Abstract
The paper reports the adsorption studies of asphaltenes of Iran’s heavy crude oil on some natural and synthetic alumino-silicates. Asphaltenes were precipitated using n-heptane. Toluene was used as a precipitating solvent of asphaltenes and several zeolites including 4A, ZSM-5, Clinoptilolite, and La-modified bentonite (Phoslock) as adsorbents. FTIR analysis indicated the asphaltenes which comprise a complex of aromatic, aliphatic, and polar compounds. The pore size and outer surface area of the adsorbents were determined by BET method and the following order was found between outer surface areas: ZSM-5 (238.27 m2 g−1) > Clinoptilolite (28.75 m2 g−1) > Phoslocks (27.92 m2 g−1) > zeolite 4A (21.11 m2 g−1) > Zeolite 13X (317.24 m2 g−1). Besides, the adsorption isotherms were investigated with the conventional isotherm models and it was indicated that the Langmuir isotherm fitted the experimental data. Zeolite 13X with the highest specific surface area and pore size exhibited the maximum adsorption capacity, indicating that there is a direct relationship between surface area and adsorption capacity. However, it seems that the pore size effect is more prominent because of the large size of asphaltene’s molecules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The precipitation of asphaltenes in oil and its negative impact on the petroleum industry is an important problem in practice [1]. The increase of asphaltenes content in crude oil increases the tendency to precipitate.
Asphaltenes are soluble in aromatic solvents but insoluble alkanes. They are polar, surface-active and densest [2]. The asphaltenes are comprised of polyaromatic cores adhered to aliphatic chains. The aliphatic chains usually include heteroatoms such as sulfur, oxygen, nitrogen, and some transition metals. Nickel, iron, and vanadium are the most common metals found in asphaltenes [4, 25]. There are multifunctional groups in asphaltenes and they have high molecular weight. Determination of molecular weight of asphaltenes by different physical methods in various solvents concluded that asphaltenes are associated and form aggregates [22].
The adsorption of asphaltenes is studied to prevent productivity losses of petroleum reservoirs.
Adsorption studies are investigated by asphaltene precipitation and then resolubilize in a solvent. According to the concentration of asphaltenes, morphology and nature of the adsorbent and adsorbed asphaltenes and nature of the adsorbents different mechanisms have been developed and multilayer and monolayer adsorptions were reported [8, 9]. Some researchers reported that the adsorption of asphaltene on mineral adsorbents mostly followed the Langmuir adsorption model [10].
Dubey and Waxman [7] studied the adsorption experiments in different solvents and on some mineral surfaces. The type I and II isotherms of Langmuir were followed by them [8, 9]. Nassar [19] studied the adsorption of asphaltenes onto alumina nanoparticles in 2010. The results agreed well with the Langmuir isotherm model. Nassar et al. [18, 20] studied the oxidation of asphaltenes and adsorption onto six different nanometal oxides. The Langmuir model was well fitted for all the six nanoparticles. In 2012, they were used from iron oxide nanoparticles for rapid adsorption and enhanced catalytic oxidation of thermally cracked asphaltenes. The results fit well to the Langmuir model. Franco and Nassar [8] chose 12 types of nanoparticles and investigated their chemical effect on asphaltenes adsorption in 2013, which the results were fitted to both the Freundlich and Langmuir models.
Different inorganic materials could be used for asphaltene adsorption. Phoslock is a type of the modified bentonite in that some Na+ are replaced by La3+ [11].
