Abstract
TbMnO3 rods-like morphology were prepared by the microwave-assisted chemical route. The samples were synthesized at different microwave heating conditions. We synthesized nanorods of size 100 nm in diameter and length in several micrometers. SEM and TEM confirmed the morphology and size of the samples. Orthorhombic phase was indexed from XRD data. The temperature profile of the magnetization (M) at H = 50 Oe indicates well defined feature around 8 K for almost all samples. Temperature dependence of dielectric constant demonstrates an upward rise in dielectric constant start near 50 K and continues up to 23 K and lowering near 10 K. Magnetization and dielectric constant (ε) was observed to be strongly influenced by microwave power level used for synthesis of the samples.
Similar content being viewed by others
Introduction
Recently multiferroic materials have been extensively studied because of their significance in basic research and in potential application using magneto-electric effects. In multiferroic systems, magnetic and ferroelectric orders co-exist and are cross correlated (Cheong and Mostovoy 2007; Kimura et al. 2003). These materials exhibit interplay between lattice distortion and electric and magnetic ordering. They have great possibilities for application in multifunctional devices. These applications are specially based on the control of magnetic state by electric fields and ferroelectric state by magnetic fields. These type of materials are called magnetoelectric, a subclass of multiferroics (Hur et al. 2004). The revival of interest in magnetoelectric materials was initiated by the theoretical investigation of Hill (2000) and by the recent discoveries of new mechanisms of ferroelectricity in perovskite TbMnO3, hexagonal YMnO3, RMn2O5, and Ni3V2O8 (Lawes et al. 2005). Efforts have been devoted to find new multiferroic materials or to investigating multiferroic properties in known oxides, such as BiCrO3 (Niitaka et al. 2004; Baettig et al. 2005), BiMnO3 (Montanari et al. 2005a, b), BiFeO3 (Wang et al. 2006; Baettig et al. 2005), BiCoO3 (Belik et al. 2006a), BiScO3 (Belik et al. 2006b) and double perovskite Bi2MnNiO6 (Azuma et al. 2005). Recently, multiferroic effects have been found in some rare earth manganates. Perovskite rare-earth manganites RMnO3 (R = Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Y, etc.) are representative examples of multiferroic materials. Kimura et al. (2003). have explored TbMnO3 to observed giant magneto-capacitance and magneto-electric.
Recently, synthesis of low-dimensional nanomaterials with well controlled size, morphology, and chemical composition has attracted considerable research interest due to their distinctive geometries, novel physical and chemical properties, which make them to be potential application in modern electronics and photonics. With this motivation, in the present work, we report the synthesis of TbMnO3 in rods like morphology at nano-scale by microwave assisted soft chemical route. According to our knowledge, this is the first efforts to synthesize multiferroics pervoskite in rods like morphology by microwave-assisted method. The effect of microwave synthesis conditions (i.e., microwave power, corresponding temperature, and irradiation time, etc.) on magnetic and electric properties are studied in view point of multiferroic.
Experimental
The starting materials used in the present work included terbium trichloride hexahydrate TbCl3·6H2O, manganese dichloride tetrahydrate MnCl2·4H2O and sodium hydroxide NaOH. All chemicals were GR grade procured from Sigma-Aldrich and used without further purifications. TbMnO3 nano-rods, were prepared using stoichiometric (1:1) amount of 0.05 M solutions of TbCl3·6H2O and MnCl2·4H2O, mixed in 500 ml flask and vigorously stirred for 2 h. This solution of the mixed salts was then drop wise added to 0.05 M solution of NaOH with vigorous stirring leading to a dark brown solution. The flask containing the solution was placed in microwave synthesis assembly having a chamber of 360 mm × 210 mm × 430 mm dimensions with a 2.45 GHz frequency multimode source reflexor. Maximum deliverable power output was 700 W. Samples were prepared for different microwave synthesis conditions by varying the scale of microwave power level from 120 to 650 W and irradiation time 15 min to 65 min. The reaction product in the form of colloidal particles was filtered and dried in vacuum. The final black product was collected for characterization.
Characterization
The phase composition of as prepared powder samples was detected by X-ray powder diffraction (D8 Advanced Bruker instrument with Cu Kα radiation (1.54 Å) at a scanning rate of 1° min−1. The morphology of the sample was observed using JEOL JSM-6360A scanning electron microscope (SEM). The TbMnO3 powder was spin-coated on to silicon substrate and a thin coating of platinum was sputter-deposited on this film in order to avoid any charging propel due to high energy electron bombardment during SEM. The transmission electron microscopy (TEM) was carried out using a Technai 20 G2. Temperature profile of the magnetization of the samples was measured by ADE-Digital measurements system model vibrating sample magnetometer. Dielectric measurements were carried out by 4192 A impedance analyzer.
