Abstract
Surface modification of cotton fabrics using graphene oxide (GO) nanostructures was reported. Scanning electron microscopic (SEM) investigations revealed that the GO nanostructure was coated onto the cotton fabric. The molecular level interaction between the graphene oxide and the cotton fabric is studied in detail using the Fourier transform infra-red (FTIR) spectra. Thermogravimetric analysis (TGA) showed that GO loaded cotton fabrics have enhanced thermal stability compared to the bare cotton fabrics. The photocatalytic activity of the GO-coated cotton fabrics was investigated by measuring the photoreduction of resazurin (RZ) into resorufin (RF) under UV light irradiation. The antibacterial activity was evaluated against both Gram-negative and Gram-positive bacteria and the results indicated that the GO-coated cotton fabrics are more toxic towards the Gram-positive ones. Our results provide a way to develop graphene oxide-based devices for the biomedical applications for improving health care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Textiles can provide suitable substrates for the growth of micro-organisms (Dastjerdi and Montazer 2010). The major problem in health care especially in hospitals is the transmission of bacteria and pathogens from the patients to the workers and viceversa through the textiles used by them. The increasing concern towards health care has been a motivating factor for the production of antimicrobial textiles towards its direct applications in the environment safety and human health care applications (Vigo 1981). Antimicrobial properties into the textiles can be developed by introducing antimicrobial material into the textiles through surface modification process. Several antimicrobial agents which can effectively destroy the microbial growth are available. In these aspects, the use of commercial antimicrobial agents for the modification of textiles has been limited due to their toxicity, need of complex post treatment processes, environmental pollution, and multidrug-resistance (MDR) property of bacteria (French 2005; Sreeprasad et al. 2011). To overcome the MDR effect of bacteria, several researches have been attempted in the recent decades. Nanoparticles (1–100 nm) are increasingly used for biomedical applications due to their ability to sense and detect pathogens (Reddy et al. 2007). The advantage of nanoparticles for antibacterial activity is that it overcomes the MDR of bacteria. Inorganic nanoparticles such as Ag, Au, TiO2, ZnO exhibited antibacterial activity towards several multidrug resistant bacteria (Kumar et al. 2008; Karthikeyan et al. 2009). The applications of nanomaterials in medicine and development of health care products are termed as “nanomedicine” which is a vast growing research for ensuring safety life.
In this scenario, the use of inorganic nanoparticles possessing antibacterial activity for the surface modification of textile becomes a suitable alternative for fabrication of antimicrobial textiles with multifunctional properties (Fu et al. 2005). The nanoparticles such as Ag, ZnO, TiO2 have been effectively reinforced to the textiles by several surface modification process such as dip coating, sonochemical method, sol–gel method, chemical reduction method, using polymeric nanocomposites, pad-dry-cure method, sputtering of nanoparticles using plasma polymerization, etc., (Mahltig et al. 2005; Hegemann et al. 2007). The significant advantages of using nanoparticles for the preparation of antimicrobial textiles are not only limited to the antibacterial property, but also significantly improve the thermal stability of the textile fabric, developing photocatalytic property in the textiles which can avoid the growth of organic contaminants in textiles leading to the self-cleaning property (Tsai et al. 2010).
Recently, the antibacterial activity of graphene oxide nanosheets was reported (Hu et al. 2010; Akhavan and Ghaderi 2010). Even though graphene has a wide range of applications in semiconductor industry, the biological applications of graphene are limited when compared to graphene oxide. This is because of the chemical structure and properties of GO such as intrinsic luminescence, hydrophilicity, tunable band gap, biocompatibility, and photocatalytic activity which makes them a suitable candidate for biological and biomedical applications (Shukla and Saxena 2011; Joung et al. 2010; Karthikeyan et al. 2011). In this paper, we report the surface modifications of cotton fabrics using GO by a facile method and evaluated their potential antibacterial activity against both Gram-negative and Gram-positive bacteria using modified Hohenstein test. The photocatalytic activity of the GO-coated cotton fabrics is also evaluated by the reduction of Resazurin (RF) into Resorufin (RZ).
