Abstract
In this work, different kinds of supported molybdenum carbide catalysts have been prepared using temperature programmed carburization method with 20 vol% CH4/H2. Cobalt and potassium were later loaded over the resultant carbides. The catalysts were evaluated on the new alcohols test rig under 70 bar pressure with syngas feed gas with a H2/CO ratio of 2.0 at various temperatures. The carbide catalysts supported over boron-modified Al2O3 produced higher alcohols in significant yields. The best catalyst achieved 46.7 % selectivity to alcohol at 31.8 % conversion of CO. This corresponds to 199.7 g/alcohol/liter of catalyst/hour. In addition, 72.5 % selectivity to alcohol (93.1 % on CO2 free basis) was achieved at lower temperature with a conversion of 14.1 %. Characterization results showed that the boron-modified supports result in smaller molybdenum carbide crystallite size which might be responsible for the high activity. Higher potassium content benefits the high alcohol selectivity, but affects the catalyst activity to some extent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last 20 years, much attention has been paid to the synthesis of higher alcohols (HAS) from syngas because alcohols are being considered as a potential alternative synthetic fuels for automotive applications and as feedstock for the synthesis of a variety of industrial chemicals and polymers [1–4]. Syngas can be produced from all sorts of carbon containing feedstock such as biomass, natural gas, biogas and coal, which can be converted to alcohols by catalytic conversion. However, the catalytic conversion of syngas to alcohols remains challenging, and no commercial process exists as of today, although the research on this topic has been pursued for the past 90 years.
Transition metal carbides have been found to have the similar property to noble metal in many aspects, which have been extensively studied for high alcohol synthesis process. It has been shown that Mo–CO adsorption is weakened by C, leading to CO dissociation over Mo2C. When promoted with alkali metals and/or Group VIII metals, the molybdenum carbide catalyst showed higher selectivity to alcohol under the typical operation conditions of 5–8 MPa, H2/CO = 2–1, and 537–603 K.
So far, most studies on carbide catalysts systems are focused on bulk catalysts. Christensen reported “catalytic conversion of syngas into Higher Alcohols over WC and Mo2C Carbide Catalysts”, and found that K2CO3 addition increases the alcohol selectivity [5]. Wang Ning et al. [6] studied that Fe and K promoted Mo2C catalyst for higher alcohol synthesis, and proposed that the Fe3Mo3Cx might be the active phase for the higher alcohol formation over the catalyst system. The effect of Fe/Mo ratio and the K2CO3 addition exerts a significant effect on the catalyst activity and selectivity [6, 7]. In contrast, Xiang’s studies suggested that the modification of K/Mo2C with Co and Ni gives higher selectivity to high alcohol selectivity with fair CO conversion [8–10].
An overview of the published papers on the molybdenum carbide catalysts for high alcohol synthesis showed that most research focuses on the bulk carbide catalyst, which showed higher selectivity to alcohol and relatively high activity, but from the practical view, the strong metallic property of the bulk molybdenum carbide does not have high strength and produce few pores in the catalyst particles when extruded into pellets for fixed bed applications. For industrial applications, it is desirable to have supported catalysts. However, so far, there have been very few studies published on the supported carbide catalyst for alcohol synthesis [11–14], and they almost totally focused on active carbon as the supports, which are also extensively studied on the molybdenum sulfide systems for alcohol synthesis [12, 15–18].
The alumina-supported molybdenum carbide-based catalysts are relatively new for alcohol synthesis; also, the supported catalysts are expected to have high strength and contain less metal. Various modifications of the catalyst are possible to improve the catalytic performance. Therefore, in this work, we have prepared series of alumina (varying the support property)-supported molybdenum carbide catalyst with different promoters. Their performances have been tested under various conditions and have been correlated with the preparation factors.
Experimental
Catalyst preparation
In this work, the HAS catalysts were prepared using CH4/H2 carburization method. Lots of studies have been reported on Mo-based carbide catalysts preparation by the carburization of bulk CoMoCx or NiMoCx [8–10], but few of them were on the supported Mo-based carbide catalysts. Here we prepare the supported carbides first and then modify with other additives and promoters. The preparation details are as follows.