Result and discussion
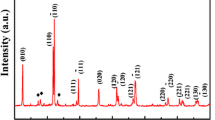
Figure 1 shows the XRD pattern of TbMnO3 sample prepared at microwave power 550 W for 15 min exposure time. All the diffraction peaks are well matched with the (JCPDS 72-0379) orthorhombic structure of TbMnO3 (space group pbnm) with unit cell constant of a = 5.378 Å, b = 5.893 Å, c = 7.399 Å. The average crystallite size calculated using Scherrer formula for these particles is found to be about 90 ± 10 nm. The morphology of the samples was examined by SEM and TEM as shown in Fig. 2 a, (b), respectively. TEM and SEM image exhibits a rod like morphology of TbMnO3 particles with average size 100 nm and length in micrometer. Almost same morphology was obtained for all the samples with change in scale of dimensions of the rods.
The field cooled temperature dependence of DC magnetization of TbMnO3 bulk in a field of 50 Oe is shown in Fig. 3 for all samples. Above 50 K, there is almost no difference between the curves of all the samples. The anomaly in the magnetization is observed near ~8 K, corresponding to the magnetic ordering of Tb3+ moments. The transitions around 40 K and 27 K observed in single crystalline TbMnO3 and is attributed to the sine wave ordering of Mn3+ moments and lock-in transition, respectively don’t appear to be strong in the rods-like morphology. There is possibility that the sine wave ordering of Mn3+ is localized due to nano-dimensions of the rods. The rods-like morphology develop intrinsic strain gradient which is responsible for softening of lock-in transition. Since these both transition are related to longer length scale. Although, the magnetization anomaly is observed at almost same temperature in all the samples, decrease trend in magnetization is detected for samples synthesized at increasing microwave power level. The samples proceed at higher microwave power level gives rise to an increase in radial dimension of the rod. This could be attributed to an Ostwald ripening process. Such a process of widening of the rods is quite possible since there is no capping agent used to cap their boundaries. This widening results in change in impact factor (diameter/length) of rods, which causes change in magnetic ordering of Tb3+ ions on the surface layer to core. The widening of rods gives rise to the decrease in surface layer attributes to low magnetic ordering of Tb3+ ions on the surface. This results out the decrease in magnetization with increasing microwave synthesis power level.
Temperature dependence of dielectric constant (ε) at H = 10 Oe and frequency 10 kHz is shown in Fig. 4. The temperature profile of dielectric constant exhibits that below 50 K, ε(t) began to increase rapidly and passes through a maximum at vicinity of ~15 K. The dielectric feature occurs at almost the same temperature at which the magnetic transition observed. It implies that the multiferroic behavior is retained in the rods-like morphology. Enhancement of ε is up to 9–15% for the samples synthesis at microwave power level from 450 to 650 W. The microwave power level is found to be diameter manipulative parameter of the TbMnO3 rods.
The influence of size on ε is understood as; at nanoscale dimension has large surface-to-volume ratio raised number of surface atoms compare to core. The termination of lattice periodicity on the surface leads to reduction in the coordination number of surface atoms. It attributes to surface bond contraction and thus the band gap expansion. The energy band gap is correlated to dielectric constant by relation Δχ/χ = −2(ΔEg/Eg) (Tsu and Babic 1994), where, ΔEg change in band gap energy, Eg original energy, Δχ change in static polarization, χ actual static polarization. It clearly indicates that decrease in size of particles increases band gap expansion and thus decreases dielectric constant.
In the present work observed trends of increase of dielectric constant with increasing microwave synthesis power level (from 450 to 650 W) can be explained as: the sample synthesis at increasing power level widen the diameter of rods and reduces the surface-to-volume ratio. Thus, the band gap expansion is decreasing and dielectric constant is increasing with increasing power level in accordance with band gap correlation with dielectric constant.
