Abstract
Substantial quantities of energy are required in conventional distillation columns applied in high-purity separation of close-boiling mixtures. To achieve energy saving of distillation, a novel different pressure thermally coupled distillation (DPTCD) was proposed for separating the close-boiling mixture of n-butanol and iso-butanol. Both this intensified energy integration technique and two other processes, namely conventional distillation (CD) and vapor recompression column (VRC), were simulated in process simulator Aspen Plus. The optimization was carried out to determine the optimal values of design and operating variables on the basis of minimizing energy consumption. Subsequently, the energy saving and economic efficiency of the DPTCD scheme were evaluated through the comparison with the other two processes. The results showed that, compared to the CD and VRC processes, the energy consumption of DPTCD process was decreased by 65.21 and 15.79%, respectively, and the total annual cost (TAC) of DPTCD process can be reduced by 33.75 and 10.46%. It demonstrated that DPTCD scheme was the most promising alternative to reduce the total energy consumption and TAC with high purity (99.1 wt%) n-butanol and iso-butanol products among these separation processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Distillation is the most common and earliest industrialized unit operation for the separation of liquid mixtures. It is a process of separating and purifying components by heating liquid mixture to form two phases of liquid and gas and taking full advantage of the relative volatility difference of different components. Due to its excellent characters of simple process, high-purity products and good reliability, distillation has been widely used in the fields of chemical, petroleum, and environmental protection, etc. However, it has one stark drawback, i.e., considerable energy consuming—40–70% of energy consumption was used in the separation process of petrochemical industry, and the distillation unit operation accounts for 95%, nearly 3% of the world’s total energy consumption [1,2,3]. With the worsening energy shortage situation and increasing energy demand in the world, process intensification technology is becoming more important in the chemical process development [4]. Meanwhile, it attracts more and more industrial groups and research teams to compete on researching how to save energy as much as possible.
To reduce energy consumption and improve energy utilization efficiency, researchers continuously put forward all kinds of energy-saving technologies. One method is to invent novel distillation column internals of excellent performance on high gas–liquid mass transfer efficiency, including new and efficient trays, random and structured packings [5,6,7,8]. Another way is to take measures to improve the thermodynamic efficiency of distillation system such as adding intermediate condensers or reboilers, adding side stream, and taking thermal coupling techniques, i.e., multi-effect distillation, heat pump distillation and internal thermally coupled distillation, etc. [9,10,11,12,13,14,15,16,17]. Recently, Li et al. [18] developed a new different pressure thermally coupled distillation technology widely used in distillation process, in which a conventional distillation column was divided into two columns, one conventional fractionator as rectifying section and the other vacuum one as stripping section. The pressure of rectifier was the same with that of conventional distillation column, while the stripper adopted decompression operation to reduce the bottom temperature of the stripper. When the temperature of top stream of rectifying section was higher than the liquid leaving the bottom of the stripper, the latent heat of top steam can be used to heat the stripping column bottom reboiler, which achieved substantial energy-saving in distillation process by virtue of thermal coupling between the two column sections. This technology was applied to the cases of propylene/propane separation and mixed C4 separation processes. The results showed that the energy consumption of different pressure thermally coupled distillation process could be reduced by 92.3 and 87.1%, respectively, compared to the conventional distillation process. Zhang et al. [19] also studied the performance of the pressure swing thermally coupled distillation (PSTCD) by separating 24 different kinds of binary hydrocarbon mixtures with various boiling point differences. The results showed that both PSTCD and heat pump distillation processes have high cost-saving efficiencies for the binary mixture with low boiling points differences. PSTCD process was more cost-conserving than the heat pump both in operating cost and TAC. Moreover, further research and optimization were conducted for the methyl cyclopentane/benzene binary mixture, and the results showed that relatively low feed temperature and high products purities can contribute to TAC saving efficiency and the optimum feed location was on the 1st stage of the stripping section. Besides, on the basis of PSTCD process,many scholars proposed some interesting structures by combing DPTCD process with other principles. Li et al. [20] presented different pressure thermally coupled reactive distillation (DPTCRD) for methyl acetate hydrolysis. The results demonstrated that the TAC and energy consumption of the DPTCRD process can be saved by 7.49 and 40.07%, respectively, compared with conventional reactive distillation. Moreover, a novel different pressure thermally coupled extractive distillation column was proposed by Sun et al. [21] for the separation of propylene/propane with aqueous acetonitrile (ACN) solution as entrainer, and 46.02% energy saving and nearly 9.7% reduction in TAC could be obtained through this attractive technology in contrast with conventional extractive distillation.