The molybdenum precursor was prepared from ammonium molybdate, to which deionized water (DI) and hydrogen peroxide (H2O2) were added to increase the molybdate solubility. The molybdate solution was impregnated with supports (γ-Al2O3 or γ-Al2O3- B2O3, B2O3 content, 2.5 wt%) by pore volume impregnation method, e.g., incipient wetness at room temperature. The supported MoO3/Al2O3 precursor was obtained after calcination at 500 °C, which is then converted into supported Mo2C after the supported oxide was carburized using CH4/H2 to 700 °C with 20 vol% CH4/H2 stream, then cooled to room temperature naturally in H2 stream, and passivated with static air for 24 h before it was unloaded from the reactor. The carburization process is monitored using TPR-TCD to study the effect of support on the supported molybdenum oxide reduction.
Finally, the desired modifier components or promoters’ metal was added to the obtained Mo-based carbide support catalyst. It is noted that the cobalt salts were loaded onto the supported carbides after carburization to avoid sintering during the carburization process. The Mo carbide was formed due to the reaction between MoO3 and CH4. Co(Ac)2 and K2CO3 were used as the promoters and modifiers.
The detailed preparation parameters of each catalyst are listed in Table 1.
Catalyst test and data analysis
The catalysts have been tested in a micro-reactor system. Each time, 2.73 ml HAS catalyst with 32.5 ml SiC was loaded in HAS reactor, plugged with quartz wool at both ends, and reduced with H2 at 4.0 MPa at 404 ml/min at 350 °C (ramp 1 °C/min) for over 16 h.
The test conditions of the carbide catalysts are set as follows:
-
Feed gas characteristic—syngas with H2:CO ratio of 2 optionally with 5–10 % CO2 with 5 vol% of N2 as for internal calibration
-
GHSV 2,000–4,000 H−1
-
Pressure 5.0–10.0 MPa
-
Temperature 220–300 °C.
After the HAS catalyst was reduced with H2 at 4.0 MPa and temperature cooled down to 200–220 °C from 350 °C, syngas was introduced into reactor at 136 ml/min. It spent about 25–30 h after the reaction pressure was increased to 7.0 Mpa from 4.0 MPa, all H2 was purged with syngas and reaction temperature reached 245 °C.
From the GC data of gas products, the compositions of H2, CO, CO2, CH4, C2–C5 hydrocarbons can be analyzed, and the CO conversion, all gas products’ selectivity and the selectivity to liquid are calculated. From another GC analysis of liquid products, we can obtain the compositions of alcohols, heavy hydrocarbons and H2O. Then we get all products’ selectivity and productivity.
Catalyst characterization
The catalyst crystalline structure was analyzed using powder XRD with a Philips X’ PeRT Pro Alpha 1 diffractometer with Cu Kα radiation (λ = 1.5406 Å) operated at a tube current of 40 mA and a voltage of 45 kV. Data were collected over 2θ values from 10° to 80°, at a scan speed of 1° min−1. The average crystal size was calculated using Scherer equation based on the strongest XRD peak of Mo2C (101).
The Scherrer equation is expressed as follows:
where τ is the mean size of the ordered (crystalline) domains, which may be smaller or equal to the grain size; K is a dimensionless shape factor, which is about 0.9 in this work; λ is the 1.5406 Å; β is the line broadening at half the maximum intensive peak, e.g., plane (101), which has less overlap with the background diffraction peaks; and θ is the Bragg angle.
Results and discussion
Effect of support on the carburization of the molybdenum oxide
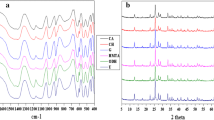
The carburization process of the bulk and supported molybdenum oxide was monitored using a TPR system and the changes of the products were detected using TCD. The results are shown in Fig. 1. It is seen that the carburization process starts at 200 °C, and most carburization process occurs at about 400 °C; this process contains two major peaks: the first at about 400 °C, during which reduction of MoO3 by H2 or CH4 occurs, while the second peak at 650 °C, where complete carburization of MoO2 or MoCx to Mo2C occurs.