Experimental procedure
Synthesis of graphene oxide nanostructures
GO nanostructures are synthesized according to the modified Hummer’s method (Karthikeyan et al. 2011). Briefly, the expandable graphite powders (2 g) were stirred in 98% H2SO4 (35 ml) for 2 h. KMnO4 (6 g) was gradually added to the above solution while keeping the temperature less than 20°C. The mixture was then stirred at 35°C for 2 h. The resulting solution was diluted by adding 90 mL of water under vigorous stirring and a dark brown suspension was obtained. The suspension was further treated by adding 30% H2O2 solution (10 mL) and 150 mL of distilled water. The resulting graphite oxide suspension was washed by repeated centrifugation, first with 5% HCl aqueous solution and then with distilled water until the pH of the solution becomes neutral. The GO nanostructures were obtained by adding 160 mL of water to the resulting precipitate and sonicated well to attain a uniform suspension of GO.
Surface modification process of cotton fabrics using GO nanostructures
The surface modification of cotton fabrics by GO nanostructures was achieved using a facile approach comprising of dip coating via stirring. Briefly, the cotton fabric (3 × 4 cm dimension) was immersed into the solution containing (0.25 g of GO in water) and allowed to vigorous stirring at 300 rpm for 24 h. After that, the GO-coated fabric was taken from the solution and washed thrice with deionized water for the removal of residual GO on the surface. The resulting fabric was allowed to dry at 35°C for 1 day in a vacuum desiccator. The amount of GO-coated onto the fabric was calculated by weight method by employing the formula given below:
where W2 is the final weight of GO-coated cotton fabrics, W1 is the initial weight of the cotton fabrics.
Characterization techniques
The UV–vis spectroscopy was performed using Hewlett Packard HP-8453 spectrophotometer. The presence of functional groups in GO, cotton fabrics and GO-coated cotton fabrics was confirmed through the Fourier transform infra-red (FTIR) spectroscopy (Model: Bruker IFS 66/S). The surface morphology of the GO nanostructures and GO-coated cotton fabrics were observed using scanning electron microscope (FE-SEM (JSM-6700F, JEOL Ltd). The thermal properties of the bare cotton fabric and GO-coated cotton fabrics were measured on Perkin Elmer instrument.
Photocatalytic activity of GO-coated cotton fabrics
The photocatalytic activity of GO-coated cotton fabrics was evaluated by measuring the photoreduction of RZ as a function of irradiation time under UV light (350 nm). RZ is a redox indicator used in various biological assays (Premanathan et al. 2011). It is a phenoxazin-3-one dye, blue in color, having intense absorption at 600 nm and a weak absorption at 380 nm. These bands are assigned to the π–π* transition of the phenoxazin-3-one and n–π* transition of the N-oxide. It can be reduced into RF which has maximum absorption at 572 nm (Porcal et al. 2009). The photocatalysis experiment was carried out in glass reactors containing 10 mL of RZ aqueous solution at a concentration of 1.5 μg/mL in the presence of GO-coated cotton fabrics. The photocatalytic reaction is monitored by the color change from blue into pink followed by the absorption spectra measured at regular time intervals.
Growth of bacterial strains
In this study, the antibacterial activity of the GO-coated cotton fabrics was studied against one Gram-positive (Streptococcus iniae) and Gram-negative bacteria (Escherichia coli DH5α). E. coli DH5α is obtained from the Department of Marine Life Sciences, Jeju National University, Republic of Korea. S. iniae was gifted by professor Sung-Ju Jung, Department of Aqualife Medicine, Chonnam National University, Republic of Korea. E. coli cells were grown on LB agar plates (lysogeny broth containing 1.5% agar), whereas S. iniae was grown in BHI medium supplemented with 1% NaCl (BHIS) plates, respectively. Petri plates were incubated at species-specific temperatures (37°C: E. coli; 30°C: S. iniae) for 18–24 h. Single colonies were inoculated into respective media (5 mL) and cultured overnight at species-specific temperatures. This culture was seeded in a final volume of 50 mL to further grow the bacteria. When optical density (OD600) reached 0.6, cells were harvested by centrifugation at 3,500 rpm for 30 min at 4°C. The harvested cells were washed twice with saline solution (0.9% NaCl) to remove the residual media components and then resuspended with saline. Finally, the cells were spectrophotometrically (Bio-Rad, USA) quantified.
