Abstract
The shell extract of green coconut (Cocos nucifera Linn) has been utilized for the synthesis of gold nanoparticles at room temperature under very mild condition without any extra stabilizing or capping agents. The size of the synthesized gold nanoparticles could be controlled by varying the concentration of the shell extract. The stabilized gold nanoparticles were analyzed by surface plasmon resonance spectroscopy, HRTEM, Energy dispersive X-ray spectroscopy and X-ray diffraction studies. The catalytic activity of the freshly synthesized gold nanoparticles was studied for the sodium borohydride reduction of 4-nitrophenol and the kinetics of the reduction reaction were studied spectrophotometrically.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gold nanoparticles (AuNPs) with its unique optical, electronic and magnetic properties have found applications in catalysis, biodiagnostics, pharmaceuticals, etc. (Alkilany et al. 2013; Zhang et al. 2012; Murphy et al. 2008; Thomas and Kamat 2003; Wunder et al. 2011). Many of such applications require the AuNPs to be dispersed in water stabilized with non-toxic biomolecules to avoid any undesired environmental effects (De et al. 2008). Among various methods reported for the synthesis of AuNPs, the plant extract-based reductive method, involving the reduction of Au(III) to Au(0) by the phytochemicals, has gained profound significance in recent years because such methods will lead to “green” and “sustainable” developments (Anastas and Kirchhoff 2002). The renewable nature of the plant extracts, eco-friendly aqueous medium and mild reaction conditions make the method advantageous over other hazardous methods. Moreover, the phytochemicals present in the plant extracts act as stabilizers for the synthesized gold nanoparticles and no additional stabilizers or capping agents are needed. The green syntheses of AuNPs from the extracts of Punica granatum (Dash and Bag 2012), Saraca indica bark (Dash et al. 2013), Ananas comosus (L.) (Basavegowda et al. 2013), Terminalia arjuna bark (Majumdar and Bag 2012), Ocimum sanctum stem (Paul and Bag 2013) have recently been reported. As newer applications of nanoparticles and nanomaterials are emerging rapidly, there is an ever growing need for the development of newer methods for the synthesis of metal nanoparticles utilizing plant resources as renewables.
Coconut tree (Cocos nucifera Linn) is a palm tree (Arecaceae family), growing up to 30 meters in height, spread in the tropical regions of the world. Usually found in the sea-shores and gardens as an ornamental plant, coconut plant is a very useful plant for its versatile uses in daily life. The green coconut water is consumed as a delicious, nutritional and refreshing natural drink. The decoction of coconut husk fiber is used as a medicine for the treatment of diarrhea and arthritis (Esquenazi et al. 2002). Coconut shell charcoal has been utilized for the removal of toxic metal ions from waste water (Pino et al. 2006; Babel and Kurniawan 2004; Hasany and Ahmad 2006). Antibacterial and antiviral (Esquenazi et al. 2002), anti-inflammatory (Rinaldi et al. 2009), leishmanicidal (Mendonça-Filho et al. 2004), and antimalarial (Al-Adhroeya et al. 2011) properties of coconut shell extract have also been reported. Herein, we report a very mild and environment-friendly method for the synthesis of AuNPs from green coconut shell (mesocarp) extract without any additional capping or stabilizing agents. The stabilized colloidal AuNPs were characterized by high resolution transmission electron microscopy (HRTEM), energy dispersive X-ray spectroscopy (EDX), selected area electron diffraction (SAED), SPR spectroscopy, X-ray diffraction and FTIR studies. Catalytic activity of the freshly synthesized colloidal AuNPs has been demonstrated, using sodium borohydride reduction of 4-nitrophenol to 4-aminophenol as a model reaction, in water at room temperature and the kinetics of the reduction reaction have been investigated spectrophotometrically.
Materials and methods
Materials
Au(III) solution
HAuCl4 was purchased from SRL (Sisco Research Laboratory) and used without further purification. HAuCl4 (36.5 mg) was dissolved in deionized water (10 mL) to obtain a 10.74 mM Au(III) stock solution.