Zeolites are called as magic materials. They have a three-dimensional crystalline structure and are considered as a type of alumino-silicates. They are comprised of units of AlO4 and SiO4 tetrahedral, arranged to result in an open structure. Some zeolites are naturally found in the environment and many of them are synthesized. The zeolites of A, X, and ZSM-5 are of three types of synthetic zeolites [14]. A-type zeolites have different pore sizes, which the most common sizes are 3, 4 and 5 Å. A-type zeolites are usually synthesized in alkali media. In the synthesis of zeolite 4A, the ratio of SiO2/Al2O3 varies between 1.92 and 2.08 to give a pore opening of 0.38 nm [21]. The pore size of zeolite X is 0.74 nm instead of 0.38 nm in zeolite A. The ratio of SiO2/Al2O3 in zeolite X is between 2.0 and 3.0. The SiO2/Al2O3 ratio of ZSM-5 varies in a wide range and range of pore size is 0.54–0.56 nm [3].
This study aimed to study the adsorption isotherms of asphaltenes over some mineral absorbents (natural and synthetic zeolites and Phoslock). The relationship between structure and sorption is investigated. The asphaltenes were precipitated from Iran crude oil. The structure of the asphaltenes is investigated by FTIR and the specific surface area of the adsorbent was studied by BET surface area.
Materials and methods
Materials
Asphaltenes in Iranian crude oil were precipitated. The general property analysis of crude oil is as Table 1. The heavy oil studied in this work is from a reservoir located in the South of Iran.
Methods
Precipitation of asphaltenes of crude oil
Asphaltenes of the crude oil were precipitated using a standardized procedure [4]. N-heptane and crude oil were mixed at a volume ratio of 20/1. At ambient temperature, the mixture was sonicated for 2 h. Then, it was stirred at 300 rpm for 22 h. The precipitated fraction was filtered using a 0.45 μm membrane filter. Suspension of asphaltenes in heptane was centrifuged at 5000 rpm for 15 min and left to rest for a day. The sample was washed with n-heptane to reach a shiny black color for asphaltenes. Then, the sample was dried at 25 °C for 12 h. It was dissolved in toluene for preparing solutions from 50 to 1500 ppm [14].
Adsorbent characterization
The pore size and BET-specific surface area of the adsorbent were determined using the N2 adsorption/desorption isotherms at − 196 °C, using an NOVA 2000. The FTIR spectra of the sample were recorded in transmittance using a Bruker spectrometer (model TENSOR 27) in the range 400–4000 cm−1. The X-ray diffraction (XRD) pattern of adsorbents was recorded at 2θ = 5–70° by a Philips PW1800 diffractometer and Cu Kα radiation (λ = 1.54 Å).
Adsorption experiments
The calibration curve of absorbance versus asphaltenes concentration at 400 nm was constructed from the prepared solutions with the known concentrations [1, 4, 15, 17]. A linear relationship was between adsorption and concentration up to 120 mg L−1. Toluene was used for dilution and a blank. In each test, 0.25 mg of the adsorbent was added to asphaltenes solutions of constant volume (10 mL) and stirred at 700 rpm for 4 h at 25 °C to reach the sorption equilibrium [14].
Results and discussion
The XRD patterns of the adsorbents are shown in Fig. 1. Generally, the characteristic peaks of zeolites appeared at 2θ around 5–10 and 20–27 degrees. In the XRD pattern of zeolite 4A, the sharp peaks at 2θ of 7, 9, 23, 26, 29, and 32 reveal the structure of A zeolite. All zeolite NaA samples were identified as a single-phase zeolite NaA (JCPDS card 430142) [13]. The presence of characteristic peaks of ZSM-5 zeolite at the XRD pattern of the sample indicated the ZSM-5 structure [24]. The appearance of characteristic peaks at 2θ around 7, 10, 12, 16, 20, 23, 27, and 32 approved the zeolite 13X [12]. Phoslock which is La-modified bentonite exhibited the main peak at 2θ of 5.8 which is in agreement with the literature [5]. Finally, the peaks at 2ϴ of 11, 15, 20, 26, 31, 33, 35, and 38 approved the clinoptilolite zeolite [16].
