Abstract
Biosynthesis of silver nanoparticles (AgNPs) with ecofriendly plant extracts contains different phytochemicals act as both reducing and capping agents forming stable and shape-controlled AgNPs. The present study deals with the synthesis of AgNPs using the aqueous leaf extract of Coleus aromaticus and to evaluate the bactericidal activity against three antibiotic resistant Escherichia coli strains. The AgNPs are characterized using UV–Visible Spectroscopy, Scanning Electron Microscope coupled with X-ray Energy Dispersive Spectroscopy, Fourier transform-infrared spectroscopy, and Atomic force microscope. The synthesized AgNPs were crystalline in nature, spherical in shape with an average of 48 ± 5 nm in size, and the stability of AgNPs is due to its high negative Zeta potential −65.7 mV. The AgNPs have outstanding bactericidal activity against three antibiotic resistant E. coli strains. This is the first report of antibacterial activity against antibiotic resistant strains using AgNPs by green route.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Integration of biosynthesis and nanotechnology has opened new possibilities, in particular for biomedical applications, and the synthesis of noble metal nanoparticles, for various applications such as catalysis, electronics, optics, environmental and biotechnology (Hussain et al. 2003; Yuan 2005; Masciangioli and Zhang 2003; Albrecht et al. 2006). Silver, gold, and copper have been used regularly for the synthesis of stable dispersions of nanoparticles, which are very efficient in areas such as biological labeling, photonics, and surface-enhanced Raman scattering (SERS) detection (Smith et al. 2006; Kearns et al. 2006). Silver nanoparticles are used in the field of biosensing, imaging, drug delivery, nanodevice fabrication, and medicine (Nair and Laurencin 2007; Lee and El-Sayed 2006; Jain et al. 2008). The biosynthesis by employing plant extracts has drawn attention as a simple, rapid, stable, inexpensive, and viable alternative to chemical procedures and physical methods. Recently, plant sources have been widely used for the synthesis of silver nanoparticles by using, Andrographis paniculata (Kotakadi et al. 2014), Cathranthus roseus (Kotakadi et al. 2013), Murraya koenigii leaf (Philip et al. 2011), Mangosteen leaf (Veerasamy et al. 2011), Mangifera indica leaf (Philip 2011), Tansy fruit (Dubey et al. 2010), Jatropha curcas (Bar et al. 2009), Cinnamomum zeylanicum leaf (Smitha et al. 2009), Camellia sinensis (Nestor et al. 2008), Aloe vera (Chandran et al. 2006), and Medicago sativa seed extract (Lukman et al. 2011) have been used for the synthesis of metal nanoparticles of different sizes.
It is well known that the silver ions and silver-based compounds are highly toxic in nature to microorganisms; this toxicity depends upon the preparation of the nanosized particles which are specific in size and shape, and exhibits different physical and chemical properties of great interest (Joguet et al. 2002). Past studies shown that highly concentrated and nonhazardous nanosized silver particles can easily be prepared in a cost-effective manner (Sondi et al. 2003), Sondi and others also worked using synthesized AgNPs against different Escherichia coli bacteria showed good bactericidal activity (Sondi and Salopek-Sondi 2004). Audra I. Lukman et al. also worked on the antimicrobial and antibacterial properties of Ag nanoparticles against Salmonella, Shigella, and Proteus (Lukman et al. 2011). Recent studies have been focused on some of the physicochemical properties of AgNPs, and these physical or chemical properties are responsible for the effect on microorganisms. It also depends on concentration of AgNPs’ involved in inactivation of E. coli (Dror-Ehre et al. 2009), and the antibiotic ability of AgNPs against E. coli, Staphylococcus aureus, B. subtilis, and K. mobilis was enhanced with increasing the silver content (Zhang et al. 2008). Flores and coworkers also studied the biocide activity of the AgNP-modified titanium substrates on Pseudomonas aeruginosa a gram-negative bacterium. P. aeruginosa is an opportunistic microorganism that can cause severe, life-threatening infections (Flores et al. 2010). And presently, the silver nanoparticles are also used as Diagnostic Antimicrobial Nanoparticles (DANs) The minimum inhibitory concentration of DANs suggests that they are slightly more effective against S. aureus than E. coli at 12 and 24 ppm, respectively (Mark Hoppens et al. 2014), whereas Ruparelia et al. determined the average MIC of SNPs against four different strains of E. coli and three different strains of S. aureus as 120 and 250 ppm, respectively (Ruparelia et al. 2008). Though there are several reports regarding antimicrobial activity of AgNPs, which can be prepared in a simple and cost-effective manner, suitable for the formulation of new types of bactericidal materials (Kotakadi et al. 2013, 2014), there are no reports regarding the studies of AgNPs on antibiotic resistant bacteria so far. So the present study deals the synthesis of AgNPs using leaf extract of Coleus aromaticus and to study bactericidal activity of these nontoxic nanomaterials.
