Abstract
2-Mercaptosuccinic acid (MSA)-capped gold nanoparticles (GNPs) were used to determine the level of concentration of lead and cadmium metals in various environmental samples. Alumina-coated MSA-capped GNPs easily remove lead and cadmium present in various samples. The absorbance spectrum was obtained at 547 nm. Effects of pH, reagent concentration, interferences, were studied. This method is simple, selective and successfully applied for the determination of lead and cadmium species in various water samples collected in and around four industries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gold nanoparticles (GNPs) play a vital role in environmental science and chemistry, because of its large surface-to-volume ratio, quantum confinement, stability and other unique properties. GNPs consist of more than a few kinds of remarkable properties (Wang et al. 2001, 2002). 1–100 nm sized GNPs show high surface energy along with high surface-to-volume ratio to endow with a steady control of a large amount of biomolecules retaining their bioactivity. GNPs may potentially be another useful material for removing contaminants, such as toxic chlorinated organic compounds, pesticides and inorganic mercury, from water.
Heavy metals like lead and cadmium are toxic even at very low exposure levels and have chronic and acute effects on human health. These two metals cause serious dangerous diseases not only for human beings but also for plants, animals and microorganisms.
A microextraction technique combining Fe3O4 nanoparticles with surfactant-mediated solid phase extraction (SM-SPE) was successfully developed for the preconcentration/separation of Cd(II) and Pb(II) in water and soil samples. The analytes were determined by flame atomic absorption spectrometry (FAAS) by Jalbani and Soylak (2014).
A facile, cost-effective, and sensitive fluorescent method for Pb2+ ion detection had been developed using catechin-synthesised GNPs (C–Au NPs). The Pb–catechin complexes and Pb–Au alloys that formed on the C–Au NPs surfaces allowed NPs to exhibit peroxidase-mimicking catalytic activity in the H2O2-mediated oxidation of Amplex UltraRed (Wu et al. 2013). A colorimetric assay for the highly sensitive and selective detection of Cd2+ using GNPs (AuNPs) cofunctionalized with 6-mercaptonicotinic acid and l-cysteine through the formation of an Au–S bond (Xue et al. 2011).
The application of sulphur-nanoparticle-loaded alumina as an efficient adsorbent for the solid phase extraction and determination of trace amounts of Cd, Cu, Zn, and Pb ions was investigated in marine samples using flame atomic absorption spectrometry (FAAS) by Ghanemi et al. (2011).
Several authors analysed lead and cadmium in various samples using nanoparticles following analytical techniques (Zhu et al. 2014; Baghban et al. 2013; Yang et al. 2011; Shakerian et al. 2013; Ebrahimzadeh et al. 2013).
There is a need to develop simple, sensitive reagent that requires less solvent preconcentration method for the determination of lead and cadmium in various environmental matrices. In the present investigation, the author successfully synthesised and applied GNPs capped with 2-mercaptosuccinic acid (MSA) for the determination of lead and cadmium present in various environmental water samples, which were collected from selected industrial areas. The interaction between alumina-supported MSA-capped GNPs with lead and cadmium metals was studied using a column setup. GNPs capped with MSA determined lead and cadmium up to ppb levels in real water samples following flame atomic absorption spectrophotometry (FAAS).
Experimental section
Chemicals
All the chemicals and solvents used were of analytical reagent grade and procured from Sigma-Aldrich Company and all samples were prepared by using double-distilled water throughout the experiment. Gold chloride (HAuCl4·3H2O) was purchased from Research Lab Fine Chem Industries India Limited. Analytical grade trisodium citrate, MSA, sodium chloride (NaCl) and other chemicals were purchased from Sigma-Aldrich Company. Aluminium oxide (Brockmann 1) was procured from Sigma-Aldrich Company.
Instrumentation
A pH meter (Elico, model LI-129, India) with combined glass electrode was used for pH measurements. A single pan analytical balance (Dhona, Model 100 DS, India) was employed for weighing the samples. A Systronics UV–Vis spectrophotometer, model 118, with 1 cm matched quartz cells was used for all absorbance measurements. Scanning electron microscopic (SEM) image was taken using a FEI QUANTA-200 SEM instrument. High resolution transmission electron microscopy (HRTEM) was carried out using a 300 kV JEOL-3011 instrument with an ultrahigh resolution pole piece. Flame atomic absorption spectrophotometer (Perkin-Elmer Model AAnalyst 100) was used to determine Pb and Cd metals using an air/acetylene flame.
