Abstract
The increasing use of nano based-products induces the potential hazards from their manufacture, transportation, waste disposal and management processes. In this report, we emphasized the acute toxicity of silver nanoparticles (AgNPs) using freshwater fish Labeo rohita as an aquatic animal model. The AgNPs were synthesized using chemical reduction method and the formation of AgNPs was monitored by UV–Visible spectroscopy analysis. The functional groups, crystaline nature and morphological characterizations were carried out by fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) and high resolution transmission electron microscopy (HRTEM) analysis. UV-Vis range was observed at 420 nm and XRD pattern showed that the particles are crystalline nature. HRTEM analysis revealed that the morphology of particles was spherical and size ranges between 50 and 100 nm. This investigation was extended to determine the potential acute toxicity, L. rohita was treated orally with the lethal concentration (LC50) of AgNPs. The antioxidative responses were studied in the three major tissues such as gill, liver and muscle of L. rohita. The results of this investigation showed that increasing the concentration of AgNPs led to bioaccumulation of AgNPs in the major tissues. The haematological parameters showed significant alterations in the treated fish. The histological changes caused by chemically synthesized AgNPs demonstrated the damages in the tissues, primary lamella and blood vessels of L. rohita. The histological study also displayed the formation of vacuolation in liver and muscle when compared with untreated tissues (control) of L. rohita.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years there is an increasing demand for the nanoparticles in the field of metal industries, and biomedical science etc. Nowadays the nanoparticles are even used in the household appliances. Nanotoxicology is a study of impact of manufactured nanomaterials on living organisms and environment. It also deals with the quantitative evaluation of severity and frequency of nanotoxic effects in relation to the exposure of the organisms. Metal nanoparticles have been used in various fields such as consumer products, industrial applications and health care technology are likely to enter the environment (Masciangioli and Zhang 2003; Nohynek et al. 2007; Benn and Westerhoff 2008) stated that the AgNPs are commonly used in the textiles and these nanoparticles might be released into the environment. The toxicological evidence of AgNPs is still lacking and the safety measurements for these nanoparticles have to be framed. The engineered nanomaterials comprise of numerous different physical forms and some of these materials including carbon nanotubes, carbon spheres called fullerenes (Zhu et al. 2006) and nanoparticles made from metals (Griffitt et al. 2007), metal oxides (Federici et al. 2007) or composites made of several metals (King-Heiden et al. 2009) have adverse effects on fish (Smith et al. 2007). To study the aquatic toxicity, fish species has been widely used as an indicator of pollutant and they strongly respond to stress conditions. An earlier report of Ramesh et al. (2013) emphasized the toxicological impact of SiO2 nanoparticles on antioxidant enzymes and DNA strand break using zebra fish (Danio rerio). Earlier literatures have also been discussed about the pathological alterations at morphological level in various severities in different fish organs (Lemaire-Gony and Lemaire 1992; Battaglini et al. 1993).
ROS-mediated oxidative damage to macromolecules namely lipids, proteins and DNA have been implicated in the pathogenicity of major diseases. The oxidative stress implicated in the pathology of a number of disorders, such as atherosclerosis, ischemia–reperfusion injury, cancer, malaria, diabetes, inflammatory joint disease, asthma, cardiovascular diseases, cataracts, immune system decline, play a role in neurodegenerative diseases and aging processes (Young and Woodside 2001). Furthermore, nanoparticles are also shown to cause toxicity by increasing concentration of intracellular ROS and decrease in antioxidant level (Singh et al. 2009; Hussain et al. 2009). The increase in ROS level is also an important indication of predominant mechanism of acute toxicity (Kaewamatawong et al. 2006). In this study, synthesized AgNPs were assessed to explore the acute toxicity effects using Labeo rohita as an in vivo model. These findings would provide essential information regarding the potential toxicities and biodistribution of AgNPs in the fish model.
