed with acetone and air dried. Carbon around 4.5 mg was collected.
Physical measurements
Electronic spectra of the samples were measured using JASCO, V-630 spectrophotometer, while pH of the solution was monitored using Systronics Digital pH meter 335. The fluorescence spectra were recorded with Photon Technology International (PTI) Quanta Master™ 300. Scanning Electron Microscopy (SEM), a SUPRA 40VP field-emission SEM (Carl Zeiss NTS GmbH, Oberkochen, Germany) equipped with an energy-dispersive X-ray (EDX) unit, in high-vacuum mode operated at 10 kV was used for the visualization of the size and morphology of isolated black stuff. The Raman data were collected on a Micro-Raman system (WITEC MODEL) with excitation energy of 2.41 eV (514 nm). FTIR were recorded using JASCO 450 model and fluorescence microscopic images were recorded using Leica DM2500. AFM images were made using Agilent 5100 AFM Molecular imaging model with AC mode imaging in the frequency range 152–172 kHz.
Cytotoxicity study
Approximately 103 cells/well skin keratinocyte (HaCaT) cells were seeded onto 96-well plates and incubated for overnight. In a typical experiment, 5 and 20 mg/ml of isolated rGO from one of the facial creams were prepared in DMEM. Cells were incubated with rGO dilutions for 12 h under 200 W light source. A blank, an untreated control (positive control), and a maximum rGO control (only rGO dilutions) were set in the study. After incubation, wells were washed with PBS (phosphate buffer saline). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) solution in fresh medium was added and was incubated for 4 h at 37 °C followed by solubilisation solution was added. Absorbance was measured in a UV–Vis microplate reader (SkanIt, Thermo Fischer Scientific) at 570 nm wave length; reference wave length was set at 670 nm.
Phalloidin staining
HaCaT cells were treated with 5 and 20 mg/ml rGO under light for 12 h. Graphene (rGO)-treated cells were washed with ice-cold PBS followed by fixed with 4% paraformaldehyde for 20 min at room temperature (RT). Therefore, cell permeabilization was done by 0.01% triton-X 100 in PBS for 7 min followed by PBS wash. Cells were incubated with 1 × Phalloidin stain for 60 min at RT followed by PBS wash. Cells were mounted and observed through red filter (ex: 512–552 nm, em: 565–615 nm) under inverted microscope (Nikon eclipse TίU, Japan) equipped with 60× (S Plan Fluor) objective.
Results and discussion
Field-emission scanning electron microscope (FESEM) images from P or N shows the presence of spherical or sheet like carbon (Fig. 1a–c) in nanosize, respectively. The corresponding atomic force microscopic (AFM) images support the presence of spherical nano carbon in sample P and graphene like sheets in sample N and G (Fig. 1d–f), respectively.
The nature of these carbon materials have been revealed by Raman and FTIR spectroscopic analyses. Raman spectra (Fig. 2a–c) of these carbon do not relate to pristine graphitic charcoal; rather, the presence of both G and D bands with overtone around 2700 cm−1 discloses their nature similar to graphene or spherical nanocarbon (Thema et al. 2013). FTIR spectra (Fig. 2d–f) despite subtle differences between these samples show the presence of C–O, C–H, and O–H vibrational similarities. The prominent C=O stretching (1732 cm−1) remained absent but retaining C–O–C epoxide linkage (Guerrero-Contreras and Caballero-Briones 2015) in the sample, P. Recent studies show that graphene oxide (GO) can exist in normal flat sheet or even as closed spherical form (Pakhira et al. 2015). This epoxide linkage (Pakhira et al. 2015) which is responsible for spherical shape is present in P which is absent in N1 and G.
In our recent studies, we have shown that reduced graphene oxide (rGO) generates ROS (Dutta et al. 2015) which can be utilized to kill hospital pathogens. ROS in biological context are formed as natural byproduct during the metabolism of oxygen and have important roles in cell signaling and homeostasis (Devasagayam et al. 2004; Dickinson and Chang 2011). However, under environmental stress (e.g., UV or heat exposure), ROS levels can increase dramatically (Devasagayam et al. 2004; Dutta et al. 2015). This may result in significant damage to cell structures (Dickinson and Chang 2011; Maiuri et al. 2007). Cumulatively, this process is known as oxidative stress (Dickinson and Chang 2011). ROS are also generated by exogenous sources such as ionizing radiation (Sosa Torres et al. 2015). The common effects of ROS are cause of aging (Muller et al. 2007; Van Raamsdonk and Hekimi 2009), cancer (Irani et al. 1997), and cell proliferation (Cheung et al. 2015). Thus, the unintentional use of nanocarbon present in the micro carbon is capable to provoke air under light to generate reactive oxygen species (ROS) which is directly related to cause skin cancer and with the formation of skin wrinkles aggravating the process of aging.
