Abstract
A very simple strategy for preparing Sb2O3 nanostructures under ambient aqueous condition is presented. Sb2O3 nanoparticles with an average size of approximately 44 nm were synthesised using wet chemical method. Senarmontite cubical structure Sb2O3 nanocrystals have been selectively harvested in high yield. The as-obtained nanoproducts were characterized by X-ray powder diffraction, Fourier transform infrared (FTIR), ultraviolet–visible near infrared spectroscopy (UV–Vis-NIR) and scanning electron microscopy (SEM) analyses. The size of the Sb2O3 nanoparticles was found to be in the range 44–385 nm from Debye–Scherrer equation. The X-ray density, surface area and porosity of Sb2O3 nanocrystals were found to be 2.969 g/cm3, 2.0208 × 1014 cm2/g and 1.0185 × 10−3 m, respectively. From the UV–Vis-NIR analysis, the band gap of Sb2O3 nanocrystals was estimated to be 5.6 eV at 220 nm. The various functional groups in the samples were identified using FTIR spectral studies. The SEM images showed a very spiky morphology. Very small spherical-shaped objects were clearly observed within these structures. The extinction coefficient, real and imaginary part of the dielectric constant, electric susceptibility and the refractive index of Sb2O3 nanocrystals were 7.605 × 10−9, 0.2851 × 10−17, 3.747 × 10−8, 0.48322 and 2.4636, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is now wide accepted that many fundamental properties of nanocrystals depend strongly on their shape, size and microstructure. Over the past decades, driven by the development of next generation low cost nano devices, researchers have been exploring ways to control shapes of nano building blocks. Recently, oxide nanoparticles have increasingly gaining interest over past decade to the possibilities of fabricating the nanostructured materials showing novel properties compared with bulk materials (Linderoth and Pedersen 1994; Iwanaga et al. 1998). Antimony trioxide (Sb2O3) is regarded as one of the important sensor materials for detecting leakage of inflammable gases because of their high sensitivity to low gas concentration (Yamazoes 1991) and wide industrial such as conductive material, functional filters, adhesives and textile back coating (Sato et al. 1998). In addition, Sb2O3 is used as a catalyst in the polyester industry and as a clarifying agent in optical glass, television tubes crystal production and in dielectric coatings (Chin et al. 2007; Liu and Iwasawa 2002; Lewis and Jordan 1970; Jenkins 1958; Honica and Krebs 1959). Upto now, utilising different synthesis, a great number of nanomaterials such as ZnO, TiO2, Co3O4 and V2O5 have been successfully obtained (Guo and Liu 2007; Nakajima et al. 2000; Lim et al. 2007; Li et al. 2001; Joseph et al. 2006; Gao and McGarthy 2006; Feng et al. 2005; Wang et al. 2006). To the best of our knowledge, only a limited number of articles pertaining to TEM, AFM, and FESEM of Sb2O3 nanocrystals have been published (Liu et al. 2004; Zhu et al. 2006; Feng et al. 2004; Wood et al. 1972; Wolfing and Hurych 1973). In this paper, we present the fabrication of nanocrystals. Further more in our case, the as-synthesized Sb2O3 nanocrystals exist relating to a smaller particle size obtained by one step solvothermal synthesis and these crystals are characterized by XRPD, FTIR, UV–Vis-NIR and SEM analyses.
Experimental procedure
Materials
Antimony trioxide (Sb2O3), ethanol (C2H5OH) and PEG-6000 were purchased from E-Merck. All the reagents used in the experiments were analytical grade and used without further purification. Double deionised distilled water was used throughout the experiment.
Synthesis of Sb2O3 nanocrystals
In a typical process, 0.1 M of Sb2O3 was dissolved in 40 ml of ethanol with vigorous stirring to form a homogeneous solution. 50 mg of PEG-6000 was added to the above solution under continuous stirring until an opalescent mixture was obtained. Then the mixture was stirred for another 30 min, and about 15 ml of mixture was transferred into a Teflon-lined stainless steel autoclave 60 ml of 70 % capacity of the total volume. The autoclave was hermitically sealed and maintained at 120 °C for 12 h, and then cooled to room temperature normally. The resultant white precipitate was centrifuged, washed several times with distilled water and CCl4 was added to remove the impurities and then dried at 60 °C for 3 h. The white product so processed was later used for different characterization studies.
