Abstract
We present a plausible mechanism of formation, encapsulation, and stabilization of gold nanoparticles (GNPs) in presence of poly(vinyl pyrrolidone) (PVP) in 1-butanol in support of UV–visible, Raman, Fourier transform infrared spectroscopy (FTIR), zetapotential, X-ray photoelectron spectrum (XPS), and transmission electron microscopy. A surface plasmon resonance band at 533 nm in the UV–visible spectrum reveals formation of ~20 nm spherical GNPs in the non-hydrocolloid. In the FTIR spectrum, selective enhancement in the intensity of C–H stretching and red-shift in the C=O band suggests that PVP encapsulate GNP by an interaction between PVP and GNP that occurs via O-atom of pyrrolidone ring. Raman and XPS spectrum well supports the findings of FTIR spectrum. Zeta potential of −15.22 mV at 7.5 pH found in PVP-capped GNP strongly recommends the role of electrosteric effect towards the observed colloidal stability. Microscopic image demonstrates a thin coating of amorphous PVP layer around GNPs in a core–shell structure. Probing the mechanism of formation, encapsulation, and stabilization of GNP could provide essential information for development of bimetallic NPs for catalytic applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Synthesis of therapeutic agent like gold nanoparticles (GNPs) of various architectures continues to be of immense academic and technical interest owing to possession of inimitable opto-electronic, magnetic, physical, chemical, and medicinal properties (Alexandridis 2011; Daniel and Astruc 2004; Hoppe et al. 2006). However, all these properties strongly depend on the type of reducing and/or stabilizing agent used in the reaction medium, synthetic route, and reaction conditions (Alexandridis 2011; Daniel and Astruc 2004). In recent years, researchers around the globe are showing plenty of interest in the use of non-toxic chemicals and environmentally friendly solvents for synthesis of GNPs (Daniel and Astruc 2004; Goy-López et al. 2010; Xian et al. 2012; Zhou et al. 2009). Out of various fundamental strategies, the two methods tested widely so far: (1) bottom-up and (2) top-down approach, the in situ synthesis of NPs by chemical reduction of gold ions in presence of a polymer matrix—a bottom-up technique, is an extensively used synthetic route to obtain stable GNPs in both aqueous and non-aqueous medium (Alexandridis 2011; Goy-López et al. 2010; Xian et al. 2012; Zhou et al. 2009). Soon after the pioneer work of Helcher (1718) on the synthesis of GNPs in presence of a polysaccharide, scientists and academia felt that polymers could be used as efficient stabilizing/reducing agent in the development of inorganic–organic nanostructure materials of various morphologies and sizes of ones interest (Hoppe et al. 2006; Goy-López et al. 2010; Xian et al. 2012; Zhou et al. 2009). In the synthesis of metal NPs, the most commonly used polymers include poly(vinyl alcohol), poly(vinyl pyrrolidone) PVP, and poly (ethylene glycol) (Hoppe et al. 2006; Goy-López et al. 2010; Tripathy et al. 2009; Xian et al. 2012; Zhou et al. 2009). On the other hand, the increased interest on the use of PVP as capping/reducing/nucleating agent has been increased a lot owing to its non-toxicity, good biodegradability, excellent film forming ability and easy processability, superb stabilizing ability, mild reducing ability, and outstanding solubility in various polar solvents (Alexandridis 2011; Hoppe et al. 2006; Xian et al. 2012; Zhou et al. 2009). It was reported that PVP reduce gold ions to Au atom and subsequently encapsulate those metal NPs via O-atom of pyrrolidone ring (Hoppe et al. 2006; Xian et al. 2012; Zhou et al. 2009). PVP forms a charge transfer (CT) complex with Pt NPs via nonbonding electron transfer from the O-atom to the electron-deficient NP (Borodko et al. 2006). As reported by Xian et al., PVP ligand interacts with Pd NPs through O-atom of pyrrolidone ring.
