Abstract
We are reporting the synthesis of surfactant wrapped Ni-doped dilute magnetic semiconductors (DMS) with their structural, optical and thermal studies with emphasis on magnetic studies in 300–100 K temperature range. The transmission electron microscopy (TEM) studies confirmed the round morphology of the nanoparticles. The wide peaks in X-ray diffraction (XRD) spectra depicted the very small granular size with zinc blend (cubic) structure of the nanoparticles. The energy dispersive spectroscopy (EDS) analysis confirmed the presence of Ni in the doped CdSe lattice. The magnetic studies showed that at 100 K the retentive magnetization is more than at 300 K. With the increased Ni doping concentration the magnetic behavior was shifted from diamagnetic to paramagnetic and then to ferromagnetic. From PL studies, the peaks were observed in at 485, 530 and 545 nm. The band gap of un-doped CdSe nanoparticles was found to be 1.78 nm and that of 5% Ni-doped CdSe nanoparticles was about 1.95 eV. The samples were found to thermally stable up to 450°C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years dilute magnetic semiconductors (DMS) have attracted the scientific fraternity in exploiting their properties at bulk as well as nanoscale. They are doped with external impurity elements to give improved photoluminescent and magnetic properties. The astonishing changes in the nanoscale DMS are due to their quantum confinement effects. They have found applications in spintronics biological labels, LEDs, lasers, bio-photonics, cellular and biological labels (Furdyna 1988; Norris et al. 2008; Erwin et al. 2005; Chen et al. 2008; Bussian et al. 2008; Bryan and Gamelin 2005). They have high luminescent properties and magnetic properties due to their hybrid nature and hence the concept of dilute magnetic semiconductors has been emerged in the due course. By doping with the external impurity element fine band gap tuning can be achieved to enhance the magnetic and optical properties.
It has been evidenced that transition metal doped semiconductor behaves as improved DMS (Chen et al. 2008; Dietl et al. 2000; Ohno 1998). The evidence of DMS is available in literature. Bhattacharjee (1995) have proposed a model of magnetic polaron by using DMS. Singh et al. (2008, 2009) have investigated the magneto-optical properties of Fe and Cu substitution in CdSe nanoparticles It was shown that room temperature is discussed in terms of F centres exchange mechanism. Fe3+ ions produce large number of F centres due to charge imbalance between Fe3+ and Cd2+ ions. Kumar et al. (2011) have studied the room temperature magnetism in Ni-doped CdSe nanoparticles. It was observed that at room temperature as the concentration of incorporated Ni impurities is increased the magnetic behavior shifts from diamagnetic to ferromagnetic. Meulenberg et al. (2009) have analyzed the paramagnetic behavior of CdSe nanoparticles enhanced by end group functionality of passivating layer. Seehra et al. (2008) have reported that smaller nanoparticles have high coercivity and saturation magnetization. Hanif et al. (2002) have studied the Co doping in CdSe nanoparticles. Sundaresan and Rao (2009) have elaborated that various inorganic nanoparticles have ferromagnetism as their universal property. Above all, Chen et al. have observed superparamagnetism in Mn-doped CdSe nanowires (Erwin et al. 2005). Doping of Mn in II–VI elements is available in literature in which the Mn is doped in CdS. Mn doping of core-shells of ZnSe-CdSe observed by Bussian et al. (2008) demonstrates the tunable magnetic sp-d interaction between excitons and Mn2+ ions i.e., the d-electrons of the guest Mn2+ ions interacts with sp-band electrons of host. Magnetic properties have been analyzed in ZnO DMS showing the ferromagnetism behavior (Norberg et al. 2004; Norton et al. 2003).
The tuning of magnetic properties can be made by the size effect and adding the doped magnetic impurity guest atoms in the host lattice. In this study, we have tuned the magnetic properties by varying the percentage of doped element Ni below the room temperature up to 100 K. There is literature on Ni-doped CdS quantum dots (Thambidurai et al. 2011) at room temperature and at the room temperature as well as at 5 K, but we could not find any magnetic studies at temperature range 300–100 K for Ni-doped CdSe nanoparticles. However, the rest of studies have been made at room temperature only.