Synthesis of gold nanoparticles
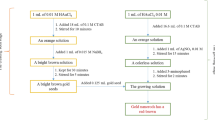
GNPs were synthesised by citrate reduction of gold chloride (HAuCl4·3H2O) solution mixed with trisodium citrate following Turkevich method; 5 mL of 1 M HAuCl4.3H2O was diluted with 90 mL of double-distilled water and heated until it began to boil. To this was added 5 mL of 1 M trisodium citrate solution and the reaction continued until the solution turned wine red. Later the surface of the GNPs was modified under stirring at 50 °C for 10 h by mixing with excess of MSA (Negishi and Tsukuda 2003) as determined by SEM. The thiol was introduced as its sodium salt by stoichiometrical neutralisation with sodium hydroxide.
Preparation of alumina-supported MSA-capped gold nanoparticles following column method
Then 5 g of neutral activated alumina was packed in a glass column (1.0 × 10 cm) to determine Pb and Cd metals in various real samples. This neutral activated alumina was saturated with 15 mL of MSA-capped GNPs suspension. Once the solution became colourless, it was replaced with another fresh 15 mL MSA-capped GNPs solution. This procedure was repeated until there was no colour change for the supernatant. After decanting the supernatant, MSA-capped GNP-coated alumina was washed thoroughly with distilled water and dried under ambient conditions.
Preparation of lead and cadmium solution
Stock solutions of lead and cadmium were prepared from lead nitrate and cadmium chloride which were procured from Merck Company, Mumbai. Standard metal solutions were prepared with different concentrations to evaluate the reagent efficiency towards the real sample analysis; 2 mL of 1.0 mg L−1 of lead and cadmium solution was spiked to the real samples for monitoring the level of lead and cadmium species in environmental water samples which were collected near selected industrial areas.
Determination of lead and cadmium using alumina-supported MSA-capped gold nanoparticles
The interaction between alumina-supported MSA-capped GNPs with lead and cadmium metals was studied using a column setup. The column was filled with 5 g of MSA-capped GNP-coated alumina and to this 1.0 mg L−1 of each lead and cadmium solution was passed at a flow rate of 5 mL min−1; 5 mL of the treated water was collected at an interval of 100 mL and analysed following flame atomic absorption spectrophotometry (FAAS).
Characterization
Conversion of Au+3 to Au0 was confirmed by ultraviolet spectrophotometer, scanning from 400 to 800 nm. FTIR spectra of GNPs capped with MSA were analysed using a Perkin–Elmer FTIR spectrum two model using KBr pellet. FTIR analysis further confirmed the presence of various functional groups in MSA-capped GNPs. Dynamic light scattering with Zetasizer analyzer (Malvern Instruments, UK) was used to measure the size and distribution of GNPs capped with MSA in a liquid. The structural and morphological changes of MSA-capped GNPs were determined by using SEM and transmission electron microscopy (TEM).
Results and discussion
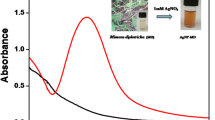
Synthesised GNPs are known in the solution by the colour changing from pale yellow to wine red. The colour change can be easily identified by the naked eye. It was clearly indicates that citrate ions along with MSA act as stabilising agent, capping agent and also they act as reducing agents to convert Au+3 to Au0. It is also noted that MSA is a stronger capping agent for GNPs which facilitates further homogeneous and strong capping with the surface-assisted reduction for growth, which is favourable for suppressing the difference in surface reduction on different particles. The presence of MSA adsorbed on modified particles and in solution played important roles in regulating the size distribution and the particle shape at different stages of growth. In addition, GNPs have a characteristic surface plasma resonance band around 300–800 nm and the maximum absorbance was found at 547 nm which is shown in Fig. 1. Temperature also plays an important role in reaction mechanism and 50 °C shows maximum absorbance under controlled conditions.
Ultraviolet (UV) spectroscopy confirmed the reduction of AuCl4 to GNPs that can be recognised from the peak obtained at 547 nm, which is the autograph for the formation of GNPs.
The size of MSA-capped GNPs which is shown in Fig. 2 clearly indicates the size distribution was maximum at 20 nm.
Structural and morphological changes of GNPs capped with MSA were done using SEM and HRTEM as shown in Fig. 3a, b. The results illustrate that the MSA-capped GNPs were spherical in shape and in the size range 20–100 nm, respectively.