Preparation of the green coconut shell extract
Green coconut was collected from the local market at Midnapore, West Bengal, India and identified at the Department of Botany and Forestry, Vidyasagar University, Midnapore. The mesocarp of green coconut was cut into pieces, dried in air and then powdered using a grinder. 10 gm of finely powdered green coconut shell was suspended in methanol (100 mL) and stirred magnetically at room temperature for 1 h and then filtered. Volatiles of the filtrate were removed under reduced pressure to afford a brown solid (0.8 g). The crude brown solid was purified by column chromatography (Silica-gel, 100–200 mesh) using methanol/ethyl acetate (0–30 %) as the eluant to afford a solid (0.4 g). The extract of green coconut shell (0.01 g) was suspended in deionized water (10 mL) and sonicated in an ultrasonicator bath for 10 min to obtain a semi-transparent solution (1,000 mg L−1).
Identification of polyphenolic compounds
The presence of the phenolic compounds present in green coconut shell extract was examined by ferric chloride test. 1 mL of green coconut shell extract was mixed with ethyl alcohol (1 mL). Then two drops of concentrated FeCl3 solution were added to the solution. Greenish color appeared instantly indicating the presence of phenolic compounds in the shell extract.
Synthesis of nanoparticles
Aliquots of Au(III) solution (0.16 mL, 10.74 mM each) were added drop-wise to the green coconut shell extract solution to prepare a series of stabilized AuNPs where concentration of the green coconut shell extract was 60, 120, 160, 200, 400 and 600 mg L−1 and the concentration of Au(III) was fixed at 0.43 mM. UV–visible spectroscopy of the solutions was carried out after 24 h of HAuCl4 and green coconut shell extract had been mixed.
Characterization
TEM images, SAED and EDX of AuNPs were taken from Technai G2 instrument. X-ray diffraction (XRD) patterns of the stabilized AuNPs were recorded in PANalytical X’pert Pro with Cu-Kα radiation (λ = 1.54 Ǻ). Mass spectra were recorded in Shimadzu GCMS QP 2100 Plus. UV–visible spectra were recorded in Shimadzu 1601 spectrophotometer. FTIR spectra of samples were analyzed using a Perkin Elmer FTIR Spectrum Two model using KBr pellet.
Results and discussion
Green coconut shell (mesocarp) is rich in different types of plant secondary metabolites especially antioxidants (Oliveira et al. 2013; Chakraborty and Mitra 2008). These protect the coconut from biotic attack that is essential for microbial infection resistance. Presence of various soluble phenolic compounds such as quercetin, catechin, 5-O-caffeoylquinic acid (chlorogenic acid), dicaffeoylquinic acid, and isomers of caffeoylshikimic acids have been reported (Oliveira et al. 2013; Chakraborty and Mitra 2008; Bankara et al. 2011). Evidence for the presence of phenolic compounds in the green coconut shell extract was obtained from a positive ferric chloride test. Mass spectral analysis of the shell extract indicated the presence of polyphenolic compounds including flavonoids (supporting information Figure S1). During our investigations on the utilization of different types of phytochemicals as renewables (Bag et al. 2013; Bag et al. 2012; Bag and Paul 2012; Bag and Majumdar 2012; Bag and Dash 2011), we felt that o-dihydroxy compounds present in the green coconut shell extract can serve as a five membered chelating ligand and also be easily oxidized to corresponding benzoquinones by air or metal ions. Chloroaurate ions having a high reduction potential can be reduced to Au(0) by phenolic hydroxyl compounds. The AuNPs can form by the collision of the neighboring atomic Au(0) and get stabilized by the resulting benzoquinone derivatives and other phytochemicals present in the shell extract. The steric bulk of the backbone of the benzoquinone derivatives and other phytochemicals wrapping around the nanoparticles will provide robustness against further aggregation of the stabilized AuNPs. To test this, we treated the aqueous mixtures of the green coconut shell contained in vials with HAuCl4 solution (Fig. 1). Violet to pinkish red coloration appeared almost instantly indicating the formation of AuNPs. The intensities of the colors increased on standing the solutions at room temperature for several hours and then remained constant and the AuNPs once formed were stable for several months at room temperature.
