Abstract
An environmental friendly route for the high yield synthesis of carbon nanofibers (CNFs) has been developed. CNFs have been synthesized using nickel formate as catalyst precursor at 680°C using chemical vapor deposition method. Upon pyrolysis this catalyst precursor yields metal catalyst nanoparticles directly. The sodium chloride and potassium chloride was used as catalyst support, it was chosen because of its non-toxic and water soluble nature. The problems such as detrimental effect, environment and even cost has been avoided by using water soluble supports. The structure of the products was characterized by scanning electron microscopy, transmission electron microscopy, Raman spectroscopy and X-ray diffraction method. The purity of as grown products and purified products was determined by thermal analysis. Here, we report the 7,800 and 7,200 wt% yield of CNFs synthesized over NaCl and KCl support. This synthetic route can be used for the large scale synthesis in industries.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Since the discovery of carbon nanotubes by Iijima (1991), an intensive research has been carried out on carbon nanofibers (CNFs) and carbon nanotubes (CNTs). These materials have unique mechanical, chemical and electrical properties and hence attracted many researchers. CNTs and CNFs have large number of potential applications which include field emission sources (Milne et al. 2004), hydrogen storage (Dillon et al. 1997), catalyst substrate (Bahome et al. 2005), chemical sensors (Douglas and Alexander 2010), scanning probe tip (Demicheva et al. 2008), electrode material (Jan et al. 2009), nanoelectronics (Brataas 2010) and mechanical reinforcements (Wang et al. 2010). Until now, various synthetic methods have been developed for the production of these nanostructures, including arc discharge (Iijima 1991), laser vaporization (Guo et al. 1995) and chemical vapor deposition (CVD) (Baker et al. 1989; Su et al. 2000). One of the remarkable advantages of CVD method is that it produces cheap CNTs and CNFs with high yields. The choice of the supporting material has been found to be important factor to synthesize these carbon nanostructures by CVD method. The support materials such as silica, alumina or magnesia are widely studied (Nagaraju et al. 2002; Kathyayini et al. 2008; Bris et al. 2010; Seifi 2011). But these supports are soluble in acids and hence acid wash is required to separate these supports from carbon structures after growth. The acid wash causes detrimental effect, environment problems and effects cost efficiency. Therefore, an important approach for the large-scale production of high quality CNTs or CNFs is (1) to avoid acids for purification and (2) to reduce the number of purification steps. In this paper, we have developed novel method to synthesize CNFs by CVD method using water soluble substrates. Nickel formate is used as catalyst precursor, as it directly yields metal catalyst nanoparticles on pyrolysis. The main advantage of using nickel formate is that it avoids the lengthy process of reduction and calcination. Sodium chloride and potassium chloride are used as supports, as they are easily soluble in water. The use of acid or base is completely avoided by using water soluble substrates. Here, we report the 7,800 wt% yield of CNFs synthesized over nickel formate as catalyst precursor on NaCl substrate.
Experimental
All the reagents used were AR-grade and purchased from Sigma Aldrich. Nickel formate was prepared by nickel acetate and formic acid at 80°C. The microcrystalline precipitate was obtained on cooling which was filtered, dried at 100°C. The formation of nickel formate was confirmed by X-ray diffraction method (Fig. 1). The supported catalyst was made by dispersing the nickel formate catalyst precursor on substrate. The obtained supported catalysts were denoted as Ni/NaCl and Ni/KCl.
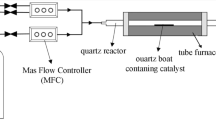
In this study, the synthesis of CNFs was carried out at atmospheric pressure in a fixed bed CVD reactor. The length and diameter of quartz tube were 900 and 20 mm, respectively. The detailed experimental set up is shown in the Fig. 2. 300 mg of catalyst precursor with support was taken in quartz boat. The boat was placed in the centre of the quartz tube after the reactor reaches the 680°C in an argon atmosphere. Acetylene gas was then released in order to grow the CNFs for 15 min. The reactor was cooled in an argon atmosphere and then carbon deposits were collected. The carbon yield is calculated by Eq. 1
where MTotal is the total mass of the carbon product and catalyst, and MCat is the mass of the catalyst.
To purify the sample, the raw material was stirred in warm water to remove the catalyst and support. The sample was finally washed with deionized water and dried in oven.