Characterization techniques
X-ray powder diffraction (XRPD) of the products was measured using a D/max (2,500 V, Rigaku, JAPAN) and X-ray powder diffractometer at a scanning rate 2θ ranging from 10° to 70° using CuKα radiation (λ = 0.15418 nm). For the optical absorption studies, UV–Vis-NIR (Hitachi U-3400) spectrophotometer was used. Fourier transform infrared spectroscopy (FTIR) spectra were recorded with a Nicolet Avatar-370 spectrometer equipped with a TGS/PE detector and a silicon beam splitter with 1 cm−1 resolution. The sizes and morphologies of the products were investigated by a scanning electron microscopy (SEM), performed on a JSM-6700F at an acceleration voltage of 16.0 kV scanning electron microanalyses.
Results and discussion
X-ray powder diffraction analysis
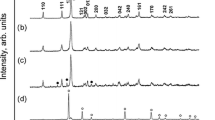
Figure 1 presents the XRPD patterned as-synthesised Sb2O3 nanoparticles. From XRPD diffractogram, all labelled reflection peaks can be readily indexed to the senarmontite structure as per the standard ASTM card. No evidence of metallic antimony signal was detected in the nanosamples. In addition, no obvious impurities could be detected in the pattern, and the sharp strong characteristic peaks suggest that the resulting products were well crystallized. The size of the Sb2O3 nanoparticles were determined by applying the Debye–Scherrer equation to the half intensity width of the (111), (121) and (012) peaks. The average size of the nanocrystallites was also estimated using Debye–Scherrer formula:
where λ, β and θ are the X-ray wavelength, the full width at half maximum (FWHM) of the diffraction peak and the Bragg diffraction angle, respectively. The estimated crystalline size was found to vary from 44 to 385 nm from XRPD data. It is known that for cubical structure, the lattice parameter can be calculated by the formula:
where h, k, and l are all integers, (hkl) in the lattice place index and a in the lattice constant. The strongest peak (111) was used to estimate the lattice expansion of the nanocrystals. The lattice parameter was estimated to be 2.3538 Å. The X-ray density for a cubical system is evaluated using the formula:
where M is the molecular weight, N is the Avogadro number and a is the lattice constant.
The density was found to be Dx = 2.96919 g/cm3. Further, the specific area of the Sb2O3 nanoflakes along the strongest peal is calculated using the formula:
where D and Dx are the particle size and X-ray density of the Sb2O3 nanocrystals, respectively. The specific area of Sb2O3 nanocrystal was found to be 2.020814 × 104 cm2/g.
Ultraviolet–visible-NIR absorption studies
The band gap of the bulk Sb2O3 crystal is 3.30 eV, which was determined by the UV–Vis-NIR transmission spectrum in the 1970’s (Ashour et al. 1995; Kaid and Ashour 2007; Gupta and Mansingh 1996). Here, an optical absorption study is carried out in order to provide a simple and an effective method to explain some features concerning the optical band gap structure. Figure 2 shows the UV–Vis-NIR absorption spectrum of spherical Sb2O3 nanoparticles measured at room temperature in the wavelength ranging from 190 to 1,100 nm. The spectrum shows that band with a prominent absorption, maximum at 220 nm, was attributed to the band gap absorption appearing in the visible region. The optical band gap (Eg) was evaluated from the transmission spectra and the optical absorption coefficient (α) near the absorption edge is given by (Chin et al. 2007):
where A is constant, Eg in the optical band gap, h is the Planck’s constant and υ is the frequency of the incident photons. The band gap of the Sb2O3 nanocrystal was estimated by plotting (αhυ)1/2 versus hυ and extrapolating the linear portion near the onset of absorption edge to the energy axis. The value of band gap is 3.3 eV. The measured transmittance (T) was used to calculate the absorption coefficient (α) using the formula:
where t is the diameter size of the nanosample. The extinction coefficient (α) is got from the following relation:
The transmittance (T) is given by,
which is constant at λ = 220 nm. The reflectance (R) in terms of the absorption coefficient is found using the following equation
The refractive index (n) is calculated using,
The refractive index n is 2.4636 for Sb2O3 nanocrystallites. From the optical constants, the electrical susceptibility (χc) is calculated using the equation,
where \(\epsilon_{\text{o}}\) is the dielectric constant in the absence of any contribution from free carriers. The value of electric susceptibility (χc) is 0.48322 at λ = 220 nm. The real part dielectric constant \((\epsilon_{\text{r}})\) and imaginary part of dielectric constant \((\epsilon_{\text{i}})\) is calculated using the following equation:
The values of real \((\epsilon_{\text{r}})\) and imaginary \((\epsilon_{\text{i}})\) dielectric constant were found to be \(\epsilon_{\text{r}}=0.2851\times 10^{-17}\) and \(\epsilon_{\text{i}}=3.747\times 10^{-8}\).