Coleus aromaticus Benth is commonly known as Indian country borage and ‘Kapparillaku’ in Telugu (Kumar et al. 2007). Coleus aromaticus is used for seasoning meat dishes and food products, and decoction of its leaves is administered in cases of chronic cough and asthma. Other important medicinal properties viz, antispasmodic, headache, epilepsy, dyspepsia, diarrhea, nervous tension, rheumatism, whooping cough, and bronchitis (Warrier et al. 1995). Coleus aromaticus is a good source of nutritious food supplement as it contains important phytochemicals like flavones salvigenin, quercetin, chrysoeriol, luteolin, apigenin, the flavanone eriodyctol and the flavanol taxifolin, triterpenic acids, oleanolic acid, 2,3-dihydroxyoleanolic acid, ursolic acid, pomolic acid, tormentic acid, etc., (Sahaykhare et al. 2011).
Materials and Methods
Synthesis of Silver Nanoparticles
The Kapparillaku leaves were collected from the plants in and around Tirupati (Supplementary Fig. S1). The leaves were shade dried at room temperature and then made into fine powder with the help of electric blender. The plant leaf extract was prepared, by taking 3 g of fine powder leaves in a 250-mL Erlenmeyer flask with 150 mL of distilled water, and the mixture was boiled for 2–3 min and filtered through sterile muslin cloth and through whatmann filter paper. The filtrate was used as source of extract for the preparation of silver nanoparticles. To 5 ml diluted leaf extract, 10 ml of 0.001 (M) AgNO3 was added and the sample was left at room temperature, until the color of solution changed from colorless to light brown and subsequently dark brown. In the present investigation, the production of AgNPs was carried out with leaf extract without any man-made chemicals.
Characterization
The bio-reduction of pure Ag+ ions done with the leaf extract of Kapparillaku was monitored periodically by sampling of the 1 mL aliquots, and the optical absorbance was recorded by UV–Vis Spectrophotometer (Nanodrop 8000 series Thermofisher) in 200–800 nm wavelength range. Scanning Electron Microscopy (SEM) and EDX was performed by Oxford Inca Penta FeTX3 EDS instrument attached to Carl Zeiss EVO MA 15 Scanning Electron Microscope (200 kV) machine with a line resolution 2.32 (in Å). The SEM images were taken by coating a drop of AgNPs on an aluminum foil. Energy dispersive absorption spectroscopy of AgNPs was carried out by the SEM equipment, as mentioned above. The FT-IR was carried using Brucker Tensor 27; Particle size and zeta potential measurement experiments were carried out using a Nanopartica SZ-100 (HORIBA). Morphological studies of AgNPs were also done by AFM. A drop of synthesized AgNPs suspension was placed on thin aluminum sheet and dried at room temperature, prior to analysis of the sample. (AFM-Solver Next, NT-MDT, Russia).
Antimicrobial Activity
The antimicrobial activity of silver nanoparticles was evaluated against three different strains of Gram-negative E. coli as follows: E. coli Strain-I (Donor) rifampin resistant, E. coli Strain-II (Recipient) streptomycin resistant, and E. coli AB1157 (Mutant) streptomycin resistant by disk method. Bacterial cultures were prepared freshly by transferring one to three colonies into a tube containing 20 ml nutrient broth (Himedia, gm/L) and grown overnight at 37 °C. Three replicates of respective microorganisms were prepared by spreading 100 μl of culture on the nutrient agar plate with the help of spreader. Disks were prepared using Whatmann No. 1 filter paper. The disks were placed on agar plates, and sample of synthesized silver nanoparticles 30 μl (30mcg) was added on the disk with the help of micropipette. The plates were incubated at 37 °C overnight. Amoxyclav (Himedia SD063, 30 mcg) disk was used as reference drug.