Materials and methods
Synthesis of AgNPs
The AgNPs were synthesized using chemical reduction method of Rashid et al. (2013), with minor modifications. Silver nitrate (0.1 M), 0.8 g of trisodium citrate and 0.1 g of sodium borohydride were freshly prepared. These three solutions were mixed with equal volume (2 mL) and finally made up to 200 mL with distilled water (pH 8.0). The reaction mixture was stirred continuously at room temperature for 3 h and the colour change of the reaction was noticed. The solution was centrifuged at 6000 rpm for 20 min followed by distilled water to purify the reaction mixture. Finally, the pellet was made into powdered form and stored for further studies.
Characterization of AgNPs
UV–Visible spectroscopy
The characterization of synthesized AgNPs was carried out using UV–Visible spectroscopy. The reduction of AgNPs was monitored by measuring the absorbance of reactions mixture at the range of 200–700 nm using synergy HT multi-mode microplate reader (Biotek Instruments, Inc, Winooksi, VT, USA).
X-ray diffraction (XRD)
The AgNPs were subjected to XRD analysis using PAN analytical-XPERT-PRO diffractometer system, Eindhoven, Netherlands and the target was Cu Kα radiation with a wavelength of 1.54 Å, the generator was operated at 40 kV and with a 30 mA current. The average grain size of AgNPs was determined using Scherrer’s formula. D = 0.94λ/β cos θ, where, D is the average crystal size, k is the Scherer coefficient (0.94), λ is the x-ray wavelength, θ is Bragg’s angle and β is the full width at half maximum in radians.
FTIR and HRTEM analysis
FTIR spectroscopy was used to identify the possible functional groups involved in the reduction of silver ions. The FTIR spectra of synthesized AgNPs were analyzed in the range of 400–4000 cm−1 and the measurement was carried out by JASCO (FTIR-6200) spectrum. The size and morphological nature of the AgNPs were determined using transmission electron microscope (JEOL-JEM-200 CX).
Experimental animal and acute toxicity assessment of AgNPs
The experimental animal L. rohita was collected from local fish pond and maintained in the aquarium separately. The experimental aquaria were aerated and test media were replaced every day. The laboratory temperature was 28 ± 2 °C and normal illumination (approx 12 h light and 12 h dark) was maintained throughout the experimental period. Acute toxicity effect of AgNPs was investigated on L. rohita. All experiments were carried out for a period of 7 days and lethal concentration (LC50) was determined with five different concentrations (25, 50, 100, 500, 1000 mg kg−1) of AgNPs. Among these concentrations, mortality rate was found at two concentrations (500 and 1000 mg kg−1). Hence, 100 mg kg−1 concentration was used as a maximum value for further experimental studies. For sub-acute toxicity tests, the LC50 concentration of AgNPs in water was maintained modestly below value such as 5, 10, 25, 50 and 100 mg kg−1 and AgNPs were orally introduced to the fish. After 7 days of sub-lethal exposure, the blood samples were collected and the experimental fishes were sacrificed and muscle, gill and liver were dissected out to assess the toxic impact of AgNPs in fish by analyzing haematological, antioxidant enzymes and histological parameters.
Analysis of haematological parameters
The Haemoglobin content, total protein, total erythrocytes and leukocytes count of the blood was estimated. The blood samples were collected and immediately subjected to haematological analysis. The RBC and WBC were diluted with appropriated diluting fluids and the total content was calculated using improved Neubauer haemocytometer (Blaxhall 1972). The Sahli’s haemoglobinometer was used to estimate haemoglobin (HB) content of the AgNPs-treated fish as well as in control.