Although a lot of research has been done and still going on the wet chemical synthesis of stable GNPs in presence of homo-polymer/block co-polymer, a small number of articles are available on the mechanistic study of their synthesis, encapsulation, and stabilization (Alexandridis 2011; Hoppe et al. 2006; Goy-López et al. 2010; Sakai and Alexandridis 2004, 2005a; Zhou et al. 2009). Schrinner et al. (2007) investigated the mechanism of formation of highly stable amorphous GNPs of about 1 nm diameter in presence of cationic polyelectrolyte brushes carrying chains of poly(2-aminoethyl methacrylate hydrochloride). They ascribed the excellent stability of GNPs to a strong attraction between negatively charged GNPs and positively charged polyelectrolyte chains. Abyaneh et al. proposed a multi-step mechanism of formation of Au cluster from AuCl4− ions via a free radical in presence of poly(methyl methacrylate) (PMMA) in a polar medium under UV irradiation. Photoreduction of AuCl4− ions offers GNPs obtained via PMMA-assisted growth process. A three-step mechanism of formation of GNPs from AuCl4− was reported in presence of a block co-polymer (Goy-López et al. 2010; Sakai and Alexandridis 2004, 2005a). Block co-polymer encapsulated GNPs formed from AuCl4− ions comprises three major steps: (1) reduction of AuCl4− ions to Au atoms/GNPs by block co-polymer with formation of oxidized organic residues; (2) chemisorption of block co-polymer on the surface of GNP and further reduction of AuCl4− ions on the surface of these gold clusters; and (3) growth of GNPs and finally stabilization by block co-polymer. As different mechanisms have been proposed for the synthesis of GNPs in presence of PVP in both aqueous and non-aqueous medium by various research groups, studying the formation of stable GNPs in presence of mild reducing agent like PVP remains a subject of debate to scientific communities. As proposed by Hoppe et al., synthesis of GNPs in presence of PVP is attributed to the following two reactions: (1) direct abstraction of H-atoms from PVP by the gold ion and (2) reduction of metal precursor by organic macroradicals formed by degradation of PVP. However, Zhou et al. proposed a similar mechanism of formation of GNPs, but in presence of base (i.e., NaOH). Here, the base assists in the degradation of PVP to form highly reactive macroradicals which are responsible for the reduction of Au3+ to Au0. Ram and Fecht had proposed a two-step mechanism of formation of GNPs in the presence of PVP in an aqueous medium. In the first step, the AuCl4− ion gets adsorbed by PVP via O-atom to form an ion–polymer unstable complex. The unstable complex later on breaks to form Au atom and a chlorinated PVP by-product in a local thermally induced transfer reaction in the second step. A mechanism of GNPs formation in presence of PVP in ethylene glycol (EG) under UV irradiation as proposed by Eustis et al. (2005) involves the reduction of the excited Au3+ to Au2+ by EG. This reaction is followed by disproportionation of Au2+ to Au3+ and Au+. Now both reduction of Au+ by EG and disproportionation reaction lead to formation of Au(0). Then, suitable nucleation and growth of Au atom form GNPs.
In this report, we proposed a step-wise path involved in the formation, encapsulation, and stabilization of GNPs in presence of PVP in an organic medium (i.e., 1-butanol). The proposed mechanism is well supported by UV–visible, Raman, Fourier transform infrared (FTIR), X-ray photoelectron (XPS) spectrum, zetapotential measurement, and transmission electron microscope (TEM).
Materials and methods
Chemicals
We used reagent-grade Au(OH)3 powder purchased from Alfa Aesar. It was dissolved in dilute nitric acid to prepare a stock solution of 0.05 M Au(NO3)3. PVP (25 kDa) and 1-butanol were purchased from Sigma Aldrich and Merck, respectively. The chemicals were used as received without further purification.
Preparation of gold colloids
At first, we prepared a mother solution of PVP (4 g/L) in 1-butanol by mechanical stirring for 3 h at 60–70 °C. After this, a specific volume of gold nitrate solution was added drop-wise during stirring to 5 mL of 40 g/L PVP solution taken in a 25-mL beaker maintained at 50 °C. After continuous hot magnetic stirring for 20 min, a purple colored nanocolloid containing a specific concentration of Au NPs with 40 g/L PVP molecules was obtained in 1-butanol. Stable colloids thus obtained were studied using UV–visible, zeta potential, FTIR, XPS, Raman, and TEM.