Results and Discussion
The water soluble ingredients present in the extract are responsible for the reduction of metal ions to form stable AgNPs. Leaves of Coleus aromaticus contain essential oil rich in carvacrol, thymol, eugenol, chavicol, and ethyl salicylate. Oil extracted through steam distillation and solid phase micro extractions has very good antimicrobial activity (Perumal et al. 2004).
UV–Visible Spectral Analysis
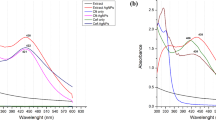
UV–Vis spectroscopy is an important tool to observe the formation of metal nanoparticles. In the present study, colorless leaf extract is known to contain several water soluble phyto-constituents which have the potential to reduce the silver ions to AgNPs by changing light yellow to dark brown colors (Fig. 1a, b). The surface plasmon resonance spectrum of synthesized AgNPs was observed at 423 nm after 10 min (Fig. 2). The particles in SPR region of around 410–450 nm can be attributed to spherical nanoparticles (Zaheer and Rafiuddin 2012).
SEM and EDX Analysis of AgNPs
The morphology of the synthesized AgNPs was determined by SEM, and it shows that the AgNPs are more uniform and spherical in shape (Fig. 3). The EDX data reveals that very strong signal to silver and weak signals to other elements like oxygen, Mn, and Iron which indicates the reduction of the silver ions to elemental silver, and the strong signal of aluminium is due to aluminium sheet being used as base for coating the silver nanoparticle sample for the study (Fig. 4). Coleus aromaticus plant leaves contain proteins, vitamins (ascorbic acid, thiamine), minerals (calcium, phosphorus, potassium, sodium, and magnesium), and trace metals (iron, zinc, copper, and chromium) (Sahaykhare et al. 2011).
IR Spectral Analysis
The IR spectrum of the silver nanoparticles is shown in (Supplementary Fig. S2), and the spectrum reveals that hydroxyl group and stretch of alkane H (O–H, 3332.25; C–H, 2896.91, 2834.33) and carbonyl group and stretch of alkenes groups (C=C, 2096.66; C=O, 1637.26 cm−1) are involved in the reduction of Ag+ to Ag. Therefore, it may be conclude that the water soluble essential oils are responsible for capping and efficient stabilization (Sahaykhare et al. 2011).
Particle Size Determination
The particle size of the AgNPs obtained is detected by intensity and laser diffraction, which are poly-dispersed mixture solution. The size of synthesized AgNPs is ranging from 48 to 125 nm, and the average diameter of the AgNPs was found to be 53.2 nm (Fig. 5).
Zeta Potential of AgNPs
The negative value of Zeta potential confirms the repulsion among the particles and thereby increases in stability of the formulation. The zeta potential was found to be −69.5 mV (Fig. 6). The electrostatic repulsive forces between the nanoparticles when they are negatively charged possibly protect them from forming an association. This prevents the particles from agglomeration in the medium leading to long-term stability (Suresh et al. 2011).
AFM Analysis
The morphology of the synthesized AgNPs was spherical in shape, and the deposited AgNPs form aggregates on the surface of the aluminum foil. The size of the synthesized AgNps varied from 40 ± 5 to 85 ± 5 nm. The average grain size of the nanoparticles was detected as 47 ± 3 nm (Fig. 7).