Experimental
All the reactants and solvents are analytical grade (SD fine chemicals) and are used without any further purification. The synthesis of CdSe nanoparticles by reflux process has been mentioned in the literature (Kumar et al. 2011). In a typical procedure, 10 mM of CdCl2 was mixed in 50 ml of dimethyl formamide (DMF) to make a solution. To this mixture, 10 ml of separately prepared solution of Na2SeO3 was added drop by drop. The guest Ni impurities were introduced by injecting NiCl2 to get mol. wt. of 1, 3 and 5% of Ni, respectively, before adding selenium selenite solution. This mixture was refluxed for 3 h at the temperature of 80°C. The solution changes its color from yellow to yellowish-green due to faster release of Se ions from its precursor at high temperature and then finely to reddish confirming the formation of CdSe nanoparticles. Similar behavior was observed by Xi and Lam (2007). The solution obtained was filtered and washed with water and ethanol repeatedly to remove the impurities.
The structural and morphological properties were studied with XRD (PANalyticals X’Perto Pro X-ray diffraction machine using copper characteristic wavelength = 1.54 Å) and TEM (Hitachi H-7500). Optical properties were studied by using PL (Cary Varian) spectra and UV–Visible (Specord-205 Analytik Jena) spectroscopy. EDS studies were carried out by Thermo EDS system coupled with variable pressure scanning electron microscope (SEM) Hitachi S3400N. Adsorbed impurity traces were recorded by using Perkin Elmer fourier transform infrared spectroscope and thermogravimetric analysis (TGA) was carried out using Perkin-Elmer Pyris1. Magnetic analysis studies were made by using SQUID (Quantum Design) magnetometer. Analysis of average size was performed using the image analysis software (Image J, National Institutes of Health, USA).
Results and discussions
TEM analysis
The morphological analysis was carried out by high resolution TEM studies. The morphological images are shown in Fig. 1.
From Fig. 1 it is clear that the particles are aggregated with almost round morphology. The average size of the particles is around 3.5–5 nm. All the particles have similar morphology. From Fig. 1e the atomic spacing of the CdSe nanoparticles was found to be around 0.32 nm which correspond to (111) lattice plane. The values were almost same in the other cases.
XRD measurements
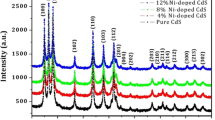
XRD spectra obtained are shown in Fig. 2. The spectra clearly indicate that diffraction peaks obtained are very broad indicating the small particle size. The diffraction data are in close agreement with the JCPDS data for CdSe (JCPDS card no. 19-0191) with peak positions at (111) (220) and (311) having zinc blend (cubic) structure.
The particle size was estimated using Debye–Scherrer formula (Patterson 1939).
where, ‘D’ is the diameter of the nanoparticles, λ = 1.54 Å (CuKα radiations wavelength), β = full width at half maxima, θ is the Bragg’s angle (2θ). The calculated size of the nanoparticles was found ~5 nm which is in close agreement with that observed with TEM results.
Electron dispersive spectroscopy studies: elemental analysis
Elemental analysis studies were carried out to confirm the presence of doped Ni in the CdSe lattice. The EDS spectra obtained are shown in Fig. 3.
The elemental analysis has been summarized in Table 1.
From the Table 1, it was observed that in all cases of Ni-doping the observed doped weight percentage of Ni was less than the actual doped percentage and this error was around 10%. It may be attributed due to some traces of impurities or some of guest ions or Ni may be lost during washing process.
Magnetic studies: SQUID analysis
The magnetic properties were studied by using SQUID (Quantum Design) up to 70,000 Oe. The obtained retentive magnetization (M) with field (H) is shown in Fig. 4. In II–VI materials, the magnetic properties depend on the magnitude of the transition metal ion-exchange coupling with the QD electronic levels. The magnitude of mixing is dependent on the d-orbital mixing with the s and p electrons in the valence band (VB) and conduction band (CB) of the II–VI QD host atom. The room temperature behavior of un-doped CdSe nanoparticles was found to be diamagnetic same to the bulk counterpart at the temperatures 100, 200 and 300 K. The results are shown in Fig. 4.
It is evident from Fig. 4I(a) that in case of un-doped CdSe nanoparticles that behavior is diamagnetic in nature at all temperatures which is similar as in bulk CdSe. The magnified magnetic curves are shown in Fig. 4I(b)–(c) at 10,000 and 1,000 Oe, respectively. In case of 1% Ni-doped CdSe nanoparticles, diamagnetic behavior was observed at 300 K. But as the temperature is lowered to 200 K, there is shift in the magnetic behavior [4 II(a)] from diamagnetic to paramagnetic behavior. With further decrease in temperature up to 100 K, the paramagnetic behavior was attained which also gives indication of mild ferromagnetism. At 3% Ni doping, a mixed paramagnetic and mild ferromagnetic behavior was observed while moving from 300 to 100 K. At 5% Ni doping the moment was found to increase threefold from 300 to 100 K.