In case for the Al2O3-supported MoO3 carburization, the first reduction of MoO3 occurs at 500 °C, which showed that the supported MoO3 is more difficult to reduce even when there are 40 wt% MoO3 loaded over the support. The difficult reduction of the supported MoO3 may result from the strong interaction between the MoO3 and Al2O3. The second carburization peak appeared at 900 °C and the span range between the two peaks is much broader, suggesting that there may be different kinds of MoO3 over the supports, the one close to the support surface would have a stronger interaction with support and more difficult to be carburized, leading to the high temperature carburization.
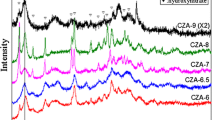
The crystalline structures of the prepared carbide catalysts were characterized using XRD and the results are shown in Fig. 2. It is noted that the aluminum plate was used as the sample holder, so the peaks at 48°, 44° and 64° are due to the diffraction of metallic Al. The diffraction peaks showed that in the bulk carbide, the main phase is Mo2C, with hcp structure [6, 14, 19]. The peak at 40.5° is assigned to the plane [101] of Mo2C, and the diffraction peak at 35.1° is ascribed to the diffraction of [002] of Mo2C. Over the Al2O3-supported Mo2C catalyst, the main phase is still Mo2C, but the intensity of the diffraction is much weaker, suggesting a smaller crystallite size of Mo2C over the alumina support. Over the B2O3-modified Al2O3 support, the resultant Mo2C [101] diffraction peak is even broader, whereas the other peaks corresponding to [002] and [110] planes are weaker, suggesting that the modification of Al2O3 with B2O3 has some effect on the molybdenum carbide phase and dimensions. The difference of the supported carbide may be due to the pore volume difference. After B2O3 was added to the Al2O3 support, the pore volume changed from 0.753 to 0.65 cm3/g, which can restrict the cluster size of MoO3, the precursor of the carbide.
The catalyst composition, its physical properties and crystallite size determined using XRD are shown in Table 1. The surface area of bulk Mo2C catalyst is 32.6 m2/g, which is due to it powder phase. The supported Mo2C carbide catalysts modified with 1.6 and 3.5 wt% Co has a surface area of 78.8 m2/g; the catalyst particle size was noted to be about 300 μm, due to the breakage of the extrudated Al2O3–B2O3 support. The pure Al2O3-supported Mo2C modified with 1.6 wt% K and 3.5 % Co has slightly higher surface area, about 83.2 m2/g. The crystallite size of Mo2C is about 18.6 nm, while in the supported carbide sample, the crystallite size decreased to <20 nm. The Al2O3–B2O3-supported carbide has smaller Mo2C crystallite, but overall the Mo2C crystallite size decreases with the content of K modification, which is in agreement with the literature results [1, 5].
Catalyst test results
The bulk and supported molybdenum carbide catalysts have been tested for higher alcohol synthesis from syngas at various temperatures with constant pressure and space velocity. The bulk-modified molybdenum carbide catalysts performance for the HAS is shown in Fig. 3. At 200 °C, when the reaction started, CO conversion was above 10 %, methane selectivity is less that 4 %, but CO2 selectivity is more than 14 %, while the selectivity to liquid is about 80 % at the initial reaction stage. The CO conversion was almost unchanged during the first 30-h reaction, while the temperature was raised to 230 °C. However, the liquid selectivity drops with the time on stream even the CO conversion does not increase. When the reactor temperature was increased to 240 °C, CO conversion increased to 25 %, CH4 selectivity increased to 7.5 vol%, and CO2 increased quickly to 40 vol%. In contrast, the liquid selectivity dropped to 40 wt%. At temperatures above 240 °C, the selectivity to liquid products dropped slowly, but selectivity to both CO2 and CH4, the undesirable products, increased gradually. When the reaction temperature is raised above 260 °C, CO conversion increased more rapidly, while the liquid selectivity decreased. In contrast, both CO2 and CH4 selectivities rose. When the CO conversion reached 75 % at 275 °C, liquid selectivity dropped below 30 %, but CH4 selectivity rose above 10 % and CO2 reached 50 %. However, the initial selectivity to liquid is higher than the bulk Mo2C carbide system.