The FTIR spectrum of the GNPs capped with MSA indicates the presence of various chemical groups. The presence of characteristic bands, i.e., at around 1,390 cm−1 for the symmetric stretching of COO− and at 1,710 cm−1 for the stretching of C=O in the free –COOH suggests a very stable carboxylic group carrying species on the modified GNPs. Another characteristic band is formed at 1,580 cm−1 due to the antisymmetric stretching of COO−. These characteristic bands, i.e., FTIR spectrum (Fig. 4), shows the presence of functional groups, such as COO− and C=O strongly supports the formation of MSA-capped GNPs in aqueous medium.
Stability
Citrate and MSA ions have negative charges and maintain stability in GNPs solution. Addition of different concentrations of NaCl solution to that of GNPs increases stability of GNPs and it was clearly observed in UV–Vis spectrophotometry. The domino effect confirms that maximum stability for GNPs was obtained at 10 M NaCl concentration.
Effect of pH on adsorption of Pb and Cd
The influence of pH on the adsorption of heavy metal ions on MSA-capped GNP-supported alumina was studied by taking 20 μg of each individual metal ion solution in the pH range from 3.0 to 8.0. The recoveries were higher at a pH value of 6.0 for both cadmium and lead metals. Hence a pH of 6.0 was chosen for the simultaneous determination of metal ions in water samples.
Effect of sample volume
Studies were performed with sample solutions of large volume to explore the possibility of enriching low concentrations of Pb and Cd ions. For this purpose 50, 100, 150 and 200 mL of sample solutions containing 5 mg of Cd, and Pb were passed through the micro-column at 2.0 mL min−1 flow rate. The recovery values were quantitative (>95 %) at 100 mL of sample volume. In this experiment, 100 mL of sample solution was adopted for the preconcentration of analytes from the water samples.
Effect of flow rate of sample solution
The flow rate of the sample solution affects the adsorption of metal ions on the MSA-capped GNP-supported alumina. Therefore, the effect of the flow rate of sample solution was examined in the flow rate range 1.0–10.0 mL min−1. It was found that the retention of the metal ions was quantitative up to 5.0 mL min−1 flow rate. The recoveries of the analytes decrease slightly when the flow rate is over 5.0 mL min−1. Thus, a flow rate of 5.0 mL min−1 is employed as an optimum flow rate.
Recoveries of Pb and Cd from spiked water samples
The efficiency of procedure was examined by determining the concentration of metal ions in spiked water samples. Deionized double-distilled water was spiked with known amounts of metal standards (10–50 μg L−1) and the percentage recoveries of each element are determined and given in Table 1. The percentage recoveries for different metal ions are 98.92 for Cd, 99.38 for Pb with a percentage of relative standard deviation (RSD) from 2.88 to 3.52. These results show that the MSA-capped GNP-supported alumina is a powerful tool for the preconcentration of Pb and Cd, from natural water samples.
Method evaluation
The proposed method, i.e., MSA-capped GNP-supported alumina was critically evaluated with regard to reproducibility, accuracy and detection limit.
Reproducibility
To test the reproducibility of the proposed method, four repetitive analysis cycles of each sample of Pb and Cd were run. The obtained %RSD is in the range 2.06–7.5, respectively.
Accuracy
The accuracy of the proposed method was evaluated by comparing the results with those obtained by the standard reagent method. The results shown in Table 4 reveal that the good correlation between the two methods indicative of present method is more sensitive than the standard reagent method.
Detection limit
Under optimum conditions, the detection limit for the determination of lead and cadmium metals in real water samples using alumina-supported MSA-capped GNPs is 0.22 and 0.36 µg/l, respectively.
Effect of coexisting ions
The effects of common coexisting ions on the adsorption of the studied metal ions on MSA-capped GNP-supported alumina were investigated. In these experiments, 100 mL solution of 3 mg mL−1 Pb and Cd containing the added interfering ions was treated according to the general procedure. The results are presented in Table 2. These are the concentration limits in μg mL−1 that caused <2 % error on recovery of studied metal ions.
Analysis of water samples
MSA-capped GNPs complexation with lead and cadmium solution was studied by using real water samples following standard procedure. These samples were collected from industrial areas, i.e., Amara Raja Batteries, Lanco Industries, Avanthi Leather Industry, Heritage Food India, Andhra Pradesh, India, at different time intervals and the results are shown in Tables 3 and 4.