The structure and morphology of the synthesized CNFs were characterized by scanning electron microscope (SUPRA 40VP, Carl Zeiss), transmission electron microscope (CM200, Philips), laser Raman spectrometer (Renishaw, RM 1000, He–Ne laser excitation line at 633.0 nm), X-ray diffractometer (JEOL JDX 8P) and thermogravimetry analysis (SDT Q600, TA, USA).
Results and discussion
In this study, the catalyst precursor with support was introduced after the CVD reactor reaches the 680°C. The pyrolysis of nickel formate in the gas flow system leads to the formation of nickel nanoparticles. Mechanism of formation of nickel nanoparticles from nickel formate is discussed in the literature (Edwards et al. 1997). The time and cost has been reduced in this process because both calcination and reduction is avoided in the preparation of catalyst. The prepared nickel formate precursor was characterized by X-ray diffraction method. Figure 1 show the XRD pattern of prepared nickel formate. They have characteristic peaks A, B, C and D (JCPDS 14-0813). The Table 1 gives summary of catalyst precursor, wt% of metal in support, CVD parameters, yields and morphology of CNFs produced.
The melting point of NaCl is 800°C, so all the reactions were performed below this temperature. A variety of experiments were conducted with argon–acetylene mixture with various volume ratios (20:1, 15:1, 10:1, 5:1, 3:1, 2:1 and 1:1) at reaction temperatures between 600 and 740°C (Figs. 3, 4). The carbon deposit at 680°C was maximum. The metal loading percentage was fixed to 3 wt% in catalyst support in order to avoid sintering effect of catalyst at high temperatures. So all the reaction were performed at 680°C with gas flow of 200 sccm: 20 sccm (Ar:C2H2) for 15 min.
Figure 5 shows the SEM morphologies of purified CNFs grown on nickel formate/NaCl and nickel formate/KCl. Because of their twisting morphology it is difficult to measure the length of the CNFs from the SEM micrographs, but the length can be estimated to be more than several tens of micrometers. As seen in SEM image, the catalytic particles and support were removed successfully by the purification. Structural details of the products were further investigated by TEM. Figure 6 show the TEM images of the purified CNFs grown on nickel formate/NaCl and nickel formate/KCl. There is clear evidence form TEM micrograph for the formation of CNFs. The diameters of the CNFs are in the range of 10–50 nm.
TGA results in Fig. 7 show the weight loss of CNFs during the oxidation under air atmosphere. The TGA curve in the temperature range of 30–700°C shows a total weight loss of 60 wt% for products grown on nickel formate/NaCl and nickel formate/KCl. There is a little bit of amorphous carbon in our samples because there are negligible mass losses below 400°C in the TGA spectrum. According to curve c, the purity of the purified CNFs increases to 99.0% after purification process.
The representative Raman spectra (Fig. 8) of the samples show the typical features of CNFs. In detail, there exist two strong peaks at 1,592, 1,596 cm−1 and 1,328, 1,334 cm−1 corresponding to the typical Raman peaks of graphitized carbon. The peak at 1,592 and 1,596 cm−1 is attributed to the Raman-active E2g in-plane vibration mode and is related to the vibration of sp2-bonded carbon atoms in a two-dimensional hexagonal lattice. The peak at 1,328 and 1,334 cm−1 is associated with vibrations of carbon atoms with dangling bonds in plane terminations of disordered graphite (Eklund et al. 1995). The intensity of D-band peak is stronger than that of G-band peak, which originates from the in-plane defects of the product preventing the layer from extending.
Figure 9 shows the XRD pattern of the purified samples. It contains two characteristic peaks at about 26.0° and 43.5°, indexed with 002 and 101 diffraction planes of hexagonal graphite (JCPDS card files, no. 41-1487), respectively. No peaks induced by impurities can be observed in the XRD pattern.
Conclusions
In this work, we have developed a simple method to synthesize CNFs in the bulk scale by CVD method. Nickel formate was used as catalyst precursor because it forms nickel nanoparticles on pyrolysis. Sodium chloride and potassium chloride which are easily soluble in water and non-toxic are used as supports. The yield of carbon nanofibers is high on sodium chloride support when compare to potassium chloride support. The uses of acid and toxic materials are completely avoided. By this route we can grow CNFs in large scale by using low cost precursors in an economical and environmental friendly way.