As reaction materials and solvents, n-butanol and iso-butanol are widely used in the fields of organic synthesis, food and medicine, etc. However, it is difficult to obtain high purity of n-butanol product using current production method, and iso-butanol is the main by-product. Generally, because the boiling points of n-butanol and iso-butanol are very close, a large amount of energy is consumed to separate them by conventional distillation. To reduce energy consumption in the separation of this close-boiling mixture, Gao et al. [22] investigated three different distillation schemes, namely conventional distillation, top mechanical vapor recompression heat pumps (MVRHP) distillation, and bottom-flashing MVRHP distillation, and the two innovative processes can achieve about 70% reduction in both energy requirement and TAC compared with the conventional distillation. Besides, top MVRHP distillation was demonstrated the most economical option. Li et al. [23] proposed an attractive self-heat recuperative distillation process for the separation of n-butanol and iso-butanol mixture. The results indicated that TAC could be reduced by 37.74 and 11.35%, respectively, in contrast with the conventional distillation process and vapor recompression column process.
The innovative DPTCD process and coupled techniques with other processes have been reported in lots of previous literatures. Unfortunately, to our knowledge, the application of DPTCD process to separate the n-butanol and iso-butanol mixture has not yet been reported. In this paper, three different distillation techniques, namely the conventional distillation column process, vapor recompression column process and DPTCD process, are used to separate the binary mixture. Afterward, the optimization of three schemes is investigated. Finally, the energy saving and economic comparison are conducted on the basis of optimal conditions.
Process description
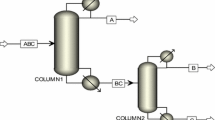
The boiling points of iso-butanol and n-butanol at atmospheric pressure are 107.3 and 117.3 °C, respectively, and the relative volatility is slightly larger than 1, which leads to much energy to be consumed in the conventional distillation. To solve this problem, two energy saving schemes are proposed: different pressure thermally coupled distillation process and vapor recompression distillation. Then, comparisons with each other and the conventional distillation sequence are conducted to find the optimal one. Figure 1 gives the schematic flowsheet diagrams of all three processes, and a single feed is adopted in every process.
The CD process is shown schematically in Fig. 1a. Feed stream enters distillation column T1 around the intermediate position, and iso-butanol and n-butanol products are taken out from the top and bottom of the column T1, respectively.
The VRC process is illustrated in Fig. 1b. The overhead vapor is pressurized and heated through a compressor. Then, this stream is used as the heat source in a heat exchanger which plays a role of the reboiler and condenser of column T2 at the same time. The bottom liquid stream is divided into two parts: one as the n-butanol product and the other used as a cooling agent into heat exchanger. After heat transfer, the reboil steam returns to the bottom of column T2 while the high-pressure stream is decompressed and cooled by a throttling valve, and it also needs to be further cooled by a cooler to saturate liquid phase state. Then, a part of saturate liquid stream is fed back to the top of the column, and the other as the iso-butanol product.
Figure 1c presents DPTCD process diagram. Thermal coupling in this structure is practical by dividing the conventional distillation column into two individual columns with different pressures, a high pressure (HP) one as rectifier and the other low pressure (LP) as stripper. The overhead vapor of HP column as the heat source heats the partial liquid leaving the bottom of LP column in main heat exchanger, where the generated boil-up vapor returns to the bottom of the stripper, and the overhead vapor is further condensed by a trim condenser before coming back to the top of the HP column. The liquid efflux from the bottom of the HP column flows into the top of the LP column by virtue of differential pressure. The overhead vapor of the LP column is compressed and then driven into the bottom of the HP column as ascending vapor. This scheme is used for separation of iso-butanol and n-butanol mixture for the first time in this work to improve the thermal efficiency. It should be highlighted that overhead vapor latent heat is the unique heat source without extra external heat.