Over 1.6 % K 3.5 % Co 40 % Mo2C/Al2O3 catalysts, the catalyst activity was lower than the bulk Mo2C catalyst system; at 210 °C, the CO conversion was <5 %, but methane selectivity was more than 6 %, but almost no CO2 detected in the products, and the selectivity to the liquid was more than 90 % at the initial reaction, but dropped to below 80 % when CO selectivity suddenly rose. Holding the reaction temperature at 220 °C for 10 h, the CO2 selectivity increased to 20 %, but CH4 also rose to 8 %. At 230 °C, the CO conversion was only 4.2 %, CO2 selectivity was almost unchanged at 20 %, but liquid selectivity dropped to about 50 %. To reach a steady state, the reactor temperature was held at 245 °C for 50 h; during this period, CO conversion reached 10 %, which is obviously lower than the bulk catalyst, liquid selectivity and CO2 selectivity all are about 33 %, while methane selectivity is about 9.2 %. Increasing the temperature to 255 °C increased CO conversion to 12 %, but the selectivity to liquid further dropped to below 30 % and CO2 selectivity increased to 35 %. This suggests that the liquid selectivity drop may be due to disproportionation of CO into CO2 and the increased yield of CH4. The catalyst activity is much lower than the bulk catalyst, which may be due to the less active site over the catalyst.
When using Al2O3–B2O3 as the support, the resultant carbide catalyst performance for HAS from syngas is shown in Fig. 5. Interestingly, the catalyst showed relatively high activity for CO conversion at 220 °C, CO conversion goes up to 20 % for a while at 220 °C, with CH4 selectivity less than 4 % and almost no CO2 produced. The selectivity to liquid is more than 90 %, but dropped with the time on stream at 220 °C. This suggests that the catalyst active site or surface property might change quickly in the initial reaction stage, which does not lead to reach the steady state, and CO conversion dropped from nearly 20 to 6 % at 220 °C without changing the other conditions, and the methane selectivity rises gradually. When increasing the reaction temperature, the CO conversion increases slowly, but the CH4 selectivity and CO2 selectivity increase when the liquid selectivity decreases rapidly. At 245 °C, CO conversion increased to 10 %, but CO2 selectivity increased to 22 %, and methane selectivity changed to 7 %; however, the liquid selectivity decreased to 62 %. When raising the reactor temperature to 255 °C, the liquid selectivity dropped to 53 %, CO2 selectivity raised to 30 %, with CH4 selectivity increase of 8.2 %. This suggests that increase of the temperature leads to more CO converting to CO2, not favoring the liquid formation. This can be understood from the thermodynamic view. As the liquid alcohol synthesis is a strong exothermic reaction, while the conversion of CO to CO2 is favored by high temperature. Increasing the reaction temperature to 265 °C leads to CO conversion to 20 %, but CO2 selectivity to 38 %, and the liquid selectivity dropped to 42 %. The CH4 selectivity slightly increases.
Comparison of Figs 3, 4 and 5 shows that the bulk molybdenum carbide catalyst has the highest activity for CO conversion, but its selectivity to liquid dropped rapidly with increasing temperature. The Al2O3-supported molybdenum carbide has the lowest activity, but the selectivity to liquid is slightly better than the bulk carbide. The modification of Al2O3 with B2O3 significantly improves the catalyst performance for liquid production from syngas. The catalyst activity is improved, while the selectivity to liquid is the best, and decrease slower than the Al2O3-supported carbide and bulk carbide catalysts. The comparison of the three catalysts’ performance is shown in Table 2.
The liquid products from the HAS process were collected from the liquid trap regularly and analyzed using GC–MS. The results are shown in Table 2 after water is excluded. It is shown that the bulk molybdenum carbide catalyst contain 0.5 % liquid hydrocarbons, with methanol selectivity in the organic phase of about 64.1 wt%. There the ethanol concentration in the organic products is 25.8 wt%, and propanol is 7.7 wt%. Butanol content is about 1.5 wt%, and pantanol about 0.1 wt%. The C2+OH/MeOH is 0.53 over the bulk carbide catalyst, suggesting that nearly half of alcohol products in the organic phase is methanol.
Over the Al2O3-supported molybdenum carbide catalysts, HCx content is 0.1 wt%, but the methanol content is only 51.9 wt% less than that over the bulk carbide catalyst, the ethanol content increases to 31.5 wt%, and the other higher carbon alcohols from C3 to C5 all increase. The C2+OH/MeOH is 0.94. Over the Al2O3–B2O3-supported molybdenum carbide catalyst, the methanol content further decreases to 50.5 % and almost no liquid hydrocarbon products were detected; the ethanol concentration increases to 32.3 wt% and propanol content decreases to some extent. The C2+OH/MeOH ratio is 0.99, suggesting that nearly half of the organic products are higher alcohol products.