Effect on tissue-damaging enzymes
Estimation of acid phosphatase (ACP) and alkaline phosphatase (ALP)
Acid phosphatase (ACP) and alkaline phosphatase (ALP) activities were estimated according to Michell et al. (1970) and Estiarte et al. (2008). The reaction medium for ACP contained 0.7 ml sodium acetate buffer (pH 5.0), 0.25 ml p-nitrophenyl phosphate (pNPP, 5 mM) as substrate and 0.05 ml of enzyme totaling to 1 ml was incubated for 30 min at 37 °C. The reaction was stopped by adding 4 ml NaOH (0.1 N) and incubated for another 30 min at 37 °C. The reaction medium for ALP contained 0.5 ml glycine buffer (pH 7.8), 0.2 ml magnesium chloride (10 mM), 0.25 ml p-nitrophenyl phosphate (pNPP, 5 mM) as substrate and 0.05 ml of enzyme totaling to 1 ml was incubated for 30 min at 37 °C. The reaction was stopped by adding 4 ml NaOH (0.02 N) and incubated for another 30 min at 37 °C. The estimation involves measurement of yellow colour of p-nitrophenol in a synergy HT Multi-Mode Microplate Reader, (Bio-Tek Instruments, Inc., Winooski, VT, USA).
Catalase (CAT)
Catalase activity was assayed by the method of Caliborne (1985). The decomposition of H2O2 was measured by the decrease in the absorbance at 240 nm. The reaction mixture contained 50 mM phosphate buffer (pH 7.2) and 50 mM H2O2, the reaction rate was measured at 240 nm. The extinction coefficient of H2O2 was 40 M−1 cm−1. One unit of catalase was defined as 1 µmol of H2O2 degraded min−1 mg−1 protein.
Glutathione-s-transferase (GST)
The GST activity was determined using the method of Habig et al. (1974). The reaction mixture (3 ml) contained 1.0 ml of 0.3 mM phosphate buffer (pH 6.5), 0.1 ml of 30 mM 1-chloro-2, 4-dinitrobenzene (CDNB) and 1.7 ml of double distilled water. After pre-incubating the reaction mixture at 37 °C for 5 min, the reaction was started by the addition of 0.1 ml of tissue homogenate and 0.1 ml of glutathione as substrate. The absorbance was followed for 3 min at 340 nm and reaction mixture without the enzyme was used as blank. The activity of GST was expressed as μmoles of GSH-CDNB conjugate formed min−1 mg −1 protein.
Superoxide dismutase (SOD)
SOD activity was analyzed using the method of Marklund and Marklund (1974). In this test, the degree of inhibition of pyrogallol autoxidation by supernatant of the lenticular homogenate was measured. The change in absorbance was read at 470 nm against blank every minute for 3 min on a Microplate Reader (Synergy HT Multi-Mode Microplate Reader, Bio-Tek Instruments, Inc., Winooski, VT, USA). The enzyme activity was expressed as units per milligram protein.
Histopathological analysis
Samples of muscle, gill and liver tissues for histological assessment were fixed in 4 % paraformaldehyde in 0.1 M phosphate-buffered solution (pH 7.4) at 4º C, dehydrated in ethanol and embedded in paraplast (Takashima and Hibiya 1995). The histological sections (5 mm thick) were cut with a rotary automatic microtome and sections were mounted on glass slides. Finally, the slides were stained with haematoxylin/eosin to visualize typical morphological features.
Results and discussion
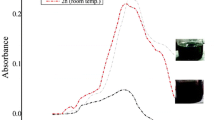
In the present study, AgNPs were synthesized by chemical reduction method and primarily confirmed by the colour change of the reaction mixture from colourless to yellowish brown colour. The appearance of a yellowish brown colour is an indication of the formation of AgNPs (Vignesh et al. 2013). Here, we have used trisodium citrate and sodium borohydrate as reducing agents. UV–Visible absorption spectra of AgNPs formed in the reaction media was recorded at 420 nm (Fig. 1). Under the UV region, AgNPs give a characteristic absorbance band due to the excitation mode of their surface plasmon which is dependent on the nanoparticle size (Vivek et al. 2012; Kanipandian and Thirumurugan 2014). The earlier reports have concluded that AgNPs production from fungi extract Rhizopus stolonifer (Banu et al. 2011) and plant extract Rhizophora apiculata (Antony et al. 2011) showed maximum absorbance at 422 and 423 nm, respectively.