To show the nature of species present in the black carbon residue, we followed the general process which we adopted earlier (Pakhira et al. 2015). The residual carbon we got was leached with 10% aqueous sodium hydroxide. The alkali leached part after extraction and on neutralization with dilute HCl acid was allowed to stand under warm condition to yield the precipitation of a blackish brown product. This residue is soluble in phosphate buffer saline (PBS) (0.01 M) having pH 6.8 and 7.4, respectively. Such buffered solution when subjected to electronic absorption spectrum resembled that of grapheme oxide (GO) as shown in Fig. 3. The insoluble remained after buffer solution leaching was black in color. The black product that was not soluble is similar in properties as reported earlier where the subtle difference between GO and rGO remained in such solubility.
The PBS solution in all the cases displayed a peak at 290 nm at pH 6.8, but this absorption gets broadened on increasing the pH to 7.4. Such electronic spectral variation under pH difference is similar to point out open and clinch fist forms of GO as reported earlier (Pakhira et al. 2015).
The FTIR spectra (Fig. 4) of light treated black carbon under aerobic condition show the appearance of new peaks and the alkali leached neutralized stuff shows C–O stretching vibrations. These can be explained in the following way. The light induces electron transfer from black insoluble carbon which is in reality reduced form of graphene oxide (rGO) that produces superoxide radical ion (ROS) with aerial oxygen and itself gets partially oxidized followed by hydrolysis to form GO (Pakhira et al. 2015, 2017). It is to be noted that GO that is leachable does not fluoresce, but the insoluble rGO does (Pakhira et al. 2015).
Fluorescence microscopic images (Fig. 5) are used to validate the role of oxygen and light in the production of fluorescent materials. Four sets of smeared glass slides contain black carbon isolated from P. In these four sets, the exposure of O2 and light were varied. After 2 h, these slides were viewed by fluorescence microscopy.
The black carbon isolated from Pi essentially contains rGO (reduced graphene oxide) and this remains indistinguishable under air or light or under argon under bright field. However, when viewed under different light source like 385,488 561 and 635 nm, the left column (Fig. 5) displays fluorescence. The rGO in this channel has been exposed to light in the presence of air. We have earlier shown that rGO reacts with O2 present in air to generate superoxide radical ion (O2 −) and rGO+. The cationic rGO+ reacts with the available moisture (water) to produce Graphene Oxide (GO). The GO has the property to fluoresce (Dutta et al. 2015). Similarly, Fig. 6 displays.
The fluorescence microscopic images caused under light and oxygen (air) exposure on the insoluble isolated black carbon from other facial cream like N1, N2, and G including their less informative bright filed images. Thus, the alleged active microcarbon present in all these facial cream generates reduced oxygen species (ROS) under exposure to visible light in the presence of air. This is because these are contaminated with rGO which has the property to get activated in air under light to produce GO (Dutta et al. 2015) with the production of toxic superoxide radical ion (O2 −).
These isolated black carbons also respond to NBT (nitro blue tetrazolium) test (Beauchamp and Fridovich 1971; Lombard et al. 2001) in the presence of air and light, which chemically confirm the production of superoxide radical, the first product amongst the reactive oxygen species (ROS).
Cytotoxicity
The standard MTT assays suggested that these isolated rGO has cytotoxic effect on HaCaT cells (Fig. 7). Viabilities of HaCaT cells were 50.53 and 36.77% for 5 and 20 mg/ml rGO treatment, respectively. The cytotoxic effect of rGO was due to its generation of superoxide radical with air under visible light. The superoxide anion radical being the first member of the ROS kills the cells. These results indicate that the alleged active microcarbon used in face cream contains enough graphene material like rGO which has high cytotoxic effect under visible light exposure and in air to the live skin cells.
Cytoskeleton study
Figure 8 shows that the isolated rGO has the property to interact with the cellular component, cytoskeleton. It has localized on F-actin filaments of HaCaT cells which leads to cell death via disruption of cytoskeleton assembly. Untreated cells showed organized cytoskeleton distribution (Fig. 8a), though cytoskeleton assembly was disrupted upon increase of rGO concentration (Fig. 8b, c).