Bactericidal Activity
The nanoparticles synthesized by green route were found very destructive against three bacterial species at a concentration of 30 μl AgNPs on different strains of antibiotic resistant Gram-negative bacteria E. coli. The results revealed superior antibacterial activity against E. coli recipient strain and donor strain, where as intermediated activity was revealed against E. coli mutant strain. The inhibitory activities in culture media of the Ag nanoparticles reported in (Table 1; Fig. 8) were comparable with reference drug viz. Amoxyclav. Several peoples investigated the antibacterial activity of silver nanoparticles against different bacterial strains (Kotakadi et al. 2013; Rout Rajesh et al. 2009; Kim et al. 2007). In olden days before introduction of penicillin in 1940, silver has been widely used for the treatment of bacterial infection (Hugo and Russell 1982; Chopra 2007). After penicillin invention, the use of silver has been minimized, whereas the use of silver nitrate in the year 1960 by Moyer proved that it possesses very good antibacterial property against S. aureus, P. aeruginosa, and E. coli (Moyer et al. 1965). There are earlier reports of mechanistic study of inhibition of silver ions against two strains of bacteria, S. aureus and E. coli (Feng et al. 2000). Other scientists also investigated the effect of silver nanoparticles and compared the results with antibiotics, and reported that the synthesis of silver nanoparticles in the size range of 10–15 nm has very good antibiotic effect on both Gram-negative and Gram-positive microorganisms (Shahverdi et al. 2007). Whereas investigations have also been carried out on antibacterial properties of silver nanoparticles of different shapes and found that the antibacterial efficacy of silver nanoparticles is also shape dependent (Pal et al. 2007). Spherical silver nanoparticles have inhibitory effect at the concentration of 12.5 ug/ml, whereas truncated triangular nanoparticles have inhibition at 1 μg/ml of silver content and rod-shaped nanoparticles at the concentration 50–100 ug/ml content of silver. The studies of silver nanoparticles of spherical-shaped nanoparticles in the size range of 20 nm showed efficient antibacterial activity against E. coli and S. aureus (Gade et al. 2008).
In the present scenario, silver nanoparticles have made a significant comeback as a potential antimicrobial agent as several pathogenic bacteria have developed resistance against various antibiotics. Silver nanoparticles have emerged up as novel antimicrobial agents due to its high surface area to volume ratio and unique chemical and physical properties (Kim et al. 2007; Morones et al. 2005). Currently, the main interest of many researchers is the development of novel metal ions which have microbial resistance, against the antibiotics resistant strains. Silver nanoparticles (AgNPs) have been proved to be most effective antimicrobial efficacy against bacteria, viruses, and other eukaryotic microorganisms (Gong et al. 2007). Similar type of study was done by (Vanaja and Annadurai 2013), with different type bacteria. But in the present study, we have carried out with antibiotic resistant bacteria.
Once the AgNPs are added to bacterial culture, the silver nanoparticles enter the bacterial cell by diffusion and also by endocytosis through the cell wall into the cytoplasm, and the AgNPs cause toxicity to the bacterial cell constituents, especially the mitochondria is damaged and so the depletion of ATP takes place, which in turn produce reactive oxygen species (ROS). The ROS along with AgNPs acts on nucleus of the bacteria, which cause oxidation of DNA and also cause chromosomal aberrations which ultimately cause the death of the bacteria, so the bacterial cell death occurs. The mechanism is shown in Fig. 9. Schematic diagram. Similar type of results is also presented by scientists (Xiu et al. 2012) on bacteria using different types of silver nanoparticles.
In the present study, the particles have been found to be spherical in size around 45 ± 5 nm, which have excellent bactericidal activity against three different strains of E. coli (recipient and mutant strains) which resistance to streptomycin and (donor strain) Rifampin resistant bacteria. The interesting thing is that so far so many scientists have worked on antimicrobial activity using silver and silver nanoparticles on different strains of bacterial species. We are the first to workout on the antibacterial activity against three different antibiotic resistant E. coli strains. The results open new avenues for the development of novel antibiotics against multidrug resistant bacteria with ecofriendly and nontoxic green synthesis.
Conclusions
Kapparillaku leaf extract is found to be appropriate for rapid extraction of AgNPs by green synthesis within 5 min. The average size of silver nanoparticles was found to be 48 ± 5 nm, which have excellent bactericidal activity against three different strains of antibiotic resistant E. coli. The nanoparticles <100 nm size are very important in biomedical applications.