X-ray diffraction analysis
The phase purity and crystalline nature of AgNPs were determined by powder XRD study (Fig. 2). The XRD spectrum showed four distinct diffraction peaks at ~38°, 46°, 64.4°, and 77° and these peaks correspond to (1 1 1), (2 0 0), (2 2 2) and (3 1 1) planes of face-centered cubic (fcc) silver phase (Kanipandian et al. 2014). The obtained XRD results were closely associated with the standard card (JCPDS file no. 65-2871). The average size of AgNPs formed in the present investigation was estimated by determining the full width at half maximum (FWHM) of the Bragg’s angle and the estimated mean size was 96 nm.
FTIR analysis
FTIR image of chemically synthesized AgNPs is given in Fig. 3 and the FTIR spectrum was analyzed between the ranges of ~400–4000 cm−1 and showed seven broad intense bands at ~3434.6, 2922.59, 2854.13, 1637.27, 1384.64, 1026.64 and 613.25 cm−1, respectively. The bands at ~3434.6 and 2922.59 cm−1 attributed to heterocyclic amine, NH stretch and methylene C–H asymmetrical/symmetrical stretch. The band at ~2854.13 cm−1 indicated the presence of asymmetric C–H stretch of the methyl and methylene groups. The FTIR peaks at ~1637.27 and 1384.64 cm−1 contributed to amide and stretching vibration of NO3− ion present in the nanocomposite (Paulraj et al. 2011). The intense peaks at ~1026.64 and 613.25 cm−1 denote the primary amine, CN stretch and alkyne C–H bends.
Electron microscopy studies
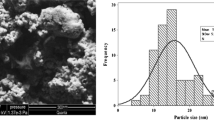
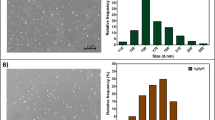
The size and morphological structure of synthesized AgNPs were studied by HRTEM (Fig. 4). These analyses revealed the presence of nano-sized materials in the suspension, which showed that the structure of nanoparticles were spherical in nature. The HRTEM clearly demonstrated that the particles were well dispersed and not severely agglomerated. The size distribution of the AgNPs displayed the average particle size ranging from 50 to 100 nm and SAED pattern confirmed the crystalline nature of the particles and agreed to XRD pattern (Fig. 4d).
Acute toxicity of AgNPs
To determine LC50 value of AgNPs, the experimental fish (L. rohita) was treated orally with different dosage levels (25, 50, 100, 500 and 1000 mg kg−1) of AgNPs. After the treatment period (7 days), the mortality in each group was observed and recorded carefully. The chemically synthesized AgNPs showed a dose dependent activity and mortality was increased with increasing the concentrations of AgNPs. The 100 % of mortality rate was observed at 500 and 1000 mg kg−1 concentration and no mortality was noticed in the lowest concentrations such as 25 and 50 mg kg−1 of AgNPs. The 50 % of mortality was observed at 100 mg kg−1 concentration and no mortality was recorded in control fishes during the tests. Hence, the further studies such as haematological impact, enzymes level and histological study were analyzed below the concentration of LC50 value. The similar LC50 value 100 mg L−1 was estimated in TiO2-treated Danio rerio (Diniz et al. 2013; Xiong et al. 2011).
Haematological parameters
The alterations in RBC, WBC, haemoglobin and total serum protein level were analyzed. From the haematological investigation, we concluded that the levels of all the haematological parameters mentioned above were reduced at 25 mg kg−1 concentration when compared with other concentrations and control fish samples (Fig. 5). The changes in the haematological parameters might be a result of stressful conditions which affect the metabolism and normal functioning of the fish physiology (Blaxhall 1972). There were no significant alterations observed at other concentrations and the level was increased with increasing the concentration of AgNPs.
Effect on enzymes activities
The tissue-damaging enzymes such as acid phosphatase (ACP) and alkaline phosphatase (ALP) levels were significantly higher in the AgNPs-treated fish tissues of gill, liver and muscle when compared with control tissues (Fig. 6). These results revealed that the tissue-damaging effect was caused by AgNPs. The elevation of these enzymes in the tissues was dose dependent and the higher concentrations of the AgNPs exhibited more enzyme activity.
