Abstract
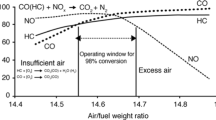
Three-way catalysts, a component of automobile converters, can control auto exhaust emissions by its capacity of converting CO, un-burnt hydrocarbon and oxides of nitrogen (NO x ) into less harmful CO2, H2O and N2 simultaneously. This process is efficient only when the A/F ratio is at 14.7. To widen the ratio for better chemical control, oxygen storage catalysts (OSC) based on CeO2, capable of three-way action, are employed in converters along with conventional catalysts. In this article, the enhanced activity of noble metal ion incorporated/dispersed CeO2 catalysts towards three-way action over conventional metal particles dispersed (in a matrix) catalysts is illustrated. The better performance of the metal ion dispersed catalysts is attributed to better dispersion of active metal ion sites over the reducible matrix like CeO2, leading to many-fold increase in the number of active sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To abate pollution due to emissions from automobiles, auto exhaust catalysts containing noble metals such as Pt, Pd and Rh dispersed in γ-Al2O3 as the support have been employed in converters. γ-Al2O3 is mixed with 30 % by weight of Ce0.7Zr0.3O2 as an oxygen storage material [1]. Although ceria-based catalysts as oxygen storage catalysts (OSC) are known since 1980s for the three way action, various approaches are adopted to widen/control the A/F ratio at 14.7 where the concentrations of oxidizing and reducing species are equal. Systems monitor the dynamic A/F ratio but monitoring through chemical control is a more versatile method. In this method, the operating window is widened by the OSC through the storage of oxygen in the oxygen-rich condition and releasing oxygen in the lean condition in situ. In this context, CeO2 has been an important oxygen storage material in TWC [2, 3]. The reactions are
The amount of oxygen released in the first reaction and the oxygen consumed in the second reaction is referred to as the oxygen storage capacity of the ceria material [4, 5]. The reversible intercalation of oxygen is derived from its structural feature.
Ceria crystallizes in fluorite structure (CaF2) in which the Ce4+ ions form the face centred cubic (FCC) structure with the tetrahedral holes occupied by oxide ions and the octahedral holes remaining vacant. The unit cell is shown in Fig. 1.
Oxygen deficient phase of CeO2-δ has been studied using X-ray diffraction (XRD), temperature programmed reduction (TPR) and magnetic measurements [6, 7]. Noble metals such as Pt, Pd and Rh, bimetallic Pt–Rh are dispersed as nanocrystalline metal particles or metallic ions into the matrix of OSC materials with gamma-alumina support (Ce0.7Zr0.3O2 with alumina)
Experimental
Synthesis of noble metal ion substituted ceria is carried out by solution combustion method [8]. A typical procedure is as follows. Stoichiometric quantities of ceric ammonium nitrate, H2PdCl6 and a fuel, urea or oxaldihydrazide are dissolved in minimum amount of water. This solution mixture is introduced into a preheated furnace kept at 500 °C. The mixture undergoes controlled combustion reaction resulting in the crystalline product within 5 min of the reaction. The products are characterized by powder XRD recorded with a Bruker D8-Advanced machine with a slow scan. X-ray photoelectron spectroscopy (XPS) was carried out by ESCA-3 Mark II VG Scientific Spectrometer using Al Kα radiation (1,486.6 eV). Binding energies reported are with respect to C (1S) at 285 eV and were measured with a precision of ≠0.2 eV. The catalytic reactions were done in a temperature programmed reaction system equipped with a quadrupole mass spectrometer SX 200 (VG Scientific Ltd, England) and a Gas Chromatograph (Mayura Analytical, India) equipped with chromosorb 101 column using a thermal conductivity detector. Catalysts were placed between glass wool plugs in the centre of a quartz tube which is inserted into the furnace appropriately. The furnace is controlled by a Eurotherm temperature controller connected by a chromel–alumel thermocouple. The gaseous products were sampled through a fine leak valve via a differential pumped sampling chamber to an ultra high vacuum (UHV) system employing a quadrupole mass spectrometer. Gases were analysed by GC. Mass balance confirmed the amount of evolved gases.
Results and discussion
Figure 2 shows the XRD pattern of nanocrystalline CeO2 by combustion method. It reveals the formation of nanocrystalline CeO2 with crystallite size of about 15–20 nm, calculated using Scherrer formula. Pd loading into ceria is carried out by the same combustion method starting from corresponding Ce and Pd salts.
Pd incorporated ceria exhibits sharper peaks indicating bigger crystallite size (100–110 nm). The cubic lattice parameters of CeO2 and Ce0.98Pd0.02O2-δ are 5.413 (2) Å and 5.4107(3) Å respectively, indicating the incorporation of smaller Pd2+ into the site of bigger Ce4+. The diffraction patterns clearly show the absence of Pd metal or PdO within the detectable limits of XRD. The XRD patterns are the same before and after CO oxidation reaction.