Conclusion
We demonstrate here that the popular cream manufactured by Ponds, Nivea, or Grainer brands face wash contains nanosized reduced graphene oxide (rGO) which are inadvertently created while producing active microcarbon powder from natural sources. The rGO present in micro carbon remains dormant but gets aggravated under normal light to excite aerial oxygen to toxic superoxide anion. The superoxide anion is the first member of ROS which will have a cascading damaging effect on living cells and would mutate the normal facial cells readily. It is true that the use of these forms of nanocarbons is beneficial in other services to human, but in contact with air and ordinary light, their use must be avoided. Therefore, this is a caveat that the manufacturer of such microcarbon should ensure that the microcarbon that they use should not contain nanocarbon like reduced graphene oxide or its carcinogenic effect on skin application by human will be disastrous.
Supporting information
Supporting Information containing FTIR spectra of carbon isolated form N2 and its light treatment parts are available from Applied Nanoscience website.
References
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. doi:10.1016/0003-2697(71)90370-8
Bhushan B (2012) Nanotribological and nanomechanical properties of skin with and without cream treatment using atomic force microscopy and nanoindentation. J Colloid Interface Sci 367:1–33. doi:10.1016/j.jcis.2011.10.019
Cheung EC, Lee P, Ceteci F, Nixon C, Blyth K, Sansom OJ, Vousden KH (2015) Opposing effects of TIGAR- and RAC1-derived ROS on Wnt-driven proliferation in the mouse intestine. Genes Dev. doi:10.1101/gad.271130.115
Devasagayam TP, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD (2004) Free radicals and antioxidants in human health: current status and future prospects. J Assoc Physicians India 52:794–804
Dickinson BC, Chang CJ (2011) Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol 7:504–511. doi:10.1038/nchembio.607
Dutta T, Sarkar R, Pakhira B, Ghosh S, Sarkar R, Barui A, Sarkar S (2015) ROS generation by reduced graphene oxide (rGO) induced by visible light showing antibacterial activity: comparison with graphene oxide (GO). RSC Adv 5:80192–80195. doi:10.1039/C5RA14061G
Ellison KL (2014) Age transcended: a semiotic and rhetorical analysis of the discourse of agelessness in North American anti-aging skin care advertisements. J Aging Stud 29:20–31. doi:10.1016/j.jaging.2013.12.003
Gamboni SE, Palmer AM, Nixon RL (2013) Allergic contact stomatitis to dodecyl gallate? A review of the relevance of positive patch test results to gallates. Australas J Dermatol 54:213–217. doi:10.1111/j.1440-0960.2012.00941.x
Gillbro JM, Olsson MJ (2011) The melanogenesis and mechanisms of skin-lightening agents—existing and new approaches. Int J Cos Sci 33:210–221. doi:10.1111/j.1468-2494.2010.00616.x
Guerrero-Contreras J, Caballero-Briones F (2015) Graphene oxide powders with different oxidation degree, prepared by synthesis variations of the hummers method. Mater Chem Phys 153:209–220. doi:10.1016/j.matchemphys.2015.01.005
Hamed SH, Assakir I, Almalty AM, Bweir S (2012) Does massage postapplication improve moisturizer’s efficacy? A 2-week regression study. J Cosmet Dermatol 11:239–244. doi:10.1111/j.1473-2165.2012.00626.x
Harris EW (1942) Activated carbon in sugar refining. Ind Eng Chem Res 34:1057–1060. doi:10.1021/ie50393a009
Idrus NI, Hymans TD (2014) Balancing benefits and harm: chemical use and bodily transformation among Indonesia’s transgender waria. Int J Drug Policy 25:789–797. doi:10.1016/j.drugpo.2014.06.012
Irani K et al (1997) Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science (New York, NY) 275:1649–1652. doi:10.1126/science.275.5306.1649
Khanna N, Datta Gupta S (2002) Rejuvenating facial massage–a bane or boon? Int J Dermatol 41:407–410. doi:10.1046/j.1365-4362.2002.01511.x
Lombard M, Houée-Levin C, Touati D, Fontecave M, Nivière V (2001) Superoxide reductase from Desulfoarculus baarsii: reaction 47 and lysine 48 in catalysis†. Biochemistry 40:5032–5040. doi:10.1021/bi0023908
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8:741–752. doi:10.1038/nrm2239