Oxidative stress is the result of an inequity in the pro-oxidant/antioxidant homeostasis. The activities of antioxidant enzymes in the treated fish tissues such as liver, gill and muscle were assessed. The oral administration of AgNPs caused a significant reduction in the activities of glutathione-s-transferase (GST), catalase and superoxide dismutase (SOD). The treated liver tissue showed the highest changes in antioxidant enzyme activity among the tissues (Fig. 7). The AgNPs can cause the oxidative stress, leading to the depletion of antioxidant enzymes activities. A significant decrease in GST, catalase and SOD activity was observed in all treated tissues when compared with control tissues. The mechanism behind this is the metallic nature of nanoparticles and the presence of transition metals encourages the production of reactive oxygen species (ROS) leading to oxidative stress (Mac Nee and Donaldson 2003; Jia et al. 2009).
Histopathological studies
The histopathological changes were observed in gill, liver and muscle tissues of L. rohita due to toxicity caused by AgNPs (Figs. 8, 9).
In control, no recognizable changes were observed in gill tissue during the experimental period. It showed primary and secondary lamellae with pillar cells and also it consists of central venous sinus, chloride cells. The AgNPs-treated fish groups exhibited proliferation of bronchial chloride cells that leads to lamellae fusion and formation of aneurism. The aneurism was localized, blood-filled balloon-like bulge of a blood vessel. It increases a significant risk of rupture, resulting in severe haemorrhage, other complications or death. These similar results were observed on deltamethrin-exposed freshwater fish Aphanius dispar that showed the changes like vacuolization, lifting of the lamellar epithelium and fusion of secondary lamellae (Al-Ghanbousi et al. 2012). The histological responses in the gills of fish were mostly associated with circulatory disturbances and regressive and progressive changes (Van Dyk et al. 2009).
In the liver section, the control tissue showed normal hepatocytes. The AgNPs-treated fish had congestive enlargement of liposomes which lead to vacuolar degenerations in liver. The necrosis were seen at higher level in liver tissues of AgNPs-exposed fish. The section of the control muscle showed normal histological structures such as fiber bundles, connective tissue and arrangement of muscle bundles. The treated fish had an abnormal arrangement of muscle bundles, vacuolization in both liver and muscle. Muscle fibre inflammations were noticed in treated L. rohita. The earlier report of Capkin et al. (2010) concluded the similar toxic effect of pesticides on the vital organs of juvenile rainbow trout (Oncorhynchus mykiss).
Conclusion
This present study deals with the chemical synthesis of AgNPs and physically characterized by UV–Visible, FTIR, XRD, and HRTEM analysis. This investigation clearly demonstrated the impact of AgNPs on aquatic organisms. These AgNPs caused alterations in the haemotology, enzymes activities and histopathological parameters. From this experimental study, we suggest that before applications of AgNPs in various industrial sectors, the toxic potential must be carefully assessed and the effluents are also to be processed before it gets entered into the environment to protect the aquatic eco-systems as well as human lives.