Design and simulation
In this work, CD process, VRC process, and DPTCD process are designed for separating a close-boiling mixture of n-butanol and iso-butanol using the commercial simulator Aspen Plus. The study of CD is divided into three steps: shortcut design, rigorous simulation and optimization. Then, based on the results of the CD scheme, the simulation and optimization of VRC process and DPTCD process are carried out to find the optimal design and operating parameters. All the optimization variables include feed location (N F), the number of the theoretical plates (N) for CD process, and compression ratio (CR) for both VRC and DPTCD processes.
The simulation of distillation columns is based on the rigorous equilibrium stage module “RadFrac”, which uses MESH equations, including material balance, vapor–liquid equilibrium, component summation and enthalpy balance equations for each stage, and the same tray type (sieve) and tray spacing are employed in every column. The Wilson model is employed to obtain indispensable vapor–liquid equilibrium data of the binary system in this work, and the effectiveness was demonstrated by previous studies [22, 24].
Conventional distillation process
For the CD process, the feed stream with 5000 kg/h, composed of 55 wt% n-butanol and 45 wt% iso-butanol, is at 130 kPa and 120 °C. The specifications for the purity of n-butanol and iso-butanol products are all set at 99.1 wt%. For a fair comparison, the feed condition and product requirement of VRC process and DPTCD process should be consistent with those of the CD process. Besides, N F and N for the other processes are the same with the optimal counterparts of CD process. The column efficiency is 80%, and top stage/condenser pressure is 100 kPa with the stage pressure drop of 0.75 kPa.
Based on known conditions, the basic parameters of CD process, namely the number of theoretical stages, feed stage, and the reflux ratio (R), are estimated by shortcut design with using the “DSTWU” block in Aspen Plus. The simulation results of N, N F and R are 54, 29 and 7.06, respectively. Then, the CD process (Fig. 1a) can be simulated with the “Radfrac” module to achieve the separation task.
Actually, the initial parameters (N = 54, N F = 29) are not optimum. Therefore, the optimizations of N and N F are carried out to obtain optimal values under the specified product purity. The optimization purpose is based on minimizing energy consumption, which is closely related with the total annual cost. And reboiler duty is the main energy consumption in CD process. Here, we choose reboiler duty as the objective function. Firstly, the influence of feed location of column on reboiler duty is investigated, and the result is shown in Fig. 2a. It is evident that, as N F increases from 20 to 40, the reboiler duty decreases first and then increases, and the minimum value is obtained at stage 27, which means optimal feed position is 27. Then, fixing the ratio of feed position to the number of theoretical plates (N F:N = 27:54), the influence of the number of theoretical plates on heat duty of the reboiler is investigated. As shown in Fig. 2b, the reboiler duty decreases continuously with the increase of the number of theoretical plates, and it declines slightly when N is greater than 60. We set the column with 62 stages (including condenser and reboiler). The rigorous simulation results are shown in Table 1 with the optimal values (N = 62, N F = 31).
To perform a meaningful comparison, N F and N for the other processes are, respectively, set to 31 and 62 in view of the optimal counterparts of CD process.