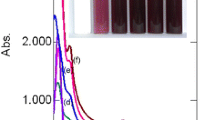
UV–visible spectra of a HAuCl4 (0.43 mM), b–g) AuNP’s at 60, 120, 160, 200, 400, 600 mg L−1 concentrations of green coconut shell extract, respectively. Inset photograph of the vials containing a HAuCl4 (0.43 mM) solution, b–g colloidal AuNP’s at 60, 120, 160, 200, 400, 600 mg L−1, respectively (after 24 h of mixing)
UV–visible spectroscopy studies
The apparently colorless solution of HAuCl4 showed a strong peak at 220 nm and a shoulder peak at 290 nm due to charge transfer interactions between the metal and the chloro ligands (Fig. 1a). The intensities of these two peaks decreased with increasing concentration of the green coconut shell extract and new peaks appeared around 535 nm. This is due to surface plasmon resonance (SPR) of the AuNPs, a phenomenon arising due to collective oscillation of the conduction band electrons interacting with the electromagnetic component of the visible light (Xie et al. 2012). The color of the AuNPs solutions was light pink to reddish brown. The shoulder peaks observed in the 270–275 nm region of AuNP colloids were due to the formation of quinone moiety formed by the oxidation of the phenolic compounds.
HRTEM, EDX, FTIR and XRD studies
High resolution transmission electron microscopy (HRTEM) was carried out to study the size distribution, shape and morphology of the AuNPs formed at different concentration of the green coconut shell extract (Fig. 2). AuNPs of spherical, triangular, tetragonal, pentagonal and hexagonal shapes were observed. The average size of the AuNPs formed at 200 mg L−1 concentration of the shell extract was 20 nm (Fig. 2a–d). At a higher concentration of the shell extract (400 mg L−1) the average particle size was 15 nm (Fig. 2e–h) and at 600 mg L−1 concentration of the shell extract the average particle size was 9.5 nm (Fig. 2i–l). At higher concentration of the shell extract, the polyphenolic compounds, quinones and other chelating phytochemicals present in the shell extract can effectively stabilize the smaller sized AuNPs. As the surrounding chelating ligands of the AuNPs prevent further aggregation, the size of the AuNPs is smaller at higher concentration of the shell extract.
X-ray diffraction analysis of the green coconut shell extract stabilized AuNPs is given in Fig. 3a. Crystallinity of the metallic face centered cubic AuNPs was confirmed from the characteristic reflections of the planes (111), (200), (220), (311) and (222) at 2θ = 38.2°, 44.5°, 64.7°, 77.9° and 81.8°, respectively, supporting the reduction of Au(III) to Au(0) by the phytochemicals present in the shell extract. The comparatively larger peak intensity of the (111) plane indicated the predominant orientation of this plane. These values are in agreement with the reported standards JCPDS file no. 04-0784. EDX analysis of the stabilized AuNPs indicated that the formed particles were gold nanoparticles (Fig. 3b) stabilized by phytochemicals.
We have compared the FTIR spectra of green coconut shell extract and stabilized AuNPs. The presence of biomolecules in the stabilized AuNPs was evident from the FTIR stretching frequencies. The peak at 3,370 cm−1 in the FTIR spectrum of green coconut shell extract is due to stretching vibration of phenolic hydroxyls (O-H bond) and broad area indicates the intermolecular hydrogen bonding among the polyhydroxy aromatic compounds (Fig. 4). The peak at 2,940 cm−1 is due to the ‘C-H’ stretching frequencies. On the other hand, the ‘O-H’ stretching frequency increases to 3,390 cm−1 in the FTIR spectrum of stabilized AuNPs. The peak at 1,633 and 1,050 cm−1 is due to the presence of aromatic rings and ‘C-O’ groups in the green coconut extract, respectively.