References
Al-Ghanbousi R, Ba-Omar T, Victor R (2012) Effect of deltamethrin on the gills of Aphanius dispar : a microscopic study. Tissue Cell 44:7–14
Antony JJ, Sivalingam P, Siva D, Kamalakkannan S, Anbarasu K, Sukirtha R, Krishnan M, Achiraman S (2011) Comparative evaluation of antibacterial activity of silver nanoparticles synthesized using Rhizophora apiculata and glucose. Colloids Surf B 88:134–140
Banu A, Vandana R, Ranganath E (2011) Silver nanoparticle production by Rhizopus stolonifer and its antibacterial activity against extended spectrum β-lactamase producing (ESBL) strains of Enterobacteriaceae. Mater Res Bull 46:1417–1423
Battaglini P, Andreozzi G, Antonucci R, Arcamone N, De Girolamo P, Ferrara L, Gargiulo G (1993) The effects of cadmium on the gills of the goldfish Carassius auratus (L) metal uptake and histochemical changes. Comp Biochem Physiol C 104:239–247
Benn TM, Westerhoff P (2008) Nanoparticle silver released into water from commercially available sock fabrics. Environ Sci Technol 42(11):4133–4139
Blaxhall PC (1972) The haematological assessment of the health of fresh water fish. A review of selected literature. J Fish Biol 4:593–604
Calibrone AL (1985) Hand book of methods for oxygen radical research. CRC, Boca Raton, p 283
Capkin E, Terzi E, Boran H, Yandi I, Altinok I (2010) Effects of some pesticides on the vital organs of juvenile rainbow trout (Oncorhynchus mykiss). Tissue Cell 42:376–382
Diniz MS, de Matos APA, Lourenço J, Castro L, Peres I, Mendonça E, Picado A (2013) Liver alterations in two freshwater fish species (Carassius auratus and Danio rerio) following exposure to different TiO2 nanoparticle concentration. J Microsc Microanal 19:1131–1140
Estiarte M, Peuuelas J, Sardans BA, Emmett A, Soweby C, Beier IK, Schmidt A, Tietema MJM, Van Mectern E, Kvacs P, Mathe P, De Angelis G, De Dato G (2008) Root-surface phosphatase activity in shrublands across a European gradient: effects of warming. J Env Bio 29:25–29
Federici G, Shaw BJ, Handy RD (2007) Toxicity of titanium dioxide nanoparticles to rainbow trout, (Oncorhynchus mykiss): gill injury, oxidative stress, and other physiological effects. Aquat Toxicol 84:415–430
Griffitt RJ, Weil R, Hyndman KA, Denslow ND, Powers K, Taylor D (2007) Exposure to copper nanoparticles causes gill injury and acute lethality in zebra fish (Danio rerio). Environ Sci Technol 41:8178–8186
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S transferases. The first enzymatic step in mercapturic acid formation. J Bio Chem 251:7130–7139
Hussain S, Boland S, Baeza-Squiban A (2009) Oxidative stress and proinflammatory effects of carbon black and titanium dioxide nanoparticles: role of particle surface area and internalized amount. J Toxicol 260:142–149
Jia HY, Liu Y, Zhang XJ, Han L, Du B, Tian Q (2009) Potential oxidative stress of gold nanoparticles by induced-NO releasing in serum. J Am Chem Soc 131(1):40–41
Kaewamatawong T, Shimada A, Okajima M, Inoue H, Morita T, Inoue K (2006) Acute and subacute pulmonary toxicity of low dose of ultrafine colloidal silica particles in mice after intratracheal instillation. Toxicol Pathol 34:958–965
Kanipandian N, Kannan S, Ramesh R, Subramanian P, Thirumurugan R (2014) Characterization, antioxidant and cytotoxicity evaluation of green synthesized silver nanoparticles using Cleistanthus collinus extract as surface modifier. Mater Res Bull 49:494–502
Kanipandian N, Thirumurugan R (2014) A feasible approach to phyto-mediated synthesis of silver nanoparticles using industrial crop Gossypium hirsutum (cotton) extract as stabilizing agent and assessment of its in vitro biomedical potential. Ind Crop Prod 55:1–10
King-Heiden TC, Wiecinski PN, Mangham AN, Metz KM, Nesbit D, Pedersen JA (2009) Quantum dot nanotoxicity assessment using the zebra fish embryo. Environ Sci Technol 43:1605–1611
