Abstract
Considerable attention has been paid recently to crystal engineering; which involves the design and preparation of new crystalline molecular solids with desired properties [1,2,3,4]. Crystalline materials with specific properties find applications in petrochemical industry for separation and purification. Moreover, crystal engineering provides products designed for manufacturing catalysts and high-valued chemicals for specific purposes. Recently, crystalline materials find application in pharmaceutical, food and microelectronic industries [5]. The main two strategies that are used for crystal engineering are based on hydrogen bonding and coordination bonding [6]. Since the hydrogen bonding is usually stronger and more directional than the other methods, more new crystal materials have been prepared based on this method. We are here able to design crystalline materials based on hydrogen bonding and study their solid state structures. N, N-dimethylformamide (DMF)-solvate of thiocyanuric acid (TCUA) and dimethyl sulfoxide (DMSO)-solvate of thiocyanuric acid (TCUA) were successfully prepared at room temperature in the presence of aqueous solution of sodium nitrate (NaNO3). To the best of our knowledge, this study presents the easy, modest, and rapid method to prepare co-crystal formation based on thiocyanuric acid (TCUA) and solvent-containing hydrogen bonding functionality. In this paper, we present the most effective method to synthesize the co-crystals of (TCUA), and as evidence, the crystal structure of (TCUA) in DMF is fully studied and presented in this paper. The N,N-dimethylformamide (DMF)-solvate of thiocyanuric acid (TCUA) was successfully prepared at room temperature, and was characterized spectroscopically by nuclear magnetic resonance (NMR) and single-crystal X-ray diffraction (SXRD). The asymmetric unit of the title compound contains one molecule of thiocyanuric acid (TCUA) features an almost planar six-membered ring having exocyclic C-S thione double bonds and one molecule of N,N-dimethylformamide (DMF). It was crystallized in the monoclinic, P21/c with unit cell parameters of a = 9.6255 (4) Å, b = 12.6864 (5) Å, c = 9.1367 (4) Å, β = 90.095 (2)°, V = 1115.71 (8) Å3, Z = 4. The structure is composed of 1-D TCUA ribbons formed via N–H–S hydrogen bonds. The ribbons are separated by DMF molecules, which are bridged to the ribbons by N–H–O hydrogen bonds. The ribbons and their DMF molecules form 2-D sheets which are in turn π-stacked to build up a layered, 3-D structure. The proton and carbon-13 NMR studies confirmed the formation of such solvate between DMF and TCUA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thiocyanuric acid (TCUA) is a tribasic, weak acid with acid first, second, and third dissociation constants of 1.99 × 10−6, 3.98 × 10−9, and 3.98 × 10−12, respectively. This polyprotic nature of TCUA permits different degree of deprotonation, and hence, various forms of thiolate and amide ligands for metals and TCUA salts. It is a planar, highly symmetric molecule (D 3h point group) and exists as two tautomers: the trithiol form (I) and the trithione form (II), as illustrated in Eq. (1) [7,8,9]:

Each tautomer has three hydrogen bond donor and three hydrogen acceptors at its nitrogen and sulfur atoms. This unique feature enables TCUA to construct self-assembled, supramolecular 1-D chain and 2-D porous structures based on hydrogen bonding [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21]. The trithiol form (I) is predominant in metal complexes [8–11, 15], whereas the trithione form(II) has been observed to exist in the mono-lithiated hexamethylphosphoramide complex and in the ammonium salts and solvent adducts of TCUA [7, 8, 12,13,14, 16,17,18, 22]. For instance, the triethylammonium salt of TCUA has two polymorphs with 1-D chain structures based on N–H–S and N–H–N hydrogen bonds [16, 23]. On the other hand, the tripropylammonium salt of TCUA has a 2-D structure stabilized by N–H–S and N–H–N hydrogen bonds. The adduct between melamine and TCUA adopts 2-D porous sheets based on planar hexamers connected by N–H–S and N–H–N hydrogen bonds. The melamine/TCUA sheets are stacked to form a 3-D channeled, layered structure [14]. The adduct of 4,4′-bipyridine with TCUA is also constructed by 2-D porous sheets layered into 3-D channeled network, where N–H–S and N–H–N hydrogen bonds are responsible for the self-assembly of 4,4′-bipyridine and TCUA into 2-D sheets [12]. The acetone-solvate of TCUA, [(TCUA)2 (Me2CO)], is made of 2-D, pseudo-hexagonal planar net, generated by three pairs of N–H–S hydrogen bonds without involvement of acetone in hydrogen bonding. The acetone molecules are hydrogen bonded to hexagons by N–H–O bonds. The acetone molecules also act as pillars between the hexagon layers and result in 3-D channeled network, where the channel cavity are capped by acetone methyl groups [7, 12]. On the other hand, the methanol solvate of TCUA, [(TCUA)3 (MeOH)], is composed of 1-D, zigzag chains of TCUA via two pairs of N–H–S hydrogen bonds. The TCUA chains are crossed by C–H–S and S–S interactions and are, at the same time, separated by methanol molecules which are also hydrogen bonded with each other and hydrogen bonded to TCUA molecules via N–H–O bonds, generating a 3-D hydrogen-bonded ntework [7].