Vapor recompression column process
Figure 1b shows a flowsheet of VRC process, including a column with 62 stages (numbered from the top stage). The feed mixture enters at stage 31 of the column T2. The feed condition, product specification, column efficiency and stage pressure drop are the same with these of conventional distillation process. In the process of simulation, compression ratio is the most important parameters of VRC process, which should be high enough to ensure a positive heat transfer of heat exchanger. In this section, in order to obtain optimal compression ratio, the influence of compression ratio on the heat transfer temperature difference (ΔT) and compressor duty (Q comp) is estimated, respectively. Generally, ΔT refers to the difference between the outflow temperature of compressor and the bottom liquid stream temperature of column, and it should not be lower than the minimum heat transfer temperature difference (ΔT min = 10 °C) [20, 22]. However, when the ΔT is equal to or larger than the lower limit of temperature difference, the temperature difference of heat exchanger between two outlet fluids may be small or even less than zero, which will lead to considerable heat transfer area or abnormal operation. Therefore, in this section, the heat transfer in heat exchanger is calculated on the basis of logarithmic mean temperature difference (LMTD), and ΔT refers to the logarithmic mean value.
The results are shown in Fig. 3, as the compressor ratio increases from 2.86 to 7, ΔT rises from 4.93 to 28.50 °C, and the dotted line represents minimum heat transfer temperature difference (10 °C). As expected, variation of compressor duty with compressor ratio is approximately linear. Since the electricity cost dominates the overall annual operation cost, it is more advisable to choose a smaller compressor ratio. In view of the cost of heat exchanger and compressor in VRC process, compressor ratio is set as 3.45, and the corresponding simulation results are shown in Table 1.
Different pressure thermally coupled distillation
The design of the DPTCD process is based on CD column, namely the high pressure one as rectifying section and the other as stripping section. Figure 1c shows a flowsheet of DPTCD process, which includes high pressure (HP) column with 30 stages and low pressure (LP) column with 32 stages. The feed mixture enters at top stage of the LP column. The feed condition, product specification and all the other design and operating variables are the same with these of CD column.
The compression ratio of compressor is numerically equal to the ratio of the bottom pressure of the HP column to the top pressure of the LP column, and pressure in HP column changes with the compression ratio, while the pressure of the LP column is fixed at 100 kPa. The stage pressure drop in each column is 0.75 kPa. The ΔT min is also set as 10 °C for this process, which is of crucial importance to implement a normal heat transfer.
Figure 4 illustrates how the compression ratio of compressor affects the heat transfer temperature difference (ΔT), compressor duty (Q comp), condenser duty (Q cond) of trim condenser and reflux ratio. In this section, ΔT refers to the logarithmic mean value of temperature difference at two ends of main heat exchanger. It shows that, as compression ratio increases from 2.25 to 5, ΔT rises from 4.92 to 34.98 °C, and in order to meet ΔT min requirement of main heat exchanger, CR should be greater than 2.58. In the meantime, both Q comp and R climb with the increase of compression ratio, which means that the energy consumption also increases gradually. As shown in Fig. 4b, Q cond is always less than zero in range of CR, which indicates that the vaporization heat of the bottom liquid of LP column can be completely provided by overhead vapor of HP column without trim reboiler.
Therefore, compression ratio should be reduced as far as possible under the precondition of the ΔT min and energy efficiency. Unfortunately, the heat-exchange area of main heat exchanger will increase with the reduction of the temperature difference supposing the heat duty is constant. Finally, 2.6 has been selected for CR in DPTCD process, and the corresponding simulation results are shown in Table 1.
Energy saving
The comparisons on energy saving of different processes are given in Table 2. Here, the energy savings of VRC and DPTCD processes are calculated, respectively, by Eq. (1). The total energy consumptions (Q total) of the VRC and DPTCD process can be acquired from reboiler duty (Q reb) and compressor duty (Q comp), and the condenser duty (Q cond) is actually negligible. The relationship between them can be described as Eq. (2), of which the factor 3 for the compression duty refers to the conversion coefficient of electric energy transferring into thermal energy [25]. Based on the energy consumption of each equipment given in Table 1, the obtained Q total of VRC and DPTCD processes are 1013.09 and 853.13 kW.
It is obvious that, compared with conventional distillation process, both VRC and DPTCD processes achieve much energy saving for separating of iso-butanol and n-butanol mixture. Among them, DPTCD scheme is the best option, which reduces energy consumption by 15.79% in contrast with VRC process.