Characterization techniques
The UV–visible spectrum of Au non-hydrocolloid with PVP molecules was measured on a Perkin Elmer double beam spectrophotometer (LAMBDA 1050). The sample was filled in a transparent cell of quartz (10 mm path length) and the spectrum was recorded against a reference of 1-butanol with 40 g/L PVP in an identical cell. FTIR spectra have been studied of the solutions with a Thermo Nicolet Corporation FTIR spectrometer (Model NEXUS-870). The spectra have been recorded in an attenuated total reflectance (ATR) mode using a ZnSe crystal as a sample holder. X-ray photoelectron spectrum (XPS) was collected on a VG ESCALAB MK-II spectrometer with a monochromatic Al Kα source (hν = 1486.6 eV) operated at 10 kV and 20 mA at 10−9 Pa. We studied the Raman spectra for the aqueous PVP solution and PVP with GNPs by using a highly sensitive Renishaw inVia Raman microscope in conjunction with a high-sensitivity ultra-low noise CCD detector (RenCam) and an Ar+ ion laser source (λex = 514.5 nm with a maximum 50 W power). Research-grade Leica microscopes are integrated with systems to ensure a high optical efficiency and high stability. Sample for XPS was prepared by drop-casting a drop of Au colloid with PVP in 1-butanol on silicon substrate and dried in desiccators by keeping overnight at room temperature. Microscopic images of PVP-encapsulated GNPs were studied with a high-resolution transmission electron microscope (HRTEM) of JEM-2100 (JEOL, Japan) operated at an accelerating voltage of 100 kV. The samples were prepared by placing one drop of diluted solution on a carbon coated 400-mesh copper grid and then allowing the samples to dry in desiccators overnight at room temperature.
Results and discussion
UV–visible, Raman, and FTIR spectra
The UV–visible spectra of 40 g/L PVP molecules and 5 μM GNPs with 40 g/L PVP in 1-butanol were recorded and are shown in the Fig. 1. The absorption spectrum of pure PVP is totally transparent in the visible region as seen from the Fig. 1a. However, a broad peak at 296 nm arises to the n → π* transition of C=O band of pyrrolidone ring (Abyaneh et al. 2007). As evident from the spectrum (b), a strong absorption band centered at 533 nm arises from localized surface plasmon resonance reveals formation of GNPs in a dielectric medium. SPR band at 533 nm corresponds to spherical GNPs of size ~20 nm as observed from the TEM image affixed as inset in Fig. 1. Absence of band near 320 nm reveals complete reduction of Au3+ ions to Au atom (Sakai and Alexandridis 2005b).
The FTIR spectra of PVP and Au nanocolloids have been studied in order to analyze the surface interaction between GNP and PVP molecules. The vibrational bands in PVP molecules (a) before and (b) after adding a specific amount of GNPs are compared in Fig. 2. As noticed from the spectrum (b), a visible amplification in band intensity in C–H (2,961, 2,936, 2,872 cm−1) stretching in the pyrrolidone ring of PVP molecules was observed and occurs only when PVP molecules get adsorbed on the surface of NP via nonbonding electron of O-atom of lactam unit (Borodko et al. 2006). Our earlier reports on gold and fullerene (C60) nanofluids showed similarly enhanced intensities in those vibrational bands of PVP owing to chemisorption via O-atom of pyrrolidone ring onto the gold and C60 nanosurfaces, respectively (Behera and Ram 2012a, b, c). The C=O stretching band of PVP is at 1,667 cm−1. Red-shift in the C=O stretching vibration from 1,667 to 1,662 cm−1 in presence of GNPs implies a charge transfer interaction of PVP molecules with Au surface via O-atom of pyrrolidone ring (Lu et al. 2005; Xian et al. 2012).
We study Raman spectra of pure PVP and PVP with GNPs to confirm the interfacial interaction between GNP and PVP molecules which are shown in Fig. 3. Raman spectrum exhibits selective enhancement of C=O (1,656 cm−1), CH2 bending (1,453, 1,442, 1,421, 1,371, 1,307 cm−1), and C–N (1,490 cm−1) vibrational modes of PVP in the presence of GNPs attributed to the surface-enhanced Raman scattering (SERS) chemical enhancement. SERS effect results in when PVP molecules get adsorbed via O-atom (C=O group) of pyrrolidone ring on the surface of GNP through a donor–acceptor type interaction between two interacting moieties (Borodko et al. 2006).