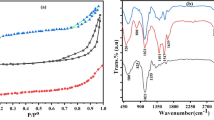
By FTIR, spectrum is shown in Fig. 2, the structure and functional groups of the asphaltenes studied. According to this figure, asphaltenes show signals that correspond to the aliphatic and aromatic structure. The aromatic, aliphatic, and polar functional groups were characterized in asphaltenes. The bands at 750, 813, and 861 cm−1 corresponded to the out-of-plane CH bending in 1,2-disubstituted aromatic, 1,4-substituted aromatic, and 1,3-disubstituted aromatic, respectively. The band at 3452 cm−1 corresponds to the OH tension band and NH. The C=C aromatic bond appears at 1596 cm−1 and heteroatom vibration frequency such as sulfur and nitrogen is displayed between 1080 and 1030 cm−1. For the aliphatic characteristic, CH-stretching vibrations of CH2 and CH3 were assigned at 2920 and 2858 cm−1, while the peaks at 1449 and 1371 cm−1 represented the CH-bending vibration of CH2 and CH3 [6, 23, 26].
The specific surface area and pore size of the adsorbent are presented in Table 2. From the specific surface area and adsorption capacity of the adsorbent, we could not find any direct relationship between them.
Equilibrium studies and adsorption isotherms
In the isotherm study of asphaltene adsorption on the adsorbents, two types of common isotherms, i.e., Langmuir and Freundlich isotherm models, were considered and the experimental data were fitted. The isotherm equations of Langmuir and Freundlich were presented in Eqs. 1 and 2, respectively:
where qe is the amount of adsorbed asphaltenes (mg/g), Ce is the equilibrium concentration of asphaltenes (mg/L), KF, Freundlich constant, is roughly an indicator of the adsorption capacity [(mg/g)(L/mg)1/n], and 1/n is the adsorption intensity factor (no unit) [14]. KL is the Langmuir equilibrium adsorption constant and is related to the affinity of binding sites (L/mg). The qm is the maximum amount of adsorbed asphaltenes per unit surface area of nano absorbents for complete monolayer coverage (mg/g) [18].
Figure 3 shows the experimental data for the rate of adsorption of the asphaltenes on different absorbents.
According to this figure, the following order results for the rate of adsorption of the asphaltenes on different absorbents:
Zeolite 13X > ZSM-5 > Clinoptilolite > Phoslocks > zeolite 4A.
Zeolite 4A due to having the small pores size has attracted very low asphaltenes. That’s why, this type of zeolites was excluded to remove asphaltenes of crude oil. Zeolite 13X due to having a relatively larger pore size and specific surface area (according to Table 2) exhibit the highest amount of asphaltenes adsorption (Fig. 3). The zeolite ZSM-5 adsorbs the asphaltenes close virtually based on qm. In higher concentrations, the adsorption rate of asphaltene onto zeolite ZSM-5 is high. According to Table 2, the pore size and specific surface area of ZSM-5 are relatively high, and the higher adsorption rate is attributed to the large pore size of ZSM-5. Generally, clinoptilolite, 4A, and phoslock due to having small pore size could not adsorb large asphaltene molecules considerably and exhibit the minimum adsorption rate. Furthermore, for the first time, the adsorptive performance of Phoslock was evaluated to remove asphaltenes of crude oil.
At low concentrations of asphaltenes (< 500 ppm), the rate of adsorption of asphaltenes on phoslock is more than that on ZSM-5, whereas at higher asphaltene concentration (> 500 ppm), the adsorption on the ZSM-5 is more than that on Phoslock. It is concluded that at high concentration of asphaltenes, the pore size directly affects the adsorption capacity of the adsorbent.
Experimental data obtained from experiments were studied with two Langmuir isotherm and Freundlich. Figure 4 shows the results of experimental data fitted with Langmuir isotherm. Figure 5 shows the results obtained from experimental data and fitted with Freundlich isotherm.
The corresponding isotherm parameters and determination coefficient (R2) for the different adsorbents are presented in Table 3. By considering the Figs. 4 and 5 and Table 3, it is observed that the experimental data are in a good agreement with Langmuir isotherm.