The XPS spectrum of Pd2+ substituted CeO2 along with that of Pd metal, PdO and PdCl2 is shown in Fig. 3. The binding energies of Pd 4d peak in 2 % doped Pd/CeO2 are close to that of Pd 4d states in PdCl2. This indicates that Pd in ceria is present as Pd2+, in contrast to metal particles dispersed in a matrix like Al2O3. The redox matrix of CeO2 facilitates the existence of ionic form of Pd in the support and such ionic species can enhance the number of active sites for the catalytic reactions.
XPS spectrum of Pd ion-doped ceria (from Ref. [10], with the permission of the corresponding author)
The XPS spectrum of Ce (Fig. 4) in 2 % Pd-doped CeO2 shows satellite peaks (marked) corresponding to CeO2 with Ce in the +4 oxidation state [9] in the as-prepared catalyst indicating indirectly that oxygen vacancies are created on substitution of Pd2+ into Ce4+ site into the lattice of CeO2.
XPS spectrum of Ce in Pd ion-doped ceria. (from Ref. [10], with the permission of the author)
To understand the oxygen storage capacity of Pd/CeO2 catalyst, H2-TPR is carried out where the uptake of hydrogen is measured as a function of temperature. It is depicted in Fig. 5. Pure CeO2 shows the uptake from about 350 °C until a peak at 500 °C, whereas the Pd ion-doped CeO2 uptakes hydrogen at a much lower temperature of 65 °C, a situation favourable for OSC.
Hydrogen TPR of OSC (from Ref [10], with the permission of the corresponding author)
These ionic catalysts are tested for various catalytic reactions. CO oxidation reaction is shown in Fig. 6. It is very evident from the figure that Pd ion-substituted ceria exhibits the highest CO conversion with the lowest activation energies compared to PdO revealing that Pd ion-doped CeO2 is an efficient OSC. The rates of conversion are 20–30 times higher than the corresponding metal particles impregnated catalysts [10] and their activation energies could be obtained from Arrhenius plots of the temperature dependence of reaction rates. These catalysts have been tested for NO x reduction also [11]. Using first principle density functional theory (DFT), it is shown that CO adsorption in these catalysts occurs at the ionic sites of Pd2+, with a decrease in the net energy of the system [12].
CO oxidation reaction of Pd ion dispersed ceria along with PdO (from Ref. [10], with the permission of the corresponding author)
Conclusion
Pd2+-dispersed CeO2 oxygen storage catalysts have been synthesized by solution combustion method resulting in noble metal ions dispersed OSC. We have shown that these ionically dispersed catalysts are catalytically more active towards exhaust emission reactions like CO oxidation.
References
Gandhi HS, Graham GW, McCabe RW (2003) Automotive exhaust catalysts. J Catal 216:433–442
Yao HC, Yu Yao YF (1984) Ceria in automotive exhaust catalysts I. Oxygen storage. J Catal 86:254–258
Taylor KC (1993) Nitric oxide catalysis on automotive exhaust system. Catal. Rev. Sci. Eng. 35:457–481
Kaspar J. Graziani, Fornasia P (2000) Ceria containing three way catalysts. In: Gschneidner KA Jr, Eyring L (eds) Hand book on the physics and chemistry of rare earths, vol 29. Elsevier, Amsterdam, pp 159–267
Kaspar J, Fornasiero P, Hickey N (2003) Automotive catalytic converters: present status and some perspective. Catal Today 77:419–449
Ranga Rao G (1999) Influence of metal particles on the reduction properties of ceria based materials studies by TPR. Bull Mater Sci 22:89–94
Ranga Rao G, Kaspar J, Merioni S, Monte RD, Graziani M (1994) NO decomposition over partially reduced metallized CeO2 –ZrO2 solid solution. Catal Lett 24:107–112
Vijayaraghavan R et al (unpublished results on ceria based perovskite catalysts)
Sarma DD, Hegde MS, Rao CNR (1981) Study of surface oxidation of rare-earth metals by photoelectron spectroscopy. J Chem Soc Faraday Trans 277:1509–1520
Bera Parthssarathy, Hegde MS (2010) Recent advances in auto exhaust catalysts. J. Indian Inst Sci 90–2:299–325
Hegde MS, Madras Giridhar, Patil KC (2009) Noble metal ionic catalysts. Acc Chem Res 42:704–712
Dutta G, Gupta A, Waghmure UV, Hegde MS (2011) CO adsorption in ionic Pt, Pd and Cu sites in Ce1-xMxO2-δ (M = Pt2+, Pd2+, Cu2+). J Chem Sci 123:509–516
Acknowledgments
The author thanks KOPRC for the invitation to 2nd KACST-Oxford forum meeting at Riyadh in 2012.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Vijayaraghavan, R. Noble metal ion-substituted ceria as efficient oxygen storage catalysts for clean combustion of fuels. Appl Petrochem Res 3, 79–82 (2013). https://doi.org/10.1007/s13203-013-0032-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-013-0032-z