Zeta potential and XPS spectra
The surface charge of the PVP-capped GNPs was studied by using zeta potential measurements. Such study helps in understanding the stability of Au colloids in a dielectric medium. As shown in Fig. 4, a freshly prepared mother solution (a) of 40 g/L PVP displays a prominent ξ-band at −6.0 mV with surface conductivity (σsc) = 0.014 mS/cm at 7.5 pH. Whereas, a freshly prepared colloid (b) consisting of 4 μM GNPs with 40 g/L PVP results in larger ξ-value of −15.22 mV, with an enhanced σsc = 0.14 mS/cm. Negative ξ-value implies formation of a negative charge on the surface of the GNP. In sample (b), a larger ξ-value with an enhanced σsc value is a result of an effectively negative charge confined on the surface of GNP in support of a thin surface PVP layer (Bianca et al. 2000; Behera and Ram 2012a, b; Sakai and Alexandridis 2005b). Transfer of C=O n-electrons from a nucleophilic PVP molecule to an electrophilic GNP share the localized surface charge density. When PVP-capped GNPs approach towards each other, the negatively charged surface of GNPs prevents flocculation; thus, increases the colloidal stability by electrostatic repulsion effect, whereas the adsorbed PVP molecules with long extended vinyl backbone chain suppress the agglomeration process by steric shielding (Alexandridis 2011; Behera and Ram 2012a; Hoppe et al. 2006).
XPS spectrum was recorded to study the surface interaction between NP and PVP molecules and the chemical state of synthesized GNPs. Figure 5a depicts the XPS spectrum recorded of 4f photoelectrons of GNP with PVP. The deconvolution of Au4f doublet band-group shows one band at binding energy of 82.6 and other one at 86.32 eV assigned of Au4f5/2 and Au4f7/2 peak, respectively. A chemical shift of 3.72 eV confirms reduction of Au3+ to Au0 valance state in the reaction medium (Abyaneh et al. 2007; Tripathy et al. 2009; Zhou et al. 2009). Decrease in binding energy of both 4f7/2 and 4f5/2 band in GNP with PVP molecules as compared to bulk Au atom reveals a charge transfer interaction between GNP and PVP polymer molecules (Abyaneh et al. 2007; Tripathy et al. 2009; Zhou et al. 2009). A similar effect has also been reported in PVP-capped GNP and Au metallized polycarbonate (Mishra et al. 2009; Patnaik and Li 1998). The peak of O1s (as shown in Fig. 5b) can be deconvoluted into two bands at 530.74 and 534.93 eV. The first band results from O-atom of C=O group of pure PVP. Appearance of a new peak at higher binding energy position (i.e., at 534.93 eV) hints some kind of interaction via O-atom (Tripathy et al. 2009). Almost unchanged in the N1s peak position of N-atom of pyrrolidone group in presence of GNP (as shown in Fig. 5c) implies that N-atoms of pyrrolidone ring do not interact directly with GNP (Frens 1973; Xian et al. 2012; Zhou et al. 2009). However, shifting of O1s peak to higher binding energy owing to decrease in the electron density around O-atom of carbonyl group confirms that an interaction between GNP and PVP occurs only via O-atom of C=O group (Frens 1973; Tripathy et al. 2009; Xian et al. 2012; Zhou et al. 2009).
Microstructural investigation
The size, shape, and distribution of PVP-encapsulated GNPs can be studied with transmission electron microscope (TEM). Figure 6 depicts a TEM image obtained from 4 μM GNPs with 40 g/L PVP. Nearly monodisperse and spherical GNPs of average 15–25 nm diameter are seen in this image. Microscopic image of GNPs moreover demonstrates a core–shell nanostructure consists of an Au core covered by a thin shell of PVP amorphous layer with whitish contrast in a PVP-capped GNP. Tripathy et al. and Quaroni et al. (Quaroni and Chumanov 1999) have reported similar kind of hybrid nanostructure in polymer-encapsulated Au- and Ag-NP, respectively. Even such feature observed in an Au hydrocolloid in presence of PVP molecules (Ram and Fecht 2011).