The maximum adsorption capacity of Zeolite 13X, ZSM-5, Clinoptilolite, and Phoslock resulted at equilibrium concentration below 500, 440, 400, and 380 mg/L, respectively, as illustrated in Fig. 4. It means that most of the equilibrium solution concentrations were relatively high. The result of Ce data indicated that the Langmuir equation was best, rather than the prediction of qe, below the equilibrium value, even though the R2 values are high.
Conclusions
The asphaltene content of crude oil was successfully precipitated from Iran crude oil, and its sorption was studied on four adsorbents including three zeolites and the modified bentonite (Phoslock). The experimental data were fitted with sorption isotherms of Freundlich and Langmuir models. The experimental data for the adsorption of asphaltenes were well fitted with Langmuir isotherm. The equilibrium studies for the asphaltene uptake by the adsorbents showed that the zeolite 13X has the highest adsorption capacity for asphaltene sorption and a direct relationship between the sorption capacity and specific surface area and pore size of the adsorbents resulted. According to the large size of asphaltene molecules, the pore size of adsorption is a prominent factor on asphaltenes adsorption. However, more detailed studies are needed to find more effective parameters on asphaltene adsorption.
References
Acevedo S, Ranaudo MA, Escobar G, Gutiérrez L, Ortega P (1995) Adsorption of asphaltenes and resins on organic and inorganic substrates and their correlation with precipitation problems in production well tubing. Fuel 74:595–598. https://doi.org/10.1016/0016-2361(95)98363-J
Adams JJ (2014) Asphaltene adsorption, a literature review. Energy Fuel 28:2831–2856. https://doi.org/10.1021/ef500282p
Alkafeef SF, Al-Marri SS (2016) Kinetics and isotherms of asphaltene adsorption in narrow pores. Curr Opin Colloid Interface Sci 24:44–51. https://doi.org/10.1016/j.cocis.2016.06.005
Cortés FB, Mejía JM, Ruiz MA, Benjumea P, Riffel DB (2012) Sorption of asphaltenes onto nanoparticles of nickel oxide supported on nanoparticulated silica gel. Energy Fuel 26:1725–1730. https://doi.org/10.1021/ef201658c
De Castro LF, Brandão VS, Bertolino LC, de Souza WFL, Teixeira VG (2019) Phosphate adsorption by montmorillonites modified with lanthanum/iron and a laboratory test using water from the Jacarepaguá Lagoon (RJ, Brazil). J Braz Chem Soc 30:641–657. https://doi.org/10.21577/0103-5053.20180236
Douda J, Alvarez R, Bolaños JN (2008) Characterization of maya asphaltene and maltene by means of pyrolysis application. Energy Fuel 22:2619–2628. https://doi.org/10.1021/ef800024p
Dubey ST, Waxman MH (1991) Asphaltene adsorption and desorption from mineral surfaces. SPERE 6:389–395. https://doi.org/10.2118/18462-PA
Franco CA, Nassar NN, Ruiz MA, Pereira-Almao P, Cortés FB (2013) Nanoparticles for inhibition of asphaltenes damage: adsorption study and displacement test on porous media. Energy Fuel 27:2899. https://doi.org/10.1021/ef4000825
Franco C, Patiño E, Benjumea P, Ruiz MA, Cortés FB (2013) Kinetic and thermodynamic equilibrium of asphaltenes sorption onto nanoparticles of nickel oxide supported on nanoparticulated alumina. Fuel 105:408–414. https://doi.org/10.1016/j.fuel.2012.06.022
Gonzalez G, Moreira MBC (1991) The wettability of mineral surfaces containing adsorbed asphaltene. Colloids Surf 58:293–302. https://doi.org/10.1016/0166-6622(91)80229-H