Effect of K content on the catalyst performance
To further increase the catalyst performance, Al2O3 and Al2O3–B2O3-supported Co and Mo2C catalysts with 3.3 and 4.6 wt% potassium have been prepared and the catalyst properties are shown in Table 1. The higher K content seems to give rise to smaller molybdenum crystallites with slightly lower surface areas. The catalysts have been tested at different reaction temperatures and 70 bars with GHSV of 3,000 h−1, H2/CO = 2. The results of the effects of K level on two different supported catalysts performance are given in Fig. 6, Al2O3 and Al2O3–B2O3 are shown in Table 1. The results are shown graphically in Fig. 6. It is interesting to observe that the catalyst activity in terms of CO conversion and alcohol STY is higher with the increase of the potassium content in the catalyst, as higher K content gives rise to smaller molybdenum particle size, thus leading to more active sites. The higher activity of Al2O3–B2O3-supported carbide catalysts can also be explained with this assumption. Overall, the yield of higher alcohol increases with the rise of the reaction temperature, although it is shown that the alcohol selectivity decreased, but compensated with the increase of CO conversion with the reaction temperature.
Figure 7 shows the changes in the higher alcohol selectivity with the CO conversion over the different supported molybdenum carbide catalysts with various potassium contents. Overall, the alcohol selectivity drops with the conversion, although the Al2O3–B2O3-supported 3.8 wt% Co 40 wt% Mo2C catalysts have slower decrease in high alcohol selectivity with increasing CO conversion. The catalyst has similar trends, even though the Al2O3–B2O3-supported catalyst has higher alcohol selectivity. When increasing the K content to 4.6 wt%, the Al2O3-supported carbide catalyst selectivity to alcohol dropped faster than the 3.3 % K 3.5 % Co 40 % Mo2C/Al2O3. In contrast, over the 4.6 wt% K, 3.5 % Co, 40 % Mo2C/Al2O3–B2O3 catalyst, the alcohol selectivity has lesser CO conversion than the ones over 3.3 % K 3.5 % Co 40 % Mo2C/Al2O3.
Catalyst stability and mass balances
To determine the long-term stability of the supported carbide, 3.3 % K 3.5 % Co 40 % Mo2C/Al2O3 was tested continuously for 550 h (23 days). Initially, the catalyst was tested at three temperatures but the majority of the test continued at 245 °C (Fig. 8). The gap of the data between the time on stream of 180–220 h due to the holiday and the GC control was stuck; however, the reaction was continued.
It is clear that the stability of the catalyst is remarkably good. This is necessary for any commercial application. The material and mass balances were calculated for the 3.3 % K 3.5 % Co 40 % Mo2C/Al2O3 catalyst at 245 °C. The results were 95.3 % for total mass balance, including 97.8 % hydrogen mass balance, 90.2 % carbon mass balance, 94.7 % oxygen mass balance and 95.1 % nitrogen mass balance. These numbers give confidence that the data produced are reliable. Further work is necessary to improve the C balance.
Conclusions
-
1.
Different supported molybdenum carbide catalysts have been prepared using temperature-programmed carburization, and Co and K were added to the carbide catalyst using impregnation methods. The catalysts supported with Al2O3–B2O3 showed smaller Mo2C crystallite size and lower surface area. However, the catalyst showed higher CO conversion and more selectivity to high alcohol synthesis from syngas. The bulk molybdenum carbide catalyst showed the higher activity, but also high selectivity to CO2 and CH4. Also, it is difficult to be shaped for fixed bed use.
-
2.
The potassium content has a significant effect on the catalyst performance. Higher K content favors the yield of higher alcohol, and the promotion effect is more significant over the Al2O3–B2O3-supported carbide catalysts. This can be explained by the relatively smaller Mo2C crystallites, which give higher Mo2C dispersion over the catalysts.
-
3.