Fourier transform infrared spectrum
FTIR spectra of Sb2O3 nanocrystal were carried out in the middle IR region between the wave number 400 and 4,000 cm−1. The spectra obtained are shown in Fig. 3. The peaks at 3,727.28 and 3,122.67 are attributed to two water bands corresponding to stretching and bending vibration. These regions indicate the hydroscopic character of the powder samples. The peaks at 733 and 601.07 are assigned to the Sb–O–Sb vibrations. The small absorption due to metal oxygen (N–O) stretching is also seen at around 601 cm−1. The spectra of this sample heated at 120 °C show the sharpening of the bands and slight shift to the higher frequency have been observed. This exhibits occurrence of the band shortening and reduction in the mean Sb–O–Sb in the bond angle (Gaffar et al. 2003; Bosshard et al. 1993; Hatton et al. 2006; Austin and Mott 1969; Benet and Gnanam 1994).
Scanning electron microscope analysis
The morphologies of the resulting products were examined using SEM. Figure 4 exhibits SEM images of the prepared Sb2O3 sample. It is well established that the majority of the nanocrystallite have spherical shape with a diameter of about 44 nm at the centre and length 385 nm on average, which is similar to the quasi-spherical Sb2O3 nanoparticles produced by the γ-ray radiation oxidation route (Dzimitrowice et al. 1982).
Conclusion
A very simple and convenient method was adapted to grown Sb2O3 nanocrystal by wet chemical method. The perfection of crystallinity was confirmed XRPD and the peaks were indexed. The band gap and the related physical parameters were evaluated using UV–Vis-NIR analysis. The various functional groups in the molecule were elucidated by FTIR spectral analysis. The size of the as-obtained Sb2O3 nanocrystals were predicted using SEM topography and the results are in good agreement with the XRPD results. FESEM, AFM and PL studies are in progress.
References
Ashour A, El-kadry N, Mahmoud SA (1995) Physical properties of spray pyrolysed CdS thin films. Thin Solid Films 26:117
Austin IG, Mott NF (1969) Band structure of magnetic semiconductors. Adv Phys 18:41
Bosshard CH, Sutter K, Schlesser R, Gunter R (1993) Influence of strontium doping on the indirect band gap and optical constants of ammonium zinc chloride crystals. Opt Soc Am B 10:867
Chin HS, Cheong KY, Brebua M, Jakabb E, Sakataa Y (2010) Review on oxides of antimony nanoparticles: synthesis, properties, and applications. J Mater Sci 45:5993
Dzimitrowice DJ, Good enough BJ, Wiseman PJ (1982) Characteristics of Sb2O3 nanoparticles synthesized from antimony by vapor condensation method. Mater Res Bull 17:971
Feng XJ, Feng L, Jin MH, Zhai J, Jiang L, Zhu BD (2004) Progress in superhydrophobic surface development. J Am Chem Soc 126:62
Feng XF, Zhai J, Jiang L (2005) The fabrication and switchable superhydrophobicity of TiO2 nanorod films. Angrew Chem 44:5115
Gaffar MA, AbuEl-Fadl A, Bin Anooz S (2003) Growth, optical, mechanical and dielectric properties of glycine zinc chloride NLO crystals. Phys B 327:43
Gao LC, McGarthy TJ (2006) A general approach for fabrication of super hydrophobic and superamphiphobic surfaces. J Am Chem Soc 128:9052
Gnanam FD (1994) Synthesis, crystal growth and characterization of novel semiorganic nonlinear optical crystal: dichlorobis(l-proline)zinc(II). Cryst Res Technol 29:707
Guo ZG, Liu WM (2007) Superhydrophobic spiral Co3O4 nanorod arrays. Appl Phys Lett 90:193108