Haghseresht F, Wang S, Do DD (2009) A novel lanthanum-modified bentonite, Phoslock, for phosphate removal from wastewaters. Appl Clay Sci 46:369–375. https://doi.org/10.1016/j.clay.2009.09.009
Hiraki T, Nosaka A, Okinaka N, Akiyama T (2009) Synthesis of zeolite-X from waste metals. ISIJ Int 49:1644–1648. https://doi.org/10.2355/isijinternational.49.1644
Hosseini SA (2015) Optimization of synthesis conditions of zeolite 4A from nepheline syenite. Int J Mater Chem Phys 1:93–98
Hosseini SA, Hagjoo R, Baninaam M (2019) Adsorption of asphaltenes onto CaO, CoO, FeO and ZnO supported on 13X zeolite: an isothermal study. Petrol Sci Technol 37:2330–2337. https://doi.org/10.1080/10916466.2018.1522336
León O, Contreras E, Rogel E, Carbognani L, Espidel J, Dambakli G, Acevedo S (2002) Adsorption of native resins on asphaltene particles: a correlation between adsorption and activity. Langmuir 18:5106–5112. https://doi.org/10.1021/la011394q
Mansour N, Rikhtegar N, Ahmad Panahi H, Atabi F, Karimi Shahraki B (2013) Porosity, characterization and structural properties of natural zeolite—Clinoptilolite—as a sorbent. Environ Protect Eng 39(1):139. https://doi.org/10.5277/EPE130111
Marczewski AW, Szymula M (2002) Adsorption of asphaltenes from toluene on mineral surface. Colloids Surf A 208:259–266. https://doi.org/10.1016/S0927-7757(02)00152-8
Nassar NN, Hassan A, Pereira-Almao P (2011) Metal oxide nanoparticles for asphaltene adsorption and oxidation. Energy Fuels 25:1017–1023. https://doi.org/10.1021/ef101230g
Nassar NN (2010) Asphaltene adsorption onto alumina nanoparticles: kinetics and thermodynamic studies. Energy Fuel 24:4116–4122. https://doi.org/10.1021/ef100458g
Nassar NN, Hassan A, Carbognani L, Linares FL, Pereira-Almao P (2012) Iron oxide nanoparticles for rapid adsorption and enhanced catalytic oxidation of thermally cracked asphaltenes. Fuel 95:257–262. https://doi.org/10.1016/j.fuel.2011.09.022
Pfenninger A (1999) Manufacture and use of zeolites for adsorption processes. Mol Sieves 2:163
Pemyeszi T, Dékány I (2001) Sorption and elution of asphaltenes from porous silica surfaces. Colloids Surf A 194:25–39. https://doi.org/10.1016/S0927-7757(01)00574-X
Rodrigues Coelho R, Hovell I, Lopez Moreno E, Lopes de Souza A, Rajagopal K (2007) Characterization of functional groups of asphaltenes in vaccum residues using molecular modelling and FTIR techniques. Petrol Sci Technol 25:41–54. https://doi.org/10.1080/10916460601054198
Wang P, Shen B, Shen D, Peng T, Gao J (2007) Synthesis of ZSM-5 zeolite from expanded perlite/kaolin and its catalytic performance for FCC naphtha aromatization. Catal Commun 8:1452–1456. https://doi.org/10.1016/j.catcom.2006.12.018
Wang S, Liu Q, Tan X, Xu C, Gray MR (2016) Adsorption of asphaltenes on kaolinite as an irreversible process. Colloids Surf A 504:280–286. https://doi.org/10.1016/j.colsurfa.2016.05.086
Wu H, Kessler MR (2015) Asphaltene: structural characterization, molecular functionalization, and application as a low-cost filler in epoxy composites. RSC Adv 5:24264–24273. https://doi.org/10.1039/C5RA00509D
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baninaam, M., Hosseini, S.A. & Abbasian, A.R. Isothermal study of asphaltene adsorption over 4A, 13X, ZSM-5, clinoptilolite zeolites, and phoslock. Appl Petrochem Res 10, 49–54 (2020). https://doi.org/10.1007/s13203-020-00243-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-020-00243-x