Mechanism of the formation of stabilized AuNPs
Green coconut shell extract is rich in different types of phytochemical including polyphenols, flavonoids, fatty acids, terpenoids, etc. (supporting information Figure S1) (Oliveira et al. 2013; Chakraborty and Mitra 2008; Bankara et al. 2011). Evidence for the presence of polyphenolic compounds including flavonoids was obtained from positive ferric chloride test. The o-dihydroxy compounds present in the green coconut shell extract can form a five member chelate ring with the Au(III) ions (Fig. 5). Au(III) ions having a very high reduction potential can be reduced to Au(0) with concomitant oxidation of the polyphenols to corresponding quinones. The freshly generated Au(0) atoms in the reaction mixture can collide with each other forming AuNPs which are stabilized by the concomitantly formed quinones, polyphenols and other coordinating phytochemicals.
Application of AuNPs in catalysis
AuNPs with very high surface to volume ratio have recently been utilized as a catalyst for various kinds of chemical transformations (Zhang et al. 2012; Wunder et al. 2011). To test whether the green coconut shell extract derived colloidal AuNPs can be utilized as a catalyst, we chose the sodium borohydride reduction of 4-nitrophenol to 4-aminophenol as a model reaction (Fig. 6). On treatment of an aqueous solution of 4-nitrophenol (0.05 mM) with sodium borohydride (15 mM) at room temperature, the absorption band of 4-nitrophenol at 317.5 nm shifted to 402 nm due to the formation of 4-nitrophenolate anion (Fig. 6b). Though the reduction of 4-nitrophenol to 4-aminophenol by sodium borohydride is a thermodynamically favorable reaction (E0 for 4-nitrophenol/4-aminophenol −0.76 and for H3BO3/BH4− −1.33 V), no reduction of the nitro group took place even on standing the mixture at room temperature for several days due to very high kinetic barrier of the reduction reaction. Interestingly, the reduction was complete in several minutes in the presence of the freshly prepared green coconut shell extract derived AuNPs. The progress of the reduction reaction was monitored spectrophotometrically. Using the UV–visible data at different time intervals, the catalytic rate constant (k) for the reduction reaction was calculated using different volume of stabilized AuNPs. When 0.10 mL of the freshly prepared colloidal AuNPs was used for the reduction of 4-nitrophenol (0.05 mM, 4 mL) containing aqueous sodium borohydride (15 mM) then the peak at 402 nm arising due to 4-nitrophenolate anion (Fig. 6b) disappeared in 4 min and 46 s with concomitant appearance of a new peak at 298.5 nm indicating the formation of 4-aminophenol (Fig. 6c). Interestingly, with 0.2 mL of the freshly prepared colloidal AuNPs, the reaction was completed within 1 min and 50 s (Fig. 6d). With large excess of sodium borohydride (300 fold), we assumed a pseudo first order rate constant for the reduction reaction and calculated the apparent rate constant (k) from the UV–visible data. From the plot of lnA vs time, the rate constants for the reduction reactions with varied amount the catalyst were calculated. With 0.1 and 0.2 mL of colloidal AuNPs (synthesized with 800 mg L−1 concentration of green coconut shell extract), the apparent rate constants were calculated to be 1.7 × 10−2 s−1 and 10 × 10−2 s−1, respectively (Table 1, supporting information Table TS1, TS2 and Figure S2, S3). The increase in effective catalytic rate constant with increasing concentration of colloidal AuNPs was due to the increased number of active sites for the chemical transformations (Wunder et al. 2011).