We report in this paper the crystal structure of N, N-dimethylformamide (DMF) solvate of TCUA, which was discovered accidently during our attempt to prepare covalent organic framework (COF) of TCUA through the oxidation of thiol functional groups to disulfide functional groups in DMF using a mild oxidant of sodium nitrate at room temperature.
Experimental
Materials
Thiocyanuricacid (TCUA, C3H3N3S3, > 98.0%, TCI), sodium nitrate (NaNO3, ReagentPlus, ≥ 99.0%, Sigma-Aldrich), N,N-dimethylformamide (DMF, HPLC, ≥ 99.9%, Sigma-Aldrich) were commercially available and were used without further purification. Ultrapure deionized water (18.2 MΩ cm) was obtained from a Milli-Q water purification system (Millipore, Billerica, MA, USA).
Synthesis procedure
The synthesis of DMF solvate of TCUA (RA5403) was carried out by combining a solution of TCUA (21.27 mg) in DMF (2.0 mL) with an aqueous solution of NaNO3 (2.0 mL, 1.0 M). The reaction solution was left at room temperature for 3 days, whereupon yellow crystals of RA5403 were obtained. The resulting crystals were filtered off and were washed three times with DMF/water (1:1 v/v). The needle-shaped crystals were obtained in 91% yield.
Analytical and physical characterizations
Proton (600 MHz) and carbon (150 MHz) nuclear magnetic resonance spectra were taken using JEOL ECA-600 Spectrometer. All chemical shifts were referenced to the residual solvent protons/carbons of the solvent indicated. 1H NMR (600 MHz, DMSO-6, δ) 7.94 (s, 1H), 2.88 (s, 3H), 2.72 (d, 3H). 13C NMR (150 MHz, DMSO-6, δ) 171.87, 162.32, 35.81, 30.79.
X-ray crystallography
Data were collected on a Bruker APEX-II D8 Venture area diffractometer, equipped with graphite monochromatic Mo K α radiation, λ = 0.71073 Å at 100 (2) K. Cell refinement and data reduction were carried out by Bruker SAINT. SHELXT [13, 14] was used to solve structure. The final refinement was carried out by full-matrix least-squares techniques with anisotropic thermal data for nonhydrogen atoms on F. CCDC 1455209 contains the supplementary crystallographic data for this compound, which can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Discussion
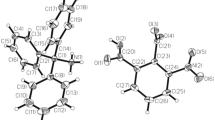
Thiocyanuric acid (TCUA) is very well known to form polymers through extensive hydrogen bonds [7,8,9,10,11,12,13,14,15,16,17,18,19,20,, 8, 16, 18, 24]. Here, we were able to prepare a layered, 3D-structure of TCUA with DMF, where the TCUA molecule N–H bonds act as H-bond donors and TCUA molecule C–S bonds and DMF molecule C=O bonds serve as H-bond acceptors. The crystal structure of DMF-solvate of TCUA, reported herein, revealed that TCUA adopts the trithion form (II, Eq. 1). The asymmetric unit of the DMF solvate of TCUA is based on a hydrogen bond (N–H–O) bridge connecting a TCUA molecule and a DFM molecule solvent, as shown below in Fig. 1. The crystallographic data and refinement information are summarized in Table 1. The selected bond lengths and bond angles are listed in Table 2. The asymmetric unit is containing of one independent molecule as shown in Fig. 1. All the bond lengths and angles are in normal ranges [25]. In the crystal packing, Figs. 2 and 3, molecules are linked via intermolecular hydrogen bonds (Table 3).