The supported molybdenum carbide catalyst showed high stability for high alcohol synthesis with a fairly good mass balance. The high alcohol yield can be up to 199.7 g/alcohol/liter of catalyst/hour under the reaction conditions, nearly matching the supported rhodium catalyst under even harsher conditions [17, 20].
References
Zaman S, Smith KJ (2012) A review of molybdenum catalysts for synthesis gas conversion to alcohols: catalysts, mechanisms and kinetics. Catal Rev Sci Eng 54:41–132
Surisetty VR, Dalai AK, Kozinski J (2011) Alcohols as alternative fuels: an overview. Appl Catal A 404:1–11
Gupta M, Smith ML, Spivey JJ (2011) Heterogeneous catalytic conversion of dry syngas to ethanol and higher alcohols on cu-based catalysts. ACS Catal 1:641–656
Herman RG (2000) Advances in catalytic synthesis and utilization of higher alcohols. Catal Today 55:233–245
Christensen JM et al (2012) Catalytic conversion of syngas into higher alcohols over carbide catalysts. Ind Eng Chem Res 51:4161–4172
Wang N et al (2010) Synthesis of higher alcohols from syngas over Fe/K/β-Mo2C catalyst. Catal Lett 136:9–13
Wang N et al (2010) Iron carbide promoted K/β-Mo2C for higher alcohols synthesis. Catal Today 158:241–245
Xiang M et al (2008) Synthesis of higher alcohols from syngas over Fischer-Tropsch elements modified K/β-Mo2C catalysts. Fuel 87:599–603
Xiang M et al (2007) Synthesis of higher alcohols from syngas over K/Co/β-Mo2C catalysts. Catal Commun 8:503–507
Xiang M et al (2007) Potassium and nickel doped β-Mo2C catalysts for mixed alcohols synthesis via syngas. Catal Commun 8:513–518
Shou H et al (2013) Use of infrared spectroscopy and density functional theory to study the influence of rubidium on alumina-supported molybdenum carbide catalyst for higher alcohol synthesis from syngas. J Catal 299:150–161
Surisetty VR, Eswaramoorthi I, Dalai AK (2012) Comparative study of higher alcohols synthesis over alumina and activated carbon-supported alkali-modified MoS2 catalysts promoted with group VIII metals. Fuel 96:77–84
Surisetty VR, Dalai AK, Kozinski JK (2012) Long term deactivation studies of novel catalysts for higher alcohols synthesis. Prepr Symp Am Chem Soc Div Fuel Chem 57:457
Wu Q et al (2011) Supported molybdenum carbides for higher alcohols synthesis from syngas. Prepr Symp Am Chem Soc Div Fuel Chem 56:153
Surisetty VR et al (2011) Structural characterization and catalytic performance of alkali (K) and metal (Co and Rh)-promoted MoS2 catalysts for higher alcohols synthesis. Appl Catal A 392:166–172
Surisetty VR, Dalai AK, Kozinski J (2011) Deactivation studies of alkali-promoted trimetallic Co-Rh-Mo sulfide catalysts for higher alcohols synthesis from synthesis gas. Energy Fuels 25:580–590
Surisetty VR, Dalai AK, Kozinski J (2010) Effect of Rh promoter on MWCNT-supported alkali-modified MoS2 catalysts for higher alcohols synthesis from CO hydrogenation. Appl Catal A 381:282–288
Li D et al (2005) Ni/ADM: a high activity and selectivity to C2+OH catalyst for catalytic conversion of synthesis gas to C1–C5 mixed alcohols. Top Catal 32:233–239
Shou H, Davis RJ (2011) Reactivity and in situ X-ray absorption spectroscopy of Rb-promoted Mo2C/MgO catalysts for higher alcohol synthesis. J Catal 282:83–93
Surisetty VR, Dalai AK, Kozinski J (2010) Alkali-Promoted Trimetallic Co-Rh-Mo sulfide catalysts for higher alcohols synthesis from synthesis gas: comparison of MWCNT and activated carbon supports. Ind Eng Chem Res 49:6956–6963
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Al-Megren, H., Xiao, T., AlKinany, M. et al. High alcohol synthesis (HAS) from syngas over supported molybdenum carbide catalysts. Appl Petrochem Res 3, 71–77 (2013). https://doi.org/10.1007/s13203-013-0031-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-013-0031-0