Mechanism of formation, encapsulation, and stabilization of GNPs
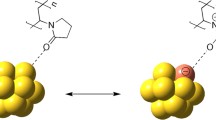
Let us exemplify how GNPs were obtained from reduction of Au3+ ions in presence of PVP molecules in 1-butanol. As proposed in Scheme 1a, a PVP molecule (a) in a weakly polar solvent such as 1-butanol undergoes keto–enol tautomerism in its pyrrolidone ring (b) to form a reactive PVP molecule (c) which is a strong nucleophilic agent (Behera and Ram 2012a, Maruthamuthu and Subramanian 1985; Yekeler 2001). In presence of electron-deficient Au3+ ion, the nucleophilic agent (i.e., enol form of PVP) can easily donate the lone-pair electron present on the O-atom of hydroxyl group and readily form Au–O–C bond in a weak polymer–metal coordinated complex (d) (Ram and Fecht 2011; Tripathy et al. 2009). As the thermodynamically stable keto form usually predominates at equilibrium in a keto–enol tautomerism, suitable rearrangement in (d) results in formation of Au atom along with PVP (keto form) and 1-butanol. So, in support of experimental findings (i.e., from FTIR, Raman, Zetapotential, XPS and TEM), we proposed that the electron rich PVP (i.e., the keto form) thus redeveloped now gets chemically adsorbed readily onto the metal surface via nonbonding electron of O-atoms (i.e., head-groups) in order to form an amorphous PVP-surface layer with long extended carbon-backbone chain (i.e., tail group) which is depicted in Scheme 1b. When two such PVP-capped negatively charged GNPs approach one another (as shown in Fig. 7) in a colloid, an electrostatic repulsion thus developed prevents two particles together (Cao 2004). Also, a polymer coat on the GNP surface further assists in preventing flocculation process by steric interaction process (Behera and Ram 2012a; Cao 2004; Hoppe et al. 2006). Such an inert adsorbed layer over highly reactive GNP makes the Au surface negatively charged by accumulation of nonbonding electrons of O-atom (as shown in Fig. 7). Hence, on the basis of our results, we strongly argued that electrosteric effect, i.e., both electrostatic and steric effects play prime role towards the observed stability of Au colloids in presence of PVP in an alcoholic medium (Cao 2004).
Models illustrate stabilization of GNPs via O-atom (i.e., head group) and long protruded carbon-backbone chain (i.e., tail group) of PVP molecules. Here, negatively charged GNP owing to accumulation of nonbonding electrons of O-atoms provide electrostatic stabilization and long and extended vinyl backbone chain offers steric stabilization to GNP
Conclusions
We proposed mechanism of formation, encapsulation, and stabilization of GNPs in presence of PVP molecules in a non-aqueous medium in support of UV–visible, Raman, FTIR, XPS spectrum, zetapotential, and TEM image. Raman, FTIR, and XPS spectrum of PVP-capped GNP suggests that GNPs are covered by a layer of PVP via O-atom of >C=O group of pyrrolidone ring in a CT complex. Zeta potential measurement suggests that electrosteric effect is responsible for the stability of Au colloid in presence of a macroscopic organic stabilizer. TEM image shows that spherical GNPs are well covered by a layer of PVP molecules in the non-hydrocolloid.
References
Abyaneh MK, Parmanik D, Varma S, Gosavi SW, Kulkarni SK (2007) Formation of gold nanoparticles in polymethylmethacrylate by UV irradiation. J Phys D Appl Phys 40:3771–3779
Alexandridis P (2011) Gold nanoparticle synthesis, morphology control, and stabilization facilitated by functional polymers. Chem Eng Technol 34:15–28
Behera M, Ram S (2012a) Solubilization and stabilization of fullerene C60 in presence of poly(vinyl pyrrolidone) molecules in water. J Incl Phenom Macrocycl Chem 72:233–239
Behera M, Ram S (2012b) Synthesis and characterization of core–shell gold nanoparticles with poly(vinyl pyrrolidone) from a new precursor salt. Appl Nanosci. doi:10-1007/s13204-012-0076-x
Behera M, Ram S (2012c) Intense quenching of fluorescence quenching of poly(vinyl pyrrolidone) molecules in presence of gold nanoparticles. Appl Nanosci. doi:10.1007/s13204-012-0159-8
Bianca MI, Jan KGD, Marcel RB, Albert PP (2000) Colloidal dispersion of gold rods characterized by dynamic light scattering and electrophoresis. Langmuir 16:459–464