The crystal structure packing of hydrogen-bonded TCUA ribbons which are hydrogen bonded to DMF molecules forming a 2-D sheets, where these sheets are turned form a layered, 3-D structure (viewed along c-axis); yellow: sulphur, red: oxygen; sky blue: nitrogen, grey: carbon, white: hydrogen, dashed lines: H-bonds
The TCUA molecules form a one-dimensional zigzag ribbons, where the TCUA molecules within each ribbon are bridged by N–H–S hydrogen bonds. The TCUA ribbons are separated by DMF molecules, which are in turn hydrogen bonded to TCUA molecules through N–H–O bridges in an alternative fashion between two TCUA ribbons, as shown in Fig. 2.
The TCUA ribbons along with their H-bonded DMF molecules form a two-dimensional sheet, as shown in Fig. 2. These sheets are in turn paralleled to other sheets through Van der Waals forces (π stacking), converting the 2-D sheets into a layered, three-dimensional structure. Figure 3 shows the TCUA ribbons which are paralleled in the different sheets.
The crystal structure pattern of our DMF solvate of TCUA is similar to those observed previously for triethylammonium thiocyanurate [9], methanol solvate of TCUA [7], tripropylammonium thiocyanurate, and the second polymorph of triethylammonium thiocyanurate [18].
The formation of the DMF solvate of TCUA was also confirmed by both liquid-state proton and carbon-13 NMR investigations. The proton NMR spectrum showed only DMF peaks at (DMSO-6, δ) 7.94, 2.88, and 2.72 which could be due to the hydrogen bond, N–H–O, formation between DMF and TCUA, reflecting the partial preservation of the DMF solvate of TCUA in the liquid state. On the other hand, carbon-13 NMR spectrum showed both TCUA and DMF carbons at (DMSO-6, δ) 171.87, 162.32, 35.81, 30.79.
Conclusion
We prepared readily a layered, 3-D DMF solvate of TCUA at room temperature in the presence of a mild oxidant of NaNO3. The unsuccessful attempt for synthesizing a COF via oxidizing the TCUA thiol functional groups into disulphide functional groups could be attributed to the thermodynamic preference of TCUA to adopt the trithione form; and the probability need for harsher conditions of temperature, pressure, and oxidation. The structure of DMF solvate of TCUA was studied by SXRD. The NMR studies confirmed the formation of this solvate (see the supporting info).
References
Liess A, Lv A, Arjona-Esteban A, Bialas D, Krause AM, Stepanenko V, Stolte M, Würthner F (2017) Exciton coupling of merocyanine dyes from H- to J-type in the solid state by crystal engineering. Nano Lett 17(3):1719–1726
Saha S, Desiraju GR (2017) Crystal engineering of hand-twisted helical crystals. J Am Chem Soc 139(5):1975–1983
Masunov AE, Tannu A, Dyakov AA, Matveeva AD, Freidzon AY, Odinokov AV, Bagaturyants AA (2017) First principles crystal engineering of nonlinear optical materials. I. Prototypical case of urea. J Chem Phys 146(24):244104
Wang C, Paul S, Wang K, Hu S, Sun CC (2017) Relationships among crystal structures, mechanical properties, and tableting performance probed using four salts of diphenhydramine. Cryst Growth Des 17(11):6030–6040
Patience DB (2002) Crystal engineering through particle size and shape monitoring, modeling, and control. University of Wisconsin-Madison, Madison
Aakeröy CB, Seddon KR (1993) The hydrogen bond and crystal engineering. Chem Soc Rev 22:397–407
Dean PAW, Jennings M, Houle TM, Craig DC, Dance IG, Hook JM, Scudder ML (2004) Crystal packing in tetraphenylphosphonium salts of trithiocyanuric acid and its methanol solvate. Cryst Eng Comm 6:543–548