References
Bahome MC, Jewell LL, Padayachy K, Hildebrandt D, Glasser D, Datye AK, Coville NJ (2005) Fischer–Tropsch synthesis over iron catalysts supported on carbon nanotubes. Appl Catal A 287:60–67. doi:10.1016/j.apcata.2005.03.029
Baker RTK (1989) Catalytic growth of carbon filaments. Carbon 27:315–323. doi:10.1016/0008-6223(89)90062-6
Biris AR, Dervishi E, Simon S, Lupu D, Misan I, Iancu C, Clichici SV, Xu Y, Watanabe F, Biris AS (2010) Analytic studies of high quality single wall carbon nanotubes synthesized on a novel Fe:Mo:MgO catalyst. Phys E 43:552–558. doi:10.1016/j.physe.2010.09.014
Brataas A (2008) Nanoelectronics: spin surprise in carbon. Nature 452:419–420. doi:10.1038/452419a
Demicheva OV, Meshkov GB, Sinitsyna OV, Tomishko AG, Yaminsky IV (2008) Multiwall carbon nanotube tips for scanning probe microscopy. Nanotechnol Russ 3:704–709. doi:10.1134/S1995078008110062
Dillon AC, Jones KM, Bekkedahl TA, Kiang CH, Bethune DS, Heben MJ (1997) Storage of hydrogen in single-walled carbon nanotubes. Nature 386:377–379. doi:10.1038/386377a0
Douglas RK, Alexander S (2010) Graphene versus carbon nanotubes for chemical sensor and fuel cell applications. Analyst 135:2790–2797. doi:10.1039/C0AN00262C
Edwards AB, Garner CD, Roberts KJ (1997) In situ QXAFS study of the pyrolytic decomposition of nickel formate dihydrate. J Phys Chem B 101:20–26. doi:10.1021/jp961488m
Eklund PC, Holden JM, Jishi RA (1995) Vibrational modes of carbon nanotubes; spectroscopy and theory. Carbon 33:959–972. doi:10.1016/0008-6223(95)00035-C
Guo T, Nicolaev P, Thess A, Colbert DT, Smalley RE (1995) Catalytic growth of single-walled nanotubes by laser vaporization. Chem Phys Lett 243:49–54. doi:10.1016/0009-2614(95)00825-O
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56–58. doi:10.1038/354056a0
Jan E, Hendricks JL, Husaini L, Burns SMR, Sereno A, Martin DC, Kotov NA (2009) Layered carbon nanotube-polyelectrolyte electrodes outperform traditional neural interface materials. Nano Lett 9:4012–4018. doi:10.1021/nl902187z
Kathyayini H, Reddy KV, Nagy JB, Nagaraju N (2008) Synthesis of carbon nanotubes over transition metal ions supported on Al(OH)3. Indian J Chem (Sec B) 47A:663–668
Milne WI, Teo KBK, Amaratunga GAJ, Legagneux P, Gangloff L, Schnell JP, Semet V, Thien VB, Groening O (2004) Carbon nanotubes as field emission sources. J Mater Chem 14:933–943. doi:10.1039/b314155c
Nagaraju N, Konya Z, Fonseca A, Nagy JB (2002) Alumina and silica supported metal catalysts for the production of carbon nanotubes. J Mol Catal A Chem 181:57–62. doi:10.1016/S1381-1169(01)00375-2
Seifi M (2011) Dependence of pore size on catalyst concentration used in synthesis carbon nanotubes. Phys E 43:998–1001. doi:10.1016/j.physe.2010.11.034
Su M, Zheng B, Liu J (2000) A scalable CVD method for the synthesis of single-walled carbon nanotubes with high catalyst productivity. Chem Phys Lett 322:321–326. doi:10.1016/S0009-2614(00)00422-X
Wang Y, Shi Z, Yin J (2010) Unzipped multiwalled carbon nanotubes for mechanical reinforcement of polymer composites. J Phys Chem C 114:19621–19628. doi:10.1021/jp107151e
Acknowledgments
The authors would like to thank Defense Research and Development Organisation (DRDO), Government of India for financial assistance and also SAIF, IITB and Instrumental facility, CECRI for providing analytical services.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Ravindra, R., Badekai Ramachandra, B. High yield synthesis of carbon nanofibers in an environmental friendly route. Appl Nanosci 1, 103–108 (2011). https://doi.org/10.1007/s13204-011-0014-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-011-0014-3