Economic evaluation
From the last section, significant energy saving of DPTCD scheme is demonstrated in Table 2, while the capital investment increases at the same time. To properly estimate the economic feasibility of DPTCD, it is chosen here to compare total annual costs (TACs) of CD, VRC and DPTCD processes. The TAC includes two parts: annual operation cost and capital investment, which can be calculated by Eq. (3):
The capital and operation cost of all three processes are estimated using the correlations given by Douglas with a payback period of 5 years [26]. The capital cost is obtained by summing up individual equipment (distillation column, heat exchangers, and compressor) costs, and the operation cost is equal to utility costs, i.e., the amount stemming from the sum of electricity (16.81$/GJ), low pressure steam (7.72$/GJ) and cooling water (0.354$/GJ) costs for a year assuming 8000 operating hours.
The estimated capital costs and operation costs for CD, VRC and DPTCD processes are summarized in Table 3. The results show that TAC of DPTCD and VRC processes can be saved up to 33.75 and 26.00%, respectively, when compared with that of CD process. And the TAC saving of DPTCD process is 10.46% larger than that of VRC process.
Finally, the main focus of this study is to investigate the economic feasibility of DPTCD process when it is used to separate the close-boiling mixture of n-butanol and iso-butanol. And the DPTCD process represents a promising technology option. To be of practical use, the high level of control over variables is necessary for its operation. However, this point beyond the scope of this study, and we will continue to study in the future work.
Conclusions
In this paper, separating the close-boiling mixture of n-butanol and iso-butanol is studied in three different sequences including CD process, VRC process and DPTCD process. Design and configuration optimization of all three schemes are conducted by adjusting important parameters. Based on the effect of such parameters on the heat transfer temperature difference and energy consumption, the optimal design and operating variables such as feed location, the number of the theoretical plates, and compression ratio are obtained.
Both the VRC and DPTCD schemes have great energy saving for the binary close-boiling mixture; fortunately, the DPTCD process can achieve 15.79% energy saving than the VRC process. At the same time, the two processes also show good economic performance in terms of TAC, and the TAC of DPTCD process exhibits a reduction of 10.46% when compared with VRC. It demonstrates that the DPTCD technology is more economic than conventional distillation and even VRC process for the separation of n-butanol and iso-butanol mixture. It is creditable that the DPTCD process can bring a large amount of energy saving and economic benefits in the practical application to industrial scale.
Abbreviations
- DPTCD:
-
Different pressure thermally coupled distillation
- CD:
-
Conventional distillation
- VRC:
-
Vapor recompression column
- TAC:
-
Total annual cost
- PSTCD:
-
Pressure swing thermally coupled distillation
- DPTCRD:
-
Different pressure thermally coupled reactive distillation
- MVRHP:
-
Mechanical vapor recompression heat pump
- N F :
-
Feed location
- N :
-
Number of the theoretical plates
- CR:
-
Compression ratio
- R :
-
Reflux ratio
- ΔT :
-
Heat transfer temperature difference
- Q cond :
-
Condenser duty
- Q total :
-
Total energy consumption
- Q reb :
-
Reboiler duty
- Q comp :
-
Compressor duty
- HP:
-
High pressure
- LP:
-
Low pressure
- MESH:
-
Material balance, vapor–liquid equilibrium, component summation and enthalpy balance
- LMTD:
-
Logarithmic mean temperature difference
- ΔT min :
-
Minimum heat transfer temperature difference
References
Jana AK (2010) Heat integrated distillation operation. Appl Energy 87:1477–1494
Waheed MA, Oni AO, Adejuyigbe SB, Adewumi BA, Fadare DA (2014) Performance enhancement of vapor recompression heat pump. Appl Energy 114:69–79
Errico M, Tola G, Mascia M (2009) Energy saving in a crude distillation unit by a preflash implementation. Appl Therm Eng 29:1642–1647