a schematic representation of the catalytic reduction of 4-nitrophenolate ion to 4-aminophenolate ion; b UV–visible spectra of a 4-nitrophenol (0.05 mM), b 4-nitrophenol in the presence of added sodium borohydride (15 mM), c after complete reduction using colloidal AuNPs as catalyst; c, d UV–visible absorption spectra of the reaction mixtures at various time intervals using 0.1 mL and 0.2 mL of freshly prepared colloidal AuNPs (containing 800 mg L−1 of green coconut shell extract), respectively
Conclusions
Colloidal gold nanoparticles have been synthesized using green coconut (Cocos nucifera Linn) shell extract in water at room temperature. The phytochemicals present in the plant extract act both as reducing as well as stabilizing agents and gold nanoparticles of 10–20 nm sizes were obtained without any additional stabilizing or capping agents. Increasing the concentration of the shell extract resulted in the formation of smaller sized nanoparticles as observed by HRTEM studies. Evidence for the presence of polyphenols has prompted us to propose a mechanism for the formation of gold nanoparticles. According to our knowledge, this is first report for the synthesis of gold nanoparticles using the shell extract of green coconut. The synthesized colloidal gold nanoparticles have also been utilized as a catalyst for the reduction of 4-nitrophenol to 4-amino phenol in water at room temperature. Kinetic studies for the reduction reaction at different concentration of the colloidal gold nanoparticles revealed that the rate increased with increasing concentration of gold nanoparticles. As green coconut shell extract has tremendous medicinal significance, the results reported here will be useful for its application in nanobiotechnology and pharmaceuticals.
References
Al-Adhroeya AH, Nora ZM, Al-Mekhlafia HM, Amranb AA, Mahmuda R (2011) Evaluation of the use of Cocos nucifera as antimalarial remedy in Malaysian folk medicine. J Ethnopharmacol 134:988–991
Alkilany AM, Lohse SE, Murphy CJ (2013) The gold standard: gold nanoparticle libraries to understand the nano bio interface. Acc Chem Res 46:650–661
Anastas PT, Kirchhoff MM (2002) Origins, current status and future challenges of green chemistry. Acc Chem Res 35:686–694
Babel S, Kurniawan TA (2004) Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and or chitosan. Chemosphere 54:951–967
Bag BG, Dash SS (2011) First self-assembly study of betulinic acid, a renewable nano-sized, 6-6-6-6-5 pentacyclic monohydroxy triterpenic acid. Nanoscale 3:4564–4566
Bag BG, Majumdar R (2012) Self-assembly of a renewable nano-sized triterpenoid 18β-glycyrrhetinic acid. RSC Adv 2:8623–8626
Bag BG, Paul K (2012) Vesicular and fibrillar gels by self-assembly of nanosized oleanolic acid. Asian J Org Chem 1:150–154
Bag BG, Garai C, Majumdar R, Laguerre M (2012) Natural triterpenoids as renewable nanos. Struct Chem 23:393–398
Bag BG, Majumdar R, Dinda SK, Dey PP, Maity GC, Mallia AV, Weiss RG (2013) Self-assembly of ketals of arjunolic acid into vesicles and fibers yielding gel-like dispersions. Langmuir 29:1766–1778
Bankara GR, Nayak PG, Bansal P, Paul P, Pai KSR, Singla RK, Bhat VG (2011) Vasorelaxant and antihypertensive effect of Cocos nucifera Linn. endocarp on isolated rat thoracic aorta and DOCA salt-induced hypertensive rats. J Ethnopharmacol 134:50–54
Basavegowda N, Sobczak-Kupiec A, Malina D, Yathirajan HS, Keerthi VR, Chandrashekar N, Dinkar S, Liny P (2013) Plant mediated synthesis of gold nanoparticles using fruit extracts of Ananas comosus (L.) (Pineapple) and evaluation of biological activities. Adv Mat Lett 4:332–337