References
Albrecht MA, Evans CW, Raston CL (2006) Green chemistry and the health implications of nanoparticles. Green Chem 8:417–432
Bar H, Bhui DK, Sahoo GP, Sarkar P, De SP, Misra A (2009) nanoparticles using latex of Jatropha curcas. Colloids Surf A 339:134–139
Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M (2006) Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog 2:577–583
Chopra I (2007) The increasing use of silver-based products as antimicrobial agents: a useful development or a cause for concern? J Antimicrob Chemother 59:587–590
Dror-Ehre A, Mamane H, Belenkova T, Markovich G, Adin A (2009) Silver nanoparticle-E. coli colloidal interaction in water and effect on E. coli survival. J Colloid Interface Sci 339:521–526
Dubey SP, Lahtinen M, Sillanpaa M (2010) Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem 45:1065–1071
Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO (2000) A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater 52:662–668
Flores CY, Diaz C, Rubert A, Benítez GA, Moreno MS, Fernández Lorenzo de Mele MA, Salvarezza RC, Schilardi PL, Vericat C (2010) Spontaneous adsorption of silver nanoparticles on Ti/TiO2 surfaces. Antibacterial effect on Pseudomonas aeruginosa. J Colloid Interface Sci 350:402–408
Gade AK, Bonde P, Ingle AP, Marcato PD, Duran N, Rai MK (2008) Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J Biob Mater Bioener 2:243–247
Gong P, Li H, He X, Wang K, Hu J, Tan W et al (2007) Preparation and antibacterial activity of Fe3O4@Ag nanoparticles. Nanotechnology 18:604–611
Hoppens MA, Wheeler ZEW, Qureshi AT, Hogan K, Wright A, Stanley GG, Young D, Savage P, Hayes D (2014) Maghemite, silver, ceragenin conjugate particles for selective binding and contrast of bacteria. J Colloid Interface Sci 413:167–174
Hugo WB, Russell AD. Principles and Practice of Disinfection, Preservation and Sterilisation. Oxford, UK: Blackwell Scientific Publications; 1982.3.8-106
Hussain I, Brust M, Papworth AJ, Cooper AI (2003) Preparation of acrylate-stabilized GOLD and silver hydrosols and gold-polymer composite films. Langmuir 19:4831–4835
Jain PK, Huang X, El-Sayed IH, EL-Sayed MA (2008) Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine.Acc. Acc Chem Res 41:1578–1586
Joguet L, Sondi I, Matijevi´c E (2002) Preparation of nanosized drug particles by the coating of inorganic cores: Naproxen and ketoprofen on alumina. J Colloid Interface Sci 251:284–287
Kearns GJ, Foster EW, Hutchison JE (2006) Nanoparticle assemblies for electron transport studies and as well-defined catalyst precursors. J Anal Chem 78:298–303
Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Kim YK, Lee YS, Jeong DH, Cho MH (2007) Antimicrobial effects of silver nanoparticles. Nanomed: Nanotechnol Biol 3:95–101
Kotakadi VS, Subba Rao Y, Susmila Aparna G, Prasad TNVKV, Varada Reddy A, Sai Gopal DVR (2013) Simple and rapid biosynthesis of stable silver nanoparticles using dried leaves of Catharanthus roseus Linn. G. Donn and its anti microbial activity. Colloids Surf B: Biointerfaces 105:194–198
Kotakadi VS, Susmila Aparna G, Subba Rao Y, Prasad TNVKV, Varada Reddy A, Sai Gopal DVR (2014) Biofabrication of silver nanoparticles by andrographis paniculata. Eur J Med Chem 73:135–140
Kumar A, Elango K, Markanday S, Undhad CV, Kotadiya AV, Savaliya BM, Vyas DN, Datta D (2007) Mast cell stabilization property of Coleus aromaticus leaf extract in rat peritoneal mast cells. Indian J Pharmacol 39:117–120
Lee KS, El-Sayed MA (2006) Gold and silver nanoparticles in sensing and imaging: sensitivity of plasmon response to size, shape, and metal composition. J Phy Chem B 110:19220–19225
Lukman AI, Gong B, Marjo CE, Roessner U, Harris AT (2011) synthesis, stabilization, and anti-bacterial performance of discrete Ag nanoparticles using Medicago sativa seed exudates. J Colloid Interface Sci 353:433–444
Masciangioli T, Zhang WX (2003) Environmental nanotechnology : Potential and pitfalls. Environ Sci Technol 37:102A–108A
Morones JR, Elechiguerra JL, Camacho A, Ramirez JT (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16:2346–2353