Antibacterial property of GO-coated cotton fabrics
The modified Hohenstein method was adopted to evaluate the antibacterial activity of GO-coated fabrics against both Gram-positive (S. iniae) and Gram-negative (E. coli DH5α) models (Vaideki et al. 2007). Bacterial strains were resuspended in saline solution with a final bacterial cell concentration of ~107 cells/mL. Cotton fabrics specimens were added into the tube and ensured it was fully immersed in inoculums. The mixture was then incubated in a shaking incubator at 200 rpm at species-specific temperatures for 24 h. A blank was arranged for each strain without adding the coated fabrics specimen and just before taking the inoculums for plating, coated cotton fabrics were added in such a way to acquire the data at ‘zero contact time’. The reduction in the bacterial concentration incubated with the specimens in different standard time intervals of 6, 12 and 24 h was measured. The anti-bacterial potential of the GO particle-coated cotton fabrics was determined by comparing the reduction in viable bacterial population of the coated fabric specimen to that of control sample and expressed as a percentage reduction in standard time intervals. The inoculum was serially diluted up to 104 cells/mL and uniformly spread on respective media plates at different cell concentrations (with blanks). Plates were incubated for overnight at species-specific temperatures. The colony forming units (CFU) were counted from a series with optimum cell population. Evaluation was carried out on the basis of a modified method of Hohenstein test and the percentage reduction was calculated using the following formula (Vaideki et al. 2007):
where R is percentage reduction, A is the number of bacteria recovered from the broth inoculated with GO-coated cotton fabric specimen and B is the number of bacteria recovered from the broth inoculated with control fabrics (pure cotton fabrics) specimen after the desired contact period (6, 12 and 24 h) immediately after inoculation.
Results and discussions
Characterization of graphene oxide nanostructures
GO nanostructures are synthesized using harsh oxidation according to the modified Hummer’s method. The characterization of GO nanostructures including the XRD, photoluminescence spectra, transmission electron microscope (TEM) is discussed in detail in our previous report (Karthikeyan et al. 2011). Figure 1a shows the UV–vis spectroscopy of GO nanostructures with maximum absorption peak at 227 nm which was attributed to the π–π* transitions of the aromatic C–C bonds (Karthikeyan et al. 2011). The band gap energy calculated from the UV–vis absorption spectra is 3.26 eV which lies in the UV range. The presence of oxygenated functional groups in GO nanostructures is confirmed using FTIR spectroscopy as shown in Fig. 1b. The FTIR spectrum of GO illustrates the presence of C=O (1,728 cm−1), C–OH (1,413 cm−1), C–O–C (1,250 cm−1), and C–O (1,050 cm−1) (Du et al. 2010). The peak at 1,600 cm−1 arises due to the C–C vibrations from the graphitic domains (Si and Samulski 2008). All these functional groups present in GO make them hydrophilic nature while the graphene and graphite are hydrophobic in nature. The surface morphology of GO nanostructures is studied using the SEM by placing a drop of GO dispersion into a glass substrate. The SEM image of GO nanostructure is shown in Fig. 2 representing the sheet-like morphology. It also resembles that some of the particles look aggregated. The aggregation behavior in GO is usual due to the strong interaction between oxygenated functional groups in the multilayered GO .
Characterization of GO-coated cotton fabrics
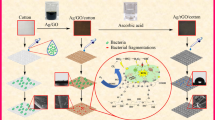
The GO nanostructures are coated onto the cotton fabrics using dip coating process. GO is an attractive material with exceptional properties such as its high stability in aqueous solution, highly electronegative, molecular level dispersion in water etc., allows it to adhere on the surface cotton fabrics very rapidly. The amount of GO coated onto the fabrics is calculated as 0.025 g using the weight method using Eq. (1). The photographic image of GO-coated cotton fabrics is shown in Fig. 3a. The white color of the control fabric changed into blackish brown color after GO coating confirms that GO is uniformly coated on the fabrics. Figure 3b shows the scanning electron microscopic (SEM) image of the GO-coated cotton fabrics. It clearly reveals the presence of GO nanosheets coated on the surface of the cotton fabrics.