Krepps MK, Parkin Sean, Atwood DA (2001) Hydrogen bonding with sulfur. Cryst Grow Des 1:291–297
Henke KR, Hutchison AR, Krepps MK, Parkin S, Atwood DA (2001) Chemistry of 2,4,6-trimercapto-1,3,5-triazine (TMT): acid dissociation constants and group 2 complexes. Inorg Chem 40(17):4443–4447
Chan C-K, Cheung K-K, Che C-M (1996) Structure and spectroscopic properties of a luminescent inorganic cyclophane from self-assembly of copper(I) and two ligand components. Chem Commun 2:227–228
Tzeng B-C, Che C-M, Peng S-M (1997) Luminescent gold(i) supermolecules with trithiocyanuric acid. Crystal structure, spectroscopic and photophysical properties. Chem Commun 18:1771–1772
Pedireddi VR, Chatterjee Swati, Ranganathan Anupama, RAo CNR (1997) Noncovalent synthesis of layered and channel structures involving sulfur-mediated hydrogen bonds. J Am Chem Soc 119:10867–10868
Clegg W, Davies JE, Elsegood MRJ, Lamb E, Longridge JJ, Rawson JM, Snaith R, Wheatley AEH (1998) The first structural studies on trithiocyanuric acid: the solid state structures of its HMPA adduct and its mono-lithiated HMPA complex. Inorg Chem Commun 1(2):58–60
Ranganathan A, Pedireddi VR, Rao CNR (1999) Hydrothermal synthesis of organic channel structures: 1:1 hydrogen-bonded adducts of melamine with cyanuric and trithiocyanuric acids. J Am Chem Soc 121:1752–1753
Hunks WJ, Jennings MC, Puddephatt RJ (1999) Gold complexes of trithiocyanuric acid: a two-dimensional polymer assembled through Gold(I). Gold(I) interactions. Inorg Chem 38(26):5930–5931
Yang Y (2010) Tripropyl-ammonium trithio-cyanurate. Acta Crystallogr Sect E 66:o2793
Li HP, Ng SW (2011) Triethyl ammonium 2,4,6-trisulfanylidene-1,3,5-triazinan-1-ide. Acta Crystallogr Sect E 67(9):o2473
Wang P, Zeng H, Wu X, Wang P, Zeng H, Wu X (2016) The second polymorph of triethylammonium 2,4,6-trisulfanylidene-1,3,5-triazinan-1-ide, C9H18N4S3. Zeitschrift für Kristallographie New Cryst Struct 231:61–63
Breuers V, Frank W, Breuers V, Frank W (2016) The crystal structure of 2-chloro-1,3-bis(2,4,6-trimethylphenyl)-4,4-dimethyl-1,3,2λ3,4-diazaphosphasiletidine. Zeitschrift für Kristallographie New Cryst Struct 231:529–532
Sheldrick GM (2007) A short history of SHELX. Acta Crystallogr A 64(1):112–122
Sheldrick GM (1997) SHELXL97. Program for the refinement of crystal structures. University of Göttingen, Germany
Altamimi R, Ghabbour HA, Aldawsari F, AlRuqi OS, Alqahtani N (2017) Crystal structure of 1,1′-(3,4-diphenylthieno[2,3-b]thiophene-2,5-diyl)bis[1-phenyl-methanone], C32H20O2S2. Zeitschrift für Kristallographie New Cryst Struct 232(2):167–169
Li HP, Ng SW (2011) Triethyl-ammonium 2,4,6-trisulfanylidene-1,3,5-triazinan-1-ide. In Acta Crystallogr Sect E 67:2473
He-Ping-Li SWN (2011) Triethyl ammonium 2,4,6-trisulfanylidene-1,3,5-triazinan-1-ide. Acta Crystallogr Sect E 67(9):02473
Allen FH, Kennard O, Watson DG, Brammer L, Orpen AG, Taylor R (1987) Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J Chem Soc Perkins Trans 2 12:S1–S19
Acknowledgements
This work was sponsored by King Abdulaziz City for Science and Technology (KACST), Saudi Arabia (Grant No. 20-0044).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Altamimi, R., Bagabas, A.A., Ghabbour, H.A. et al. Synthesis and crystal structure of N,N-dimethylformamide solvate of thiocyanuric acid. Appl Petrochem Res 7, 181–186 (2017). https://doi.org/10.1007/s13203-017-0191-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-017-0191-4