Abstract
In the present work, some Mannich bases have been prepared by using different polyethylenepolyamines. Phosphosulphurized Mannich bases have been also prepared by reaction of prepared Mannich bases with P2S5. Structure of the prepared compounds was confirmed by infrared spectroscopy and the molecular weights determination. The efficiency of the prepared additives as antioxidants and detergents/dispersants was investigated. It was found that the efficiency increases with increasing the NH groups in the amine used. The best prepared additive as antioxidant and detergent/dispersant was obtained by using triethylenetetramine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lubrication is the act of applying lubricants and lubrication substances, which are capable of reducing friction between moving mechanical parts. The main function of a lubricant is forming a film that separates two surfaces that roll or slide between one another, to reduce friction and eliminate wear. Lubricant additives are compounds or mixtures when incorporated in base lubricating fluid, supplement its natural characteristics and improve their field service performance in existing applications. The functions of lubrication additives are to reduce the oxidative or thermal degradation of oil, reduce wear, minimize rust and corrosion, lessen the deposition of harmful deposits on lubricated parts and prevent destructive metal-to-metal contact [4]. Detergent and dispersant additives are used primarily in internal combustion engines to keep metal surfaces clean by preventing deposition of oxidation products. Dispersants are typically the highest treat additives in an engine oil formulation. They are similar to detergents in that they have a polar head group with an oil-soluble hydrocarbon tail. While detergents are used to clean engine surfaces and neutralize acidic byproducts, their effectiveness is limited when it comes to dispersing oil-insoluble products resulting from the byproducts of combustion. Detergents are an integral part of any engine oil formulation. They are rarely the sole additive in a lubricant package and are often blended together with other types of additives [1]. Typically, they are blended with antiwear (AW) and extreme-pressure (EP) additives for use in engine oils, hydraulic fluids and metalworking fluids. Several studies have examined the effects of combining detergents with AW/EP additives such as zinc dialkyl dithiophosphates (ZDDPs), one of the most important chemically-active additives used in engines oils today. The purpose of detergents in crankcase oils is suspend/disperse oil-insoluble combustion products, such as sludge or soot and oxidation products, to neutralize combustion products (inorganic acids), to neutralize organic acids products of oil degradation processes and to control rust, corrosion, and deposit-forming resinous species [2]. One of the most important modes of lubricant degradation is oxidation. This oxidation is the primary cause of increase in viscosity, pour point, sludge, and enhanced engine corrosion and conventional liquid lubricants show poor oxidative and thermal stability at higher temperature ranges, which result in the formation of a vast quantity of volatile and solid products in the lubrication system [8]. The oxidation reactions that occur in a lubricant at elevated temperatures in the presence of atmospheric oxygen may lead to declined lubricant performance such as significant increase in kinematic viscosity observed in severe service conditions or during extended drain intervals [7]. In general, all types of base oil require addition of antioxidants depending on the amount of unsaturation and “natural inhibition” present. The refined mineral base oils contain “natural inhibitors” in the form of sulphur and nitrogen compounds sufficient for many applications [10]. Oxidation stability plays only a minor role in the process by which combustion chamber deposits are formed.
In the present work, some lube oil additives were prepared by synthesis of Mannich bases; which prepared by using alkyl phenol, formaldehyde and amines, then phosphosulphurization of the prepared Mannich bases by using phosphorous pentsulphide (P2S5). The efficiency of the prepared compounds as antioxidant and detergent/dispersant additives for lube oil was investigated [9].
Experimental
Preparation of Mannich Bases
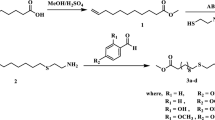
In a 5-necked round bottom flask, fitted with an efficient mechanical stirrer, a ground joint thermometer, a condenser and a dropping funnel, the appropriate amounts of alkyl phenol in methanol and aqueous solution of polyethylenepolyamines were added. A formaldehyde solution (37 %) was then added dropwise during 1 h at 25–30 °C under nitrogen gas. After complete addition of the formaldehyde, the temperature of the reactants was elevated up to reflux temperature for 3 h. The mixture was then cooled to room temperature and the upper methanol/water layer was separated from the yellow viscous lower layer. The product (lower layer) was dissolved in 500 ml benzene and washed four times with 300 ml portions of distilled water to eliminate the excess of amine and formaldehyde.