The mechanism of the adhesion of GO into the fabric is described as follows: GO has various functional groups such as carboxyl, carbonyl and hydroxyl, epoxyl groups make them hydrophilic nature (Karthikeyan et al. 2011; Si and Samulski 2008). The hydrophilicity of GO allows them to be readily soluble in water at molecular levels with high surface capacity for adsorption which results in adhering strongly onto the surface of cotton fabrics during the reaction time. This strong adhesion of GO into the surface of the fabric is confirmed from FTIR spectroscopy of control and GO-treated fabrics as shown in Fig. 4. The FTIR spectra of untreated fabrics exhibit the vibrational modes of C=O stretching, C–O stretching and O–H deformation mode in the region of 1,200 to 1,700 cm−1 (Vaideki et al. 2007). The presence of the trace amount of HO-C=O shows peak in the 2,800 cm−1 region. The FTIR spectra of GO-coated fabric show significant changes compared to the free cotton fabric. The FTIR of GO-coated textile fabrics shows the presence of C–C stretching mode at 1,600 cm−1 proves that the GO material is coated onto the fabrics. The usual carboxyl peak at 1,651 cm−1 in the cotton fabric is shifted towards the higher wavenumber at 1,671 cm−1 after GO is coated. And also the appearance of new peak at 2,300 cm−1 is due to the O–H stretching modes of vibration which formed as a result of binding of O–H group present in the GO with the cotton fabric. These changes occurred ensure that GO is coated onto the cotton fabrics successfully.
The UV–vis spectrum of the pure cotton fabrics and the GO-coated cotton fabrics is shown in Fig. 5. As compared to the pure cotton fabrics, the absorbance of UV light (200–400 nm) is significantly increased in the spectrum of GO-coated cotton fabrics which is due to the good UV absorbance property of GO.
Multifunctional properties of GO-coated cotton fabrics
Thermal stability of GO-coated cotton fabrics
The thermal stability of the control fabric and the GO-coated fabric is examined using thermogravimetric analysis (TGA) and is shown in Fig. 6. TGA of control fabric exhibited a minor weight loss at 132.27°C is probably due to the removal of moisture content. The onset of melting peak arises gradually from 200°C. After 250°C, the major decomposition of the control peak starts and it reaches the peak melting point at 365.32°C. In the TGA of GO-coated fabrics, the small transition at 153.45°C is due to the removal of moisture content. The onset of the melting peak arises at 330°C and the corresponding peak melting transition is observed at 391.55°C. The transition of GO which usually occurs around 200°C is not observed in the TGA curve of GO-coated cotton fabrics due to the lower weight fraction of GO present in the cotton surface. The increase in the peak melting point is about 36°C signifies the strong molecular level interaction between the GO and the fabric which is obvious from the result of FTIR and also due to the C–C skeleton in the GO nanostructures.
Photocatalytic activity of GO-coated cotton fabrics
The photocatalytic activity of GO nanostructures was discussed recently in our previous report (Karthikeyan et al. 2011). The photocatalytic activity of the prepared GO-cotton fabrics was studied by the reduction experiments using RZ dye as a model compound. Figure 7 shows spectroscopic changes occurred during the photoreduction in RZ into RF in the presence of GO-coated cotton fabrics under UV irradiation at regular time intervals. It clearly shows that upon regular time intervals of UV irradiation, the intensity of the RZ (absorption peak at 600 nm) decreased steadily. Simultaneously, another peak at 572 nm starts arising after UV exposure which corresponds to the formation of RF by the reduction of RZ catalyzed by GO-coated cotton fabrics. In the control experiments, there is no reduction in RZ that takes place in the conditions, viz., (a) RZ without any fabric in the presence of UV irradiation (b) RZ with bare-coated fabrics in the presence of visible light and (c) RZ with GO-coated fabrics in the presence of visible light. These observations confirmed that the reduction of RZ into RF occurs only in the presence of GO-coated cotton fabrics under UV irradiation.