Klemeš JJ, Varbanov PS (2013) Process intensification and integration: an assessment. Clean Technol Environ 15:417–422
Wilkinson P, Vos E, Konijn G et al (2007) Distillation trays that operate beyond the limits of gravity by using centrifugal separation. Chem Eng Res Des 85:130–135
Bravo JL, Kusters KA (2000) Tray technology for the new millennium. Chem Eng Prog 96:33–37
Schultes M (2003) Raschig super-ring: a new fourth generation packing offers new advantages. Chem Eng Res Des 81:48–57
Darakchiev S, Semkov K (2008) A study on modern high effective random packings for ethanol-water rectification. Chem Eng Technol 31:1039–1045
Lee SH, Shamsuzzoha M, Han M et al (2011) Study of the structural characteristics of a divided wall column using the sloppy distillation arrangement. Korean J Chem Eng 28:348–356
Zhai J, Liu Y, Li L, Zhu Y et al (2015) Applications of dividing wall column technology to industrial-scale cumene production. Chem Eng Res Des 102:138–149
Donahue MM, Roach BJ, Downs JJ et al (2016) Dividing wall column control: common practices and key findings. Chem Eng Process 107:106–115
Sun L, Bi X (2014) Shortcut method for the design of reactive dividing wall column. Ind Eng Chem Res 53:2340–2347
Agrawal R, Fidkowski ZT (1996) On the use of intermediate reboilers in the rectifying section and condensers in the stripping section of a distillation column. Ind Eng Chem Res 35:2801–2807
Díez E, Langston P, Ovejero G et al (2009) Economic feasibility of heat pumps in distillation to reduce energy use. Appl Therm Eng 29:1216–1223
Olujić Ž, Sun L, De Rijke A et al (2006) Conceptual design of an internally heat integrated propylene-propane splitter. Energy 31:3083–3096
Sayyaadi H, Saffari A (2010) Thermoeconomic optimization of multi effect distillation desalination systems. Appl Energy 87:1122–1133
You X, Rodriguez-Donis I, Gerbaud V (2016) Reducing process cost and CO2 emissions for extractive distillation by double-effect heat integration and mechanical heat pump. Appl Energy 166:128–140
Li H, Li X, Luo M (2008) Different pressure thermally coupled distillation technology for energy saving. Chem Ind Eng Prog 27:1125–1128
Zhang L, Liu J, Li X et al (2015) Separations of different binary hydrocarbon mixtures using pressure swing thermally coupled distillation process. Sep Sci Technol 50:148–157
Li L, Sun L, Wang J et al (2015) Design and control of different pressure thermally coupled reactive distillation for methyl acetate hydrolysis. Ind Eng Chem Res 54:12342–12353
Sun L, He K, Liu Y et al (2014) Analysis of different pressure thermally coupled extractive distillation column. Open Chem Eng J 8:12–18
Gao X, Chen J, Ma Z et al (2014) Simulation and optimization of distillation processes for separating a close-boiling mixture of n-butanol and isobutanol. Ind Eng Chem Res 53:14440–14445
Li L, Liu Y, Zhai J et al (2015) Design and control of self-heat recuperative distillation process for separation of close-boiling mixtures: n-butanol and iso-butanol. China Pet Process Petrochem Technol 17:111–120
Fu C, King C, Chang Y, Xue C (1979) Vapor–liquid and liquid–liquid phase equilibria of n-butanol-isobutanol-water system. Chem Ind Eng 5:97–106
Iwakabe K, Nakaiwa M, Huang K et al (2006) Energy saving in multicomponent separation using an internally heat-integrated distillation column (HIDiC). Appl Therm Eng 26:1362–1368
Douglas JM (1988) Conceptual design of chemical processes. Mcgraw-Hill, New York
Acknowledgements
This work was supported by the Natural Science Foundation of Scosthandong Province (ZR2013EEM024) and a Project of Shandong Province Higher Educational Science and Technology Programme (J14LA08).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Liu, L., Zhu, L., Sun, L. et al. Simulation and optimization of different pressure thermally coupled distillation for separating a close-boiling mixture of n-butanol and iso-butanol. Appl Petrochem Res 7, 143–150 (2017). https://doi.org/10.1007/s13203-017-0186-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-017-0186-1