Moyer CA, Brentano L, Gravens DL, Margraf HW, Monafo WW (1965) Treatment of large human burns with 0.5 per cent silver nitrate solution. Arch Surg 90:812–867
Nair LS, Laurencin CT (2007) Silver nanoparticles: synthesis and therapeutic applications. J Biomed Nanotechnol 3:301–316
Nestor ARV, Mendieta VS, Lopez MAC, Espinosa RMG, Lopez MAC, Alatorre JAA (2008) Alatorre, Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis. Mater Lett 62:3103–3105
Pal S, Tak YK, Song JM (2007) Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl Environ Microbiol 27:1712–1720
Perumal G, Subramanyam C, Natrajan D, Srinivasan K, Mohanasundari C, Prabakar K (2004) Antifungal activities of traditional medicinal plant extracts: A preliminary survey. J Phytolog Res 17:81–83
Philip D (2011) Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract Spectrochimica. Spectrochimica Acta Part A 78:327–331
Philip D, Unni C, Aswathy Aromal S, Vidhu VK (2011) Murraya Koenigii leaf-assisted rapid green synthesis of silver and gold nanoparticles. Spectrochim Acta Part A 78:899–904
Rout Rajesh W, Lakkakula Jaya R, Kolekar Niranjan S, Mendhulkar Vijay D, Kashid Sahebrao B (2009) Phytosynthesis of Silver Nanoparticle Using Gliricidia sepium (Jacq.). Curr Nanosci 5:117–122
Ruparelia JP, Chatterjee AK, Duttagupta SP, Mukherji S (2008) Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater 4:707–716
Sahaykhare R, Banerjee S, Kundu K (2011) A Nutritive Medicinal Plant of Potential therapeutic value. Int J Pharma Bio Sci 2:488–500
Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S (2007) Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanomed: Nanotechnol Biol Med 3:168
Smith AM, Duan H, Rhyner MN, Ruan G, Nie SA (2006) A systematic examination of surface coatings on the optical and chemical properties of semiconductor quantum dots. Phys Chem Chem Phys 8:3895–3903
Smitha SL, Philip D, Gopchandran KG (2009) Green synthesis of gold nanoparticles using Cinnamomum zeylanicum leaf broth. Spectrochim Acta, Part A 74:735–739
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci 275:177–182
Sondi I, Goia DV, Matijevi´c E (2003) Preparation of highly concentrated stable dispersions of uniform silver nanoparticles. J Colloid Interface Sci 260:75–81
Suresh AK, Doktycz MJ, Wang W, Moon JW, Gu B, Meyer HM III, Hensley DK, Allison DP, Phelps TJ, Pelletier DA (2011) Monodispersed biocompatible silver sulfide nanoparticles: facile extracellular biosynthesis using the γ-proteobacterium, Shewanella oneidensis. Acta Biomater 7:4253–4258
Vanaja M, Annadurai G (2013) Coleus aromaticus leaf extract mediated synthesis of silver nanoparticles and its bactericidal activity. Appl Nanosci 3:217–223
Veerasamy R, Xin TZ, Gunasagaran S, Xiang TFW, Yang EFC, Jeyakumar N, Dhanaraj SA (2011) Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J Saudi Chem Soc 15:113–120
Warrier PK, Nambiar VP, Ramankutty C (1995) Indian medicinal plants, 1st edn. Orient Longman Limited, Madras, pp 315–317
Yuan G (2005) Environmental nanomaterials: occurrence, synthesis, characterization, health effect and potential applications. J Environ. Sci. Health A 39:2545–2548
Zaheer Z, Rafiuddin (2012) Silver nanoparticles to self-assembled films: green synthesis and characterization. Colloids Surf B 90:48–52
Zhang Yongwen, Peng Huashong, Huanga Wei, Zhou Yongfeng, Yan Deyue (2008) Facile preparation and characterization of highly antimicrobial colloid Ag or Au nanoparticles. J Colloid Interface Sci 325:371–376
Xiu ZM, Zhang QB, Puppala HL, Colvin VL, Alvarez PJJ (2012) Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett 12(8):4271–4275. doi:10.1021/nl301934w
Acknowledgments
The authors are grateful to DST-PURSE, Department of Science and Technology, New Delhi for providing the Research Fellowships.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kotakadi, V.S., Gaddam, S.A., Venkata, S.K. et al. New generation of bactericidal silver nanoparticles against different antibiotic resistant Escherichia coli strains. Appl Nanosci 5, 847–855 (2015). https://doi.org/10.1007/s13204-014-0381-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-014-0381-7