Lemaire-Gony S, Lemaire P (1992) Interactive effects of cadmium and benzo (a) pyrene on cellular structure and biotransformation enzymes of the liver of the European eel Anguilla anguilla. Aquat Toxicol 22:145–160
Mac Nee W, Donaldson K (2003) Mechanism of lung injury caused by PM10 and ultrafine particles with special reference to COPD. Eur Respir J 21(40):47–51
Marklund S, Marklund G (1974) Involvement of superoxide anion radical in the autooxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Masciangioli T, Zhang WX (2003) Environmental technologies at the nanoscale. Environ Sci Technol 37(5):102–108
Michell RH, Karnovsky MJ, Karnovsky ML (1970) The distributions of some granule-associated enzymes in guineapig polymorph nuclear leucocytes. Biochem J 116:207–216
Nohynek GJ, Lademann J, Ribaud C, Roberts MS (2007) Grey Goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Crit Rev Toxicol 37(3):251–277
Paulraj P, Janaki N, Sandhya S, Pandian K (2011) Single pot synthesis of polyaniline protected silver nanoparticles by interfacial polymerization and study its application on electrochemical oxidation of hydrazine. Colloids Surf A 377:28–34
Ramesh R, Kavitha P, Kanipandian N, Arun S, Thirumurugan R, Subramanian P (2013) Alteration of antioxidant enzymes and impairment of DNA in the SiO2 nanoparticles exposed zebra fish (Danio rerio). Environ Monit Assess 185:5873–5881
Rashid MU, Bhuiyan MKH, Quayum ME (2013) Synthesis of silver nano particles (Ag-NPs) and their uses for quantitative analysis of vitamin C tablets. J Pharm Sci 12(1):29–33
Singh N, Manshian B, Jenkins GJS (2009) Nano genotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials 30(23):3891–3914
Smith CJ, Shaw BJ, Handy RD (2007) Toxicity of single walled carbon nanotubes to rainbow trout, (Oncorhynchus mykiss): respiratory toxicity, organ pathologies, and other physiological effects. Aquat Toxicol 82:94–109
Takashima F, Hibiya T (1995) An Atlas of Fish Histology. Normal and Pathological Features, 2nd edn. Kodansha Ltd., Tokyo
Van Dyk JC, Marchand MJ, Pieterse GM, Barnhoorn IEJ, Bornman MS (2009) Histological changes in the gills of Clarias gariepinus (Teleostei: Clariidae) from a polluted South African urban aquatic system. Afr J Aqua Sci 34(3):283–291
Vignesh V, Anbarasi KF, Karthikeyeni S, Sathiyanarayanan G, Subramanian P, Thirumurugan R (2013) A superficial phyto-assisted synthesis of silver nanoparticles and their assessment on hematological and biochemical parameters in Labeo rohita (Hamilton, 1822). Colloids Surf A 439:184–192
Vivek R, Thangam R, Muthuchelian K, Gunasekaran P, Kaveri K, Kannan S (2012) Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxicity effect on MCF-7 cells. Process Biochem 47(12):2405–2410
Xiong D, Fang T, Yu L, Sima X, Zhu W (2011) Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebra fish: acute toxicity oxidative stress and oxidative damage. Sci Total Env 409:1444–1452
Young IS, Woodside JV (2001) Antioxidants in health and disease. J Clin Pathol 54:176–186
Zhu S, Oberdörster E, Haasch ML (2006) Toxicity of an engineered nanoparticle (fullerene, C60) in two aquatic species, Daphnia and fathead minnow. J Mar Env Res 62:5–9
Acknowledgments
The authors are thankful to UGC-DAE CSR Indore for financial assistance through collaborative research project (Ref No: CSR-I/CRS-71/2012-2014/1016) and we are also grateful to DST-Fast Track (Ref. No: SR/FT/LS-21/2012) and UGC-SAP-DRS-II for the Instrumentation facilities in the Department of Animal Science, Bharathidasan University, Tiruchirappalli. K. S. Rajkumar thank DST for the award of INSPIRE FELLOWSHIP (IF140546).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Rajkumar, K., Kanipandian, N. & Thirumurugan, R. Toxicity assessment on haemotology, biochemical and histopathological alterations of silver nanoparticles-exposed freshwater fish Labeo rohita . Appl Nanosci 6, 19–29 (2016). https://doi.org/10.1007/s13204-015-0417-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-015-0417-7