Chakraborty M, Mitra A (2008) The antioxidant and antimicrobial properties of the methanolic extract from Cocos nucifera mesocarp. Food Chem 107:994–999
Dash SS, Bag BG (2012) Synthesis of gold nanoparticles using renewable punica granatum juice and study of its catalytic activity. Appl Nanosci. doi: 10.1007/s13204-012-0179-4
Dash SS, Majumdar R, Sikder AK, Bag BG, Patra BK (2013) Saraca indica bark extract mediated green synthesis of polyshaped gold nanoparticles and its application in catalytic reduction. Appl Nanosci. doi: 10.1007/s13204-013-0223-z
De M, Ghosh PS, Rotello VM (2008) Applications of nanoparticles in biology. Adv Mater 20:4225–4241
Esquenazi D, Wigg MD, Miranda MMFS, Rodrigues HM, Tostes JBF, Rozental S, da Silva AJR, Alviano CS (2002) Antimicrobial and antiviral activities of polyphenolics from Cocos nucifera Linn. (Palmae) husk fiber extract. Res Microbiol 153:647–652
Hasany SM, Ahmad R (2006) The potential of cost-effective coconut husk for the removal of toxic metal ions for environmental protection. J Environ Manag 81:286–295
Majumdar R, Bag BG (2012) Terminalia arjuna bark extract mediated size controlled synthesis of polyshaped gold nanoparticles and its application in catalysis. Int J Res Chem Environ 2:338–342
Mendonça-Filho RR, Rodrigues IA, Alviano DS, Santos ALS, Soares RMA, Alviano CS, Lopes AHCS, Rosa MSS (2004) Leishmanicidal activity of polyphenolic-rich extract from husk fiber of Cocos nucifera Linn. (Palmae). Res Microbiol 155:136–143
Murphy CJ, Gole AM, Stone JW, Sisco PN, Alkilany AM, Goldsmith EC, Baxter SC (2008) Gold nanoparticles in biology: beyond toxicity to cellular imaging. Acc Chem Res 41:1721–1730
Oliveira MB, Valentim IB, de Vasconcelos CC, Omena CM, Bechara EJ, da Costa JG, Freitas Mde L, Sant’Ana AE, Goulart MO (2013) Cocos nucifera Linn. (Palmae) husk fiber ethanolic extract: antioxidant capacity and electrochemical investigation. Comb Chem High Throughput Screen 16:121–129
Paul K, Bag BG (2013) Ocimum sanctum (Tulasi) stem extract mediated size controlled green synthesis of polyshaped gold nanoparticles and its application in catalysis. Int J Res Chem Environ 3:15–19
Pino GH, de Mesquita LMS, Torem ML, Pinto GAS (2006) Biosorption of cadmium by green coconut shell powder. Miner Eng 19:380–387
Rinaldi S, Silva DO, Bello F, Alviano CS, Alviano DS, Matheus ME, Fernandes PD (2009) Characterization of the antinociceptive and anti-inflammatory activities from Cocos nucifera L. (Palmae). J Ethnopharmacol 122:541–546
Thomas KG, Kamat PV (2003) Chromophore-functionalized gold nanoparticles. Acc Chem Res 36:888–898
Wunder S, Lu Y, Albrecht M, Ballauff M (2011) Catalytic activity of faceted gold nanoparticles studied by a model reaction: evidence for substrate-induced surface restructuring. ACS Catal 1:908–916
Xie X, Xu W, Liu X (2012) Improving colorimetric assays through protein enzyme-assisted gold nanoparticle amplification. Acc Chem Res 45:1511–1520
Zhang Y, Cui X, Shi F, Deng Y (2012) Nano-gold catalysis in fine chemical synthesis. Chem Rev 112:2467–2505
Acknowledgments
BGB thanks CSIR for funding. KP thanks CSIR, New Delhi for a research fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Paul, K., Bag, B.G. & Samanta, K. Green coconut (Cocos nucifera Linn) shell extract mediated size controlled green synthesis of polyshaped gold nanoparticles and its application in catalysis. Appl Nanosci 4, 769–775 (2014). https://doi.org/10.1007/s13204-013-0261-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-013-0261-6