In all doped samples the magnetization follows reverse trend with temperature whereas this trend is proportional with the doping amount. This trend is obvious from contour and 3-D plots shown in Fig. 5a and b. This behavior may be ascribed due to the fact that at low temperature the magnetic domains are aligned in the direction of magnetic field and as the temperature is increased these spins are random in the host lattice and nickel being a ferromagnetic material. Another reason for the magnetism in Ni-doped CdSe may be due to magnetic exchange of guest Ni2+ ions in the CdSe host lattice.
Optical studies
The room temperature PL emission UV–VIS spectra of pure CdSe and Ni-doped CdSe are shown in Fig. 6. The PL spectra of the nanoparticles were recorded at excitation wavelength of 450 nm (λex = 450 nm).
Sharp peaks were observed in at 485, 530 and 545 nm. Peak at 485 nm is due to blue emission whereas peaks at 530 and 545 nm are due to green emission and greenish yellow emission, respectively. The peak at 485 nm is due to direct band transition of CdSe. Figure 6a clearly indicates that with increased Ni impurity percentage the intensity of photoluminescence also increases which may be attributed to radiative recombination of luminescent process. Similar behavior was observed by Taheri et al. (2010) while tuning the luminescence of CdS by Ni doping.
The room temperature UV–Vis spectra of pure and Ni-doped CdSe nanoparticles are shown in the Fig. 6b. The absorption spectra of doped CdSe nanoparticles were blue shifted with respect to the pure CdSe nanoparticles as indicated in Fig. 6b. The band gap was calculated by plotting the graphs between hν and (αhν) (Norris et al. 2008) using the relation:
where, A is a constant, α is absorption coefficient, h is plank’s constant, ν is frequency of incident radiation, Eg is band gap and n is ½ in case of CdSe. The plots for the same are shown in Fig. 7.
The band gap of the pure CdSe found to be greater than the bulk value which may be ascribed due to quantum confinement effects. Also it is found that as the doping concentration of Ni is increased, the band gap increases to 1.95 eV for 5% Ni doping. The increased values of band gap with increased Ni-doped amount may be ascribed due to high band gap of NiSe i.e., 2 eV (Moloto et al. 2009).
Thermogravimetric analysis
The thermal stability studies were conducted to check the thermal behavior of doped CdSe nanoparticles. The studies were made in the range of 50–450°C. The spectra obtained have been shown in Fig. 8.
The spectra show a sharp decrease of 4% up to 150°C which may be attributed to adsorbed water traces. At 300°C there is minute weight loss of less than 2% which may be ascribed due to surface adsorbed impurities. From spectra it is clear that Ni-doped CdSe nanoparticles are not showing any distinct trend as compared to its pure counterpart which again may confirm that Ni is well fitted in the CdSe lattice.
Conclusions
A facile method of nanoparticles synthesis was used to synthesize Ni-doped CdSe nanoparticles. The XRD spectra confirmed that the Ni was doped in the CdSe lattice as no separate peaks were observed in XRD pattern of Ni-doped CdSe nanoparticles. The elemental analysis confirmed the presence of Ni in CdSe. It was also found that observed percentage of Ni in the samples is less than the actual amount by 10% in the doped samples. The un-doped CdSe nanoparticles show diamagnetic behavior and retentive magnetization increases and ferromagnetic behavior comes into picture as the temperature is lowered from 300 to 100 K. It also increases as the doping amount of Ni is increased. The band gap analysis from UV–VIS shows enhanced band gap with Ni-doping due to quantum confinement effects. In PL spectra, peaks were observed in at 485, 530 and 545 nm. Similar thermal behavior was shown by doped and un-doped nanoparticles as confirmed by TGA analysis.