Borodko Y, Habas SE, Koebel M, Yang P, Frei H, Somorjai GA (2006) Probing the interaction of poly(vinylpyrrolidone) with platinum nanocrystals by UV—Raman and FTIR. J Phys Chem B 110:23052–23059
Cao G (2004) Nanostructures and nanomaterials: synthesis, properties and applications. Imperial College Press, London
Daniel MC, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-sized-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104:293–346
Eustis S, Hsu H-Y, El-Sayed MA (2005) Gold nanoparticle formation from photochemical reduction f au3+ by continuous excitation in colloidal solutions. A proposed molecular mechanism. J Phys Chem B 109:4811–4815
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci 241:20–22
Goy-López S, Taboada P, Cambón A, Juárez J, Alvarez-Lorenzo C, Concheiro A, Mosquera V (2010) Modulation of size and shape of Au nanoparticles using amino-X-shaped poly(ethylene oxide)–poly(propylene oxide) block copolymers. J Phys Chem B 114:66–76
Helcher HH (1718) Aurum potabile oder gold tinstur. J Herbord Klossen, Breslau and Leipzig
Hoppe CE, Lazarri M, Pardiñas-Blanco I, López-Quintela MA (2006) One-step synthesis of gold and silver hydrosols using poly(N-vinyl-2-pyrrolidone) as a reducing agent. Langmuir 22:7027–7034
Lu X, Li L, Zhang W, Wang C (2005) Preparation and characterization of Ag2S nanoparticles embedded in polymer fibre matrices by electrospinning. Nanotechnology 16:2233–2237
Maruthamuthu M, Subramanian E (1985) Binding of Evans blue onto poly(N-vinyl pyrrolidone). Polym Bull 14:207–212
Mishra A, Ram S, Ghosh G (2009) Dynamic light scattering and optical absorption in biological nanofluids of gold nanoparticles in poly(vinyl pyrrolidone) molecules. J Phys Chem C 113:6976–6982
Patnaik A, Li C (1998) Evidence for metal interaction in gold metallized polycarbonate films: an X-ray photoelectron spectroscopy investigation. J Appl Phys 83:3049
Quaroni L, Chumanov G (1999) Preparation of polymer-coated functionalized silver nanoparticles. J Am Chem Soc 121:10642–10643
Ram S, Fecht H-J (2011) Modulating up-energy transfer and violet-blue light emission in gold nanoparticles with surface adsorption of poly(vinyl pyrrolidone) molecules. J Phys Chem C 115:7817–7828
Sakai T, Alexandridis P (2004) Single-step synthesis and stabilization of metal nanoparticles in aqueous pluronic block copolymer solutions at ambient temperature. Langmuir 20:8426–8430
Sakai T, Alexandridis P (2005a) Mechanism of gold metal ion reduction, nanoparticle growth, size control in aqueous amphiphilic block copolymer solutions at ambient condition. J Phys Chem B 109:7766–7777
Sakai T, Alexandridis P (2005b) Size- and shape-controlled synthesis of colloidal gold through autoreduction of the auric cation by poly(ethylene-oxide)-poly(propylene oxide) block copolymers in aqueous solutions at ambient conditions. Nanotechnology 16:S334
Schrinner M, Polzer F, Mei Y, Lu Y, Haupt B, Ballauff M, Göldel A, Dreschler M, Preussner J, Glatzel U (2007) Mechanism of the formation of amorphous gold nanoparticles within spherical polyelectrolyte brushes. Macromol Chem Phys 208:1542–1547
Tripathy P, Mishra A, Ram S, Fecht H-J, Bansmann J, Behm RJ (2009) X-ray photoelectron spectrum in surface interfacing of gold nanoparticles with polymer molecules in a hybrid nanocomposite structure. Nanotechnology 20:075701–075709
Xian J, Hua Q, Jiang J, Ma Y, Huang W (2012) Size-dependent interaction of the PVP capping ligand with Pd nanocrystals. Langmuir 28:6736–6741
Yekeler H (2001) An investigation of keto–enol tautomerism in N-vinyl-2-, and 3-pyrrolidones using ab initio and density functional theory calculations. Chem Phys 270:391–403
Zhou M, Wang B, Rozynek Z, Xie Z, Fossum JO, Yu X, Raeen S (2009) Minute synthesis of extremely stable gold nanoparticles. Nanotechnology 20:505606
Acknowledgments
This work has been supported by Silicon Institute of Technology, Silicon hills, Bhubaneswar, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Behera, M., Ram, S. Inquiring the mechanism of formation, encapsulation, and stabilization of gold nanoparticles by poly(vinyl pyrrolidone) molecules in 1-butanol. Appl Nanosci 4, 247–254 (2014). https://doi.org/10.1007/s13204-013-0198-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-013-0198-9