Gupta V, Mansingh A (1996) Temperature dependent optical properties of (002) oriented ZnO thin film using surface plasmon resonance. J Appl Phys 80:1063
Hatton BD, Landskron K, Hunks WJ, Bennett MR (2006) Dielectric materials research for advanced microelectronic devices. Mater Today 9:22
Honica G, Krebs K (1959) Interference filters for the far ultraviolet (1,700 Å to 2,400 Å). Physik 156:117
Iwanaga H, Fujii M, Takeuchi S (1993) Growth model of tetrapod zinc oxide particles. J Crys Grow 134:275
Jenkins FA (1985) Multilayer reflection coatings on a metal mirror. J Phys Radium 19:301
Joseph P, Cottin-Bizonne C, Benoit JM, Ybert C, Journet C, Tabeling P (2006) Anisotropic flow in striped superhydrophobic channels. Phys Rev Lett 97:156104
Kaid MA, Ashour A (2007) Sol-gel preparation, characterization and electrical properties of nanosized gallium doped zinc-oxide. Appl Surf Sci 253:3029
Lewis B, Jordan MR (1970) Morphological changes in Se films deposited on KI irradiated by electrons. Thin Solid Films 6:289
Li HJ, Wang HB, Song YL, Liu YQ, Li QS, Jiang L, Zhu DB (2008) Wettability switching between high hydrophilicity at low pH and high hydrophobicity at high pH on surface based on pH-responsive polymer. Chem Commun 10:1199
Lim HS, Kwak D, Lee DY, Lee SG, Cho K (2007) Reversible ultraviolet light-manipulated superhydrophobic-to-superhydrophilic transition on a tubular SiC nanostructure film. J Am Chem Soc 129:4128
Linderoth S, Pedersen MS (1994) Fe-Al2O3 nanocomposites prepared by high energy ball milling. J Appl Phys 75:5867
Liu H, Iwasawa Y (2002) Synthesis and purple-blue emission of antimony trioxide single-crystalline nanobelts with elliptical cross section. J Phys Chem B 106:2319
Liu H, Feng L, Zhai J, Jiang L, Zhu B (2010) Nanometric multiscale rough Zn–ZnO superhydrophobic thin films: Self-diffusion of zinc and effect of UV irradiation. Appl Phys Lett 97:183112
Nakajima A, Hashimoto K, Watanabe T, Takai K, Yamauchi G, Fujishima A (2005) pH-dependent structure and properties of TiO2/SiO2 nanoparticle multilayer thin films. Langmuir 16:7044
Sato H, Kondo K, Tsuge S, Ohtani H, Sato N (1998) Flame retardant mechanism of polydimethylsiloxane material containing platinum compound studied by analytical pyrolysis techniques and alkaline hydrolysis gas chromatography. Polym Degrad Stab 62:41
Wang ST, Feng XJ, Yao JN, Jiang L (2010) Reversible ultraviolet light-manipulated superhydrophobic-to-superhydrophilic transition on a tubular SiC nanostructure film. Appl Phys Lett 97:183112
Wolfing B, Hurych Z (1973) Atomically clean surfaces of elemental solids. J Phys Status solids A 16:161
Wood C, Van Pelt B, Dwight A (1972) Controllable synthesis and formation mechanism of bow-tie-like Sb2O3. J Phys Status solids B 54:701
Yamazoe N et al (1991) New approaches for improving semiconductor gas sensors. Sens Actuators B 5:7
Zhu WQ, Feng XJ, Feng L, Jiang L (2010) Reversible ultraviolet light-manipulated superhydrophobic-to-superhydrophilic transition on a tubular SiC nanostructure film. Appl Phys Lett 97:183112
Acknowledgments
One of the authors (K. Kaviyarasu) would like to thank Dr. S. Paulraj, Head of the Department of Physics, St. Xavier’s College, Palayamkottai for his constant support, help and encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kaviyarasu, K., Sajan, D. & Devarajan, P.A. A rapid and versatile method for solvothermal synthesis of Sb2O3 nanocrystals under mild conditions. Appl Nanosci 3, 529–533 (2013). https://doi.org/10.1007/s13204-012-0156-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-012-0156-y