References
Bhattacharjee AK (1995) Nanocrystals of diluted magnetic semiconductors: model for magnetic polaron. Phys Rev B 51:9912–9916
Bryan JD, Gamelin DR (2005) Doped semiconductor nanocrystals: synthesis, characterization, physical properties, and applications. Prog Inorg Chem 54:47–126
Bussian DA, Crooker SA, Yin M, Brynda M, Efros AL, Klimov VI (2008) Tunable magnetic exchange interactions in manganese-doped inverted core–shell ZnSe–CdSe nanocrystals. Nat Mater 8:35–40
Chen CC, Hsu YJ, Lin YF, Lu SY (2008) Superparamagnetism found in diluted magnetic semiconductor nanowires: Mn-doped CdSe. J Phys Chem C 112:17964–17968
Dietl T, Ohno H, Matsukura F, Cibert J, Ferrand D (2000) Zener model description of ferromagnetism in zinc-blende magnetic semiconductors. Science 287:1019
Erwin SC, Zu L, Haftel MI, Efros AL, Kennedy TA, Norris DJ (2005) Doping semiconductor nanocrystals. Nature 436:91–94
Furdyna JK (1988) Diluted magnetic semiconductors. J Appl Phys 64:R29–R64
Hanif KM, Meulenberg RW, Strouse GF (2002) Magnetic ordering in doped Cd1-x Co x Se diluted magnetic quantum dots. J Am Chem Soc 124:11495–11502
Kumar S, Kumar S, Verma N, Chakarvarti S (2011) Room temperature magnetism in Ni-doped CdSe nanoparticles. J Mater Sci Mater Electron 22:901–904
Meulenberg RW, Lee JRI, McCall SK, Hanif KM, Haskel D, Lang JC, Terminello LJ, van Buuren T (2009) Evidence for ligand-induced paramagnetism in CdSe quantum dots. J Am Chem Soc 131:6888–6889
Moloto N, Moloto M, Coville N, Sinha Ray S (2009) Optical and structural characterization of nickel selenide nanoparticles synthesized by simple methods. J Cryst Growth 311:3924–3932
Norberg NS, Kittilstved KR, Amonette JE, Kukkadapu RK, Schwartz DA, Gamelin DR (2004) Synthesis of colloidal Mn2+: ZnO quantum dots and high-TC ferromagnetic nanocrystalline thin films. J Am Chem Soc 126:9387–9398
Norris DJ, Efros AL, Erwin SC (2008) Doped nanocrystals. Science 319:1776
Norton D, Overberg M, Pearton S, Pruessner K, Budai J, Boatner L, Chisholm M, Lee J, Khim Z, Park Y (2003) Ferromagnetism in cobalt-implanted ZnO. Appl Phys Lett 83:5488
Ohno H (1998) Making nonmagnetic semiconductors ferromagnetic. Science 281:951
Patterson A (1939) The Scherrer formula for X-ray particle size determination. Phys Rev 56:978
Seehra MS, Dutta P, Neeleshwar S, Chen YY, Chen CL, Chou SW, Chen CC, Dong CL, Chang CL (2008) Size controlled ex nihilo ferromagnetism in capped CdSe quantum dots. Adv Mater 20:1656–1660
Singh SB, Limaye MV, Lalla NP, Kulkarni SK (2008) Copper-ion-induced photoluminescence tuning in CdSe nanoparticles. J Lumin 128:1909–1912
Singh SB, Limaye MV, Date SK, Gokhale S, Kulkarni SK (2009) Iron substitution in CdSe nanoparticles: magnetic and optical properties. Phys Rev B 80:235421
Sundaresan A, Rao CNR (2009) Ferromagnetism as a universal feature of inorganic nanoparticles. Nano Today 4:96–106
Taheri SM, Yousefi MH, Khosravi AA (2010) Tuning luminescence of 3d transition-metal doped quantum particles: Ni+2: CdS and Fe+3: CdS. Brazilian J Phys 40:301–305
Thambidurai M, Muthukumarasamy N, Agilan S, Sabari Arul N, Murugan N, Balasundaraprabhu R (2011) Structural and optical characterization of Ni-doped CdS quantum dots. J Mater Sci 46:3200–3206
Xi LF, Lam YM (2007) Synthesis and characterization of one-dimensional CdSe by a novel reverse micelle assisted hydrothermal method. J Colloid Interf Sci 316:771–778
Acknowledgments
The authors are grateful for DST for its generous funding. They would also like to acknowledge the contribution of Dr. Ramesh Chandra from IIT, Roorkee for providing SQUID facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kumar, S., Kumari, N., Kumar, S. et al. Synthesis and characterization of Ni-doped CdSe nanoparticles: magnetic studies in 300–100 K temperature range. Appl Nanosci 2, 437–443 (2012). https://doi.org/10.1007/s13204-011-0056-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-011-0056-6