The magnetization and dielectric constant TbMnO3 nanorods are observed to be strongly influenced by microwave power used for the synthesis of the samples. In the microwave-assisted synthesis, molecular level dielectric heating is key factor of processing of reaction. The size and the morphology of the product depend on rate of crystal nucleation and growth. Microwave heating maintain uniformity of temperature in the reaction by avoiding thermal gradient, which generally occur in the reaction process by conventional way of heating. Uniform thermal conditions during the reaction keep control on rate of crystal nucleation and growth. It indicates that the size and morphology of the product can be manipulated by controlling microwave thermal conditions. The microwave thermal condition is decided by microwave power. In the present work, it is observed that the products synthesized by different microwave power level have same morphology (rod-like). Microwave power has not any significant influence on the morphology. The size of the rods gets affected by variation of microwave power. The increase in size with microwave power is responsible for enhancement of magnetization and dielectric constant of the samples.
Conclusion
Nanorods of TbMnO3 were synthesized by microwave-assisted soft chemical route for different microwave power and exposure time. Temperature of solution was observed to be increasing with microwave power at initial stages of exposure time around 15 min by microwave-assisted processing of the reaction. For constant time and with increasing power, temperature is closely monitored to steeply rising. All samples were indexed to the orthorhombic structure from the XRD data. SEM and TEM confirmed nanorods of diameter 100 nm. Magnetization value was observed to be decreasing and dielectric value was observed to be increasing with increasing microwave power used for the synthesis of the TbMnO3 samples. The simple technique using a multimode microwave source may prove to be a potential tool for growing similar nanostructures of other Perovskite oxide.
References
Azuma M, Takata K, Saito T, Ishiwata S, Shimakawa Y, Takano M (2005) Designed Ferromagnetic, Ferroelectric Bi2MnNiO6. J Am Chem Soc 127:8889–8892
Baettig P, Ederer C, Spaldin NA (2005) First principles study of multiferroics BiFeO3, Bi2FeCrO6, and BiCrO3: structure, polarization, and magnetic ordering temperature. Phys Rev B 72:214105
Belik AA, Iikubo S, Kodama K, Igawa N, Shamoto S, Niitaka S, Azuma M, Shimakawa Y, Takano M, Izumi F, Takayama-Muromachi E (2006a) Neutron powder diffraction study on the crystal and magnetic structure of BiCoO3. Chem Mater 18:798–803
Belik AA, Iikubo S, Kodama K, Igawa N, Shamoto S, Maie M, Nagai T, Matsui Y, Stefanovich SY, Lazoryak BI, Takayama-Muromachi E (2006b) BiScO3: centrosymmetric BiMnO3-type oxide. J Am Chem Soc 128:706–707
Cheong SW, Mostovoy M (2007) A magnetic twist for ferroelectricity. Nat Mater 6:13–20
Hill N (2000) Why are there so few magnetic ferroelectrics. J Phys Chem B 104:6694–6709
Hur N, Park S, Sharma PA, Ahn JS, Guha S, Cheong S-W (2004) Electric polarization reversal and memory in a multiferroic material induced by magnetic fields. Nature 429:392–395
Kimura T, Goto T, Shintani H, Ishizaka K, Arima T, Tokura Y (2003) Magnetic control of ferroelectric polarization. Nature 426:55–58
Lawes G, Harris AB, Kumara T, Rogado N, Cava RJ, Aharony A, Entin-Wohlman O, Yildirim T, Kenzelmann M, Broholm C, Ramirez AP (2005) Magnetically driven ferroelectric order in Ni3V2O8. Phys Rev Lett 95:087205–087209
Montanari E, Calestani G, Migliori A, Dapiaggi M, Bolzoni F, Cabassi R, Gilioli E (2005a) High temperature polymorphism in metastable BiMnO3. Chem Mater 17:6457–6467
Montanari E, Righi L, Calestani G, Migliori A, Gilioli E, Bolzoni F (2005b) Room Temperature Polymorphism in metastable BiMnO3 prepared by high-pressure synthesis. Chem Mater 17:1765–1773
Niitaka S, Azuma M, Takano M, Nishibori E, Takata M, Sakata M (2004) Crystal structure and dielectric and magnetic properties of BiCrO3 as a ferroelectromagnet. Solid State Ionics 172:557–559
Tsu R, Babic D (1994) Doping of a quantum dot. Appl Phys Lett 64:1806–1808
Wang Y, Jiang QH, He HC, Nan CW (2006) Multiferroic BiFeO3 thin film prepared via a simple sol-gel method. Appl Phys Lett 88:142503–142505
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Acharya, S.A., Khule, S.M. A multiferroic behavior of TbMnO3 nanorods prepared by microwave-assisted chemical route. Appl Nanosci 2, 31–34 (2012). https://doi.org/10.1007/s13204-011-0039-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-011-0039-7