McRill C, Boyer LV, Flood TJ, Ortega L (2000) Mercury toxicity due to use of a cosmetic cream. J Occup Environ Med 42:4–7
Monheit GD, Dreher F (2013) Comparison of a skin-lightening cream targeting melanogenesis on multiple levels to triple combination cream for melasma. J Drugs Dermatol 12:270–274
Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H (2007) Trends in oxidative aging theories. Free Radic Biol Med 43:477–503. doi:10.1016/j.freeradbiomed.2007.03.034
Pakhira B, Ghosh S, Maity S, Sangeetha DN, Laha A, Allam A, Sarkar S (2015) Extraction of preformed graphene oxide from coal: its clenched fist form entrapping large molecules. RSC Adv 5:89076–89082. doi:10.1039/C5RA15699H
Pakhira B, Samanta A, Das GS, Sarkar S (2017) Graphene oxide: a no-acid low-temperature synthesis from graphite. ChemistrySelect 2:5564–5569. doi:10.1002/slct.201700751
Palmer RB, Godwin DA, McKinney PE (2000) Transdermal kinetics of a mercurous chloride beauty cream: an in vitro human skin analysis. J Toxicol Clin Toxicol 38:701–707 doi:10.1081/CLT-100102383
Rodgers R, Chabrol H (2009) The impact of exposure to images of ideally thin models on body dissatisfaction in young French and Italian women. Encephale 35:262–268. doi:10.1016/j.encep.2008.05.003
Roy GM (1994) Activated carbon applications in the food and pharmaceutical industries. CRC Press, Lancaster, Basel
Shannon MA, Bohn PW, Elimelech M, Georgiadis JG, Marinas BJ, Mayes AM (2008) Science and technology for water purification in the coming decades. Nature 452:301–310. doi:10.1038/nature06599
Sin KW, Tsang HF (2003) Large-scale mercury exposure due to a cream cosmetic: community-wide case series. Hong Kong Med J 9:329–334
Sosa Torres M, Saucedo-Vázquez J, Kroneck PH (2015) The magic of dioxygen. In: Kroneck PMH, Sosa Torres ME (eds) Sustaining life on planet earth: metalloenzymes mastering dioxygen and other chewy gases, vol 15. Metal ions in life sciences. Springer International Publishing, pp 1–12. doi:10.1007/978-3-319-12415-5_1
Spiewak R, Dutkiewicz J (2002) Occupational airborne and hand dermatitis to hop (Humulus lupulus) with non-occupational relapses. Ann Agric Environ Med 9:249–252
Szayna M, Kuhn W (1998) In vivo and in vitro investigations of hydration effects of beauty care products by high-field MRI and NMR microscopy. J Eur Acad Dermatol Venereol 11:122–128. doi:10.1111/j.1468-3083.1998.tb00764.x
Thema FT, Moloto MJ, Dikio ED, Nyangiwe NN, Kotsedi L, Maaza M, Khenfouch M (2013) Synthesis and characterization of graphene thin films by chemical reduction of exfoliated and intercalated graphite oxide. J Chem 2013:6. doi:10.1155/2013/150536
Tlacuilo-Parra A, Guevara-Gutierrez E, Luna-Encinas JA (2001) Percutaneous mercury poisoning with a beauty cream in Mexico. J Am Acad Dermatol 45:966–967. doi:10.1067/mjd.2001.117243
Trattner A, Slodownik D, Jbarah A, Ingber A (2009) Questionnaire study of the prevalence of allergic contact dermatitis from cosmetics in Israel. Dermatitis 20:284–286
Tzanck (1952) Dermatitis caused by beauty cream (illustration). Ann Dermatol Syphiligr 79:40
Van Raamsdonk JM, Hekimi S (2009) Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet 5:e1000361. doi:10.1371/journal.pgen.1000361
Weldon MM et al (2000) Mercury poisoning associated with a Mexican beauty cream. West J Med 173:15–18 (discussion 19)
Acknowledgements
SM thanks UGC for SRF, BP acknowledges the support of CSIR, New Delhi for SRF, and SS thanks Cromoz Inc., USA and SERB-DST (EMR/2015/001328), New Delhi, India for funding. We acknowledge the help of Mr. Sudipta Bera and Dr. Rupa Mukhopadhay of Department of Biological Chemistry, Indian Association for the Cultivation of Science, India for AFM. RS thanks her college authorities to permit her for summer internship. RS (Ripon) acknowledges the support of UGC-RGNF for his doctoral studies and AB thanks DST-SERB for funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Maity, S., Pakhira, B., Ghosh, S. et al. Microcarbon-based facial creams activate aerial oxygen under light to reactive oxygen species damaging cell. Appl Nanosci 7, 607–616 (2017). https://doi.org/10.1007/s13204-017-0604-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-017-0604-9