The reduction of RZ into RF in the presence of UV irradiation catalyzed by GO-coated cotton fabrics can be explained as follows: When UV- light of energy (3.54 eV) which is significantly greater than the energy gap of GO (3.26 eV as seen in UV spectra of GO) is irradiated on the GO-coated cotton fabrics, electrons and hole pairs will be produced on the surface of GO. This photoexcited electron generated from the GO-cotton surface reduces the RZ molecule (blue) into RF molecule (pink) by destabilizing the N–O bond in the RZ molecule. This is supported well with the time-dependent UV–vis spectra shown in Fig. 7 showing the quenching of peak at 380 nm with increase in time.
Antibacterial activity of GO-coated cotton fabrics
The antibacterial activity of GO-coated cotton fabric was examined against Gram-negative bacteria (E. coli DH5α) and Gram-positive bacteria (S. iniae) and results are shown in Fig. 8. The quantitative bacterial reduction test was performed using modified Hohenstein method for the antibacterial efficacy of the GO modified cotton fabrics (Vaideki et al. 2007).
The experimental results revealed the time-dependent reduction of bacterial growth when exposed to GO-coated fabrics. The percentage reduction of Gram-negative bacteria and Gram-positive bacteria after 6, 12, 24 h exposure with GO-coated cotton fabrics is 46, 62, 74% and 68, 86, 100%, respectively. It shows that GO-coated cotton fabrics are more effective towards the Gram-positive bacteria than the Gram-negative bacteria. Since the GO-coated fabric is more sensitive towards Gram-positive bacteria, to verify the nature of its toxicity towards bacterial species we also examined the antibacterial activity of GO nanostructures towards the same species and found that the GO is more toxic to Gram-positive ones. This is in agreement with the previous studies on the antibacterial activity of GO nanostructures (Akhavan and Ghaderi 2010). The differential toxicity of the GO-coated cotton fabrics may be due to many factors such as the primary difference between the Gram-negative and Gram-positive bacteria with respect to the nature of their cell wall (Applerot et al. 2011). In addition to this, the Gram-negative bacteria possess an additional outer membrane comprising of lipopolysaccharide which protects the peptidoglycan layer from chemical attacks (Eaton et al. 2008).
The antibacterial mechanism of the GO-coated cotton fabrics may be either due to the direct contact mechanism or oxidative stress which have been observed in the cytotoxic effects of carbon nanomaterials such as CNTs, graphene oxide, graphene, etc., (Manna et al. 2005; Liao et al. 2011). The former is due to the chemical composition of GO which contains several oxygenated functional groups such as carbonyl, carboxyl and epoxyl groups on their surfaces which makes them negatively charged, and easily bind to the bacterial cell walls and damage them. The latter is due to the reactive oxygen species produced by GO which can also contributes to the antibacterial activity of the GO-coated cotton fabrics.
Conclusions
In summary, GO nanostructures were successfully coated onto the cotton fabrics using a facile method. SEM observations reveals the presence of GO in the surface of the cotton fabrics. The FTIR analysis confirmed the molecular level interaction between the cotton fabrics and GO nanostructures. The results of TGA analysis showed that GO-coated cotton fabrics possess enhanced thermal stability compared to the bare cotton fabrics. Moreover, the GO-coated cotton fabrics showed photocatalytic activity by the reduction of RZ into RF under UV irradiation. The antibacterial activity of the GO-coated fabric shows better toxicity towards Gram-positive bacteria than the Gram-negative ones. Hence our efforts in this research level may find more practical applications of developing graphene oxide based antimicrobial coating for improving human health care.