Conclusion
MSA-capped GNPs were successfully synthesised in water medium under ordinary room temperature. MSA acts as a reducing agent as well as a capping agent, and GNPs of 20 nm sizes were obtained. This was confirmed by SEM and HRTEM images. According to our knowledge, this is the first report on the synthesis of GNPs using the MSA in water at room temperature for the analysis of Pb and Cd metals up to ppb levels in real water samples. The results obtained show that the present method is successfully applied for the determination of lead and cadmium metals in various environmental water samples with low detection limit, high accuracy and precision.
References
Baghban N, Shabani AMH, Dadfarnia S (2013) Solid phase extraction and flame atomic absorption spectrometric determination of trace amounts of cadmium and lead in water and biological samples using modified TiO2 nanoparticles. Int J Environ Anal Chem 93(13):1367–1380. doi:10.1080/03067319.2012.727811
Ebrahimzadeh H, Moazzen E, Amini MM, Sadeghi O (2013) Pyridine-2,6-diamine-functionalized Fe3O4 nanoparticles as a novel sorbent for determination of lead and cadmium ions in cosmetic samples. Int J Cosmet Sci 35(2):176–182. doi:10.1111/ics.12023
Ghanemi K, Nikpour Y, Omidvar O, Maryamabadi A (2011) Sulfur-nanoparticle-based method for separation and preconcentration of some heavy metals in marine samples prior to flame atomic absorption spectrometry determination. Talanta 85(1):763–769. doi:10.1016/j.talanta.2011.04.066
Jalbani N, Soylak M (2014) Ligandless surfactant mediated solid phase extraction combined with Fe3O4 nanoparticle for the preconcentration and determination of cadmium and lead in water and soil samples followed by flame atomic absorption spectrometry: multivariate strategy. Ecotoxicol Environ Saf 102:174–178. doi:10.1016/j.ecoenv.2013.11.018
Negishi Y, Tsukuda T (2003) One- pot preparation of subnanometer-sized gold clusters via reduction and stabilization by meso-2,3-dimercaptosuccinic acid. J Am Chem Soc 125:4046–4047. doi:10.1021/ja0297483
Shakerian F, Dadfarnia S, Shabani AMH, Esfahani GS (2013) Preconcentration and determination of lead(II) by microextraction based on suspended cadion covered zirconia nanoparticles in a surfactant media. Microchim Acta 180(13–14):1225–1232. doi:10.1007/microchimicaacta.2013.s00604
Wang J, Polsky R, Xu D (2001) Silver enhanced colloidal gold electrochemical stripping detection of DNA hybridization. Langmuir 17:5739–5741. doi:10.1021/la011002f
Wang J, Xu D, Polsky R (2002) Magnetically induced electrical DNA detection. J Am Chem Soc 124:4208–4212. doi:10.1021/ja0255709
Wu YS, Huang FF, Lin YW (2013) Fluorescent detection of lead in environmental water and urine samples using enzyme mimics of catechin-synthesized Au nanoparticles. ACS Appl Mater Interfaces 5(4):1503–1509. doi:10.1021/am3030454
Xue Y, Zhao H, Wu Z, Li X, He Y, Yuan Z (2011) Colorimetric detection of Cd2+ using gold nanoparticles cofunctionalized with 6-mercaptonicotinic acid and l-cysteine. Analyst 136(18):3725–3730. doi:10.1039/c1an15238f
Yang B, Gong Q, Zhao L, Sun H, Ren N, Qin J, Xu J, Yang H (2011) Preconcentration and determination of lead and cadmium in water samples with a MnO2 coated carbon nanotubes by using ETAAS. Desalination 278(1–3):65–69. doi:10.1016/j.desal.2011.05.010
Zhu L, Xu L, Huang B, Jia N, Tan L, Yao S (2014) Simultaneous determination of Cd(II) and Pb(II) using square wave anodic stripping voltammetry at a gold nanoparticle-graphene-cysteine composite modified bismuth film electrode. Electrochim Acta 115:471–477. doi:10.1016/j.electacta.2013.10.209
Acknowledgments
This project was sanctioned by the Department of Science and Technology under SERB-Fast track scheme with reference number SR/FTP/ES/125/2009(G). I especially thank Umesh Kumar Sharma, Scientist-D, who encouraged and monitored my project from time to time.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kiran, K. MSA-capped gold nanoparticle-supported alumina for the determination of Pb and Cd in various environmental water samples. Appl Nanosci 5, 795–800 (2015). https://doi.org/10.1007/s13204-014-0377-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-014-0377-3