References
Akhavan O, Ghaderi E (2010) Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 4:5731–5736
Applerot G, Lipovsky A, Dror R, Perkas N, Nitzan Y, Lubart R et al (2011) Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Adv Funct Mater 19:842–852
Dastjerdi R, Montazer M (2010) A review on the application of inorganic nano-structured materials in the modification of textiles: focus on anti-microbial properties. Colloids Surf B 79:5–18
Du Q, Zheng M, Zhang L, Wang Y, Chen J, Xue L, Dai W, Ji G, Cao J (2010) Preparation of functionalized graphene sheets by a low-temperature thermal exfoliation approach and their electrochemical supercapacitive behaviors. Electrochim Acta 55:3897–3903
Eaton P, Fernandes JC, Pereira E, Pintado ME, Malcata FX (2008) Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 108:1128–1134
French GL (2005) Clinical impact and relevance of antibiotic resistance. Adv Drug Deliv Rev 57:1514–1527
Fu G, Vary P, Lin CT (2005) Anatase TiO2 nanocomposites for antimicrobial coatings. J Phys Chem B 109:8889–8898
Hegemann D, Hossain MM, Balazs DJ (2007) Nanostructured plasma coatings to obtain multifunctional textile surfaces. Prog Org Coat 58:237–240
Hu W, Peng C, Luo W, Lv M, Li X, Li D, Huang Q, Fan C (2010) Graphene-based antibacterial paper. ACS Nano 4:4317–4323
Joung D, Chunder A, Zhai L, Khondaker SI (2010) Space charge limited conduction with exponential trap distribution in reduced graphene oxide sheets. Appl Phys Lett 97:093105-1–093105-3
Karthikeyan K, Poornaprakash N, Selvakumar N, Jeyasubrmanian K (2009) Thermal properties and morphology of MgO-PVA nanocomposite film. JNPN 5:83–88
Karthikeyan K, Mohan R, Kim S.-J (2011) Graphene oxide as a photocatalytic material. Appl Phys Lett 98:244101-1–244101-3
Kumar A, Vemula PK, Ajayan PM, John G (2008) Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat Mater 7:236–242
Liao KH, Lin YS, Macosko CW, Haynes CL (2011) Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl Mater Interfaces 3:2607–2615
Mahltig B, Audenaert F, Böttcher H (2005) Hydrophobic silica sol coatings on textiles-the influence of solvent and sol concentration. J Sol Gel Sci Tech 34:103–109
Manna SK, Sarkar S, Barr J, Wise K, Barrera EV, Jejelowo O, Rice-Ficht AC, Ramesh GT (2005) Single-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor-B in human keratinocytes. Nano Lett 5:1676–1684
Porcal GV, Previtali CM, Bertolotti SG (2009) Photophysics of the phenoxazine dyes resazurin and resorufin in direct and reverse micelles. Dyes Pigments 80:206–211
Premanathan M, Karthikeyan K, Jeyasubramanian K, Manivannan G (2011) Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomedicine NBM 7:184–192
Reddy KM, Feris K, Bell J, Wingett DG, Hanley C, Punnoose A (2007) Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl Phys Lett 90:213902-1–213902-3
Shukla S, Saxena S (2011) Spectroscopic investigation of confinement effects on optical properties of graphene oxide. Appl Phys Lett 98:073104-1–073104-3
Si Y, Samulski ET (2008) Synthesis of water soluble graphene. Nano Lett 8:1679–1682
Sreeprasad TS, Maliyekkal MS, Deepti K, Chaudhari K, Xavier PL, Pradeep T (2011) Transparent, luminescent, antibacterial and patternable film forming composites of graphene oxide/reduced graphene oxide. ACS Appl Mater Interfaces 3:2643–2654
Tsai TM, Chang HH, Chang KC, Liu YL, Tseng CC (2010) Comparative study of the bactericidal effect of photocatalytic oxidation by TiO2 on antibiotic-resistant and antibiotic-sensitive bacteria. J Chem Technol Biotechnol 85:1642–1653
Vaideki K, Jayakumar S, Thilagavathi G, Rajendran R (2007) A study on the antimicrobial efficacy of RF oxygen plasma and neem extract treated cotton fabrics. Appl Surf Sci 253:7323–7329
Vigo T (1981) Antibacterial fiber treatment and disinfection. Text Res J 51:454–465
Acknowledgments
This research was supported by National Research Foundation of Korea Grant under Contract Nos. 2009-0087091 and 2011-0015829, through the Human Resource Training Project for Regional Innovation. A part of this work was carried out at the Research Instrument Center (RIC), Jeju National University, Jeju, Republic of Korea.
Conflict of interest
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Krishnamoorthy, K., Navaneethaiyer, U., Mohan, R. et al. Graphene oxide nanostructures modified multifunctional cotton fabrics. Appl Nanosci 2, 119–126 (2012). https://doi.org/10.1007/s13204-011-0045-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-011-0045-9