Abbreviation of prepared Mannich bases
Mannich Bases | Abbreviation | Polyethylenepolyamines used |
|---|---|---|
I | A1 | Ethylenediamine |
II | A2 | Diethylenetriamine |
III | A3 | Triethylenetetramine |
Phosphosulphurization of prepared Mannich Bases
A 4-necked round bottom flask, fitted with a mechanical stirrer, efficient condenser, thermometer and inlet for passing nitrogen gas was used. The reactor was charged with four moles of each of prepared Mannich bases and one mole of P2S5. The reaction mixture was maintained at about 185–190 °C with continuous stirring for 4 h. The whole reaction has been carried out under nitrogen gas atmosphere. Three products of phosphosulphurized Mannich bases have been prepared using three Mannich bases to give B1, B2 and B3, respectively.
Elemental analysis
The composition of each product has been determined quantitatively in weight percentages using the following standard methods of analysis:
-
(a)
Carbon: Dumas method.
-
(b)
Hydrogen: Dumas method.
-
(c)
Nitrogen: Kjeldahl method.
-
(d)
Phosphorus: IP 148/58 method.
-
(e)
Sulphur: X-Ray Sulphur Meter Model RX-500S TANAKA Scientific instrument.
-
(f)
Oxygen: By difference.
IR spectroscopic analysis
IR spectra of the synthesized compounds were determined by using FTIR spectrometer Model Type Mattson Infinity Series Top 961.
Determination of the Molecular Weights
The molecular weights of the prepared compounds were determined by using Gel Permeation Chromatography (GPC), Water 600 E.
Evaluation of the prepared compounds as lube oil additives
As antioxidants
The lube oil samples as well as its blends with 2 % by weight of each of the prepared additives were subjected to severe oxidation condition in the presence of copper and iron strips at 165.5 °C for 72 h. The Indiana test method of oxidation was used [5]. The oxidation stability of the lube oil blends was determined by taking samples at intervals of 24 h and up to 72 h of oxidation and the samples were tested for:
-
1.
Variation of viscosity ratio V/Vo
The variation of viscosity ratio (V/Vo) was determined using IP 48/86 method, where V = Kinematic viscosity at 40 °C of sample after oxidation, Vo = Kinematic viscosity at 40 °C of sample before oxidation.
-
2.
Change in total acid number (ΔT.A.N.)
The change was calculated according to IP 177/83 method, where ΔT.A.N. = total acid number of sample after oxidation—total acid number of sample before oxidation.
-
3.
Optical density using IR technique
The IR spectra of oxidized oils have been determined in the range of the carbonyl group absorbance (1,500–1,900 cm−1) (Fig. 1). The spectra have been superimposed upon that of the unoxidized oil. The absorbance (A) has been calculated according to:
where I = % transmittance of the oil after oxidation, Io = % transmittance of the oil before oxidation.
As dispersants
By using spot method [3] and [6]. Drops were taken from the Indiana oxidation apparatus after each 24 h intervals of oxidation and up to 72 h to make spots on special filter paper (Durieux 122) and the dispersancy of the sample were measured as follows:
The efficiency of dispersants has been classified as follows:
-
Up to 30 % no dispersancy
-
30–50 % medium dispersancy
-
50–60 % good dispersancy
-
60–70 % very good dispersancy
-
Above 70 % excellent dispersancy.
Results and discussions
Preparation of Mannich Bases
Three Mannich bases have been prepared by using dodecylphenol, formaldehyde and three types of polyethylenepolyamines, namely, ethylenediamine, diethylenetriamine and triethylenetetramine. The molar ratio of dodecylphenol, formaldehyde and amine is always 1:1:1.
The determined mean molecular weights of all products were found to be very near from that calculated theoretically for the chemical structures of prospected Mannich bases which could be illustrated as follows:

Elemental analysis of the prepared products was found also to be in accordance with that of prospected Mannich bases. Calculated mean numbers of different elements of prepared products, are given in Tables (1, 3), support the preparation of Mannich bases of chemical structures as given here in above. Results show an increase in molecular weight of Mannich bases with increasing the molecular weight of used ethylenepolyamines. The weight percentage of nitrogen- as well as- the mean number of nitrogen atoms/mole has also been found to be in accord with the above mentioned chemical structures of prepared Mannich bases.
Infra red spectrum of prepared Mannich bases is given in Fig. (2), which illustrate the following:
The N–H and O–H regions are overlapping (O–H at 3,650–3,200 cm−1 and the N–H at 3,500–3,300 cm−1). C–N band is in the range from 1,350 to 1,000 cm−1, C–O band is in the range from 1,250 to 1,000 cm−1, C–H alkanes in the range from 3,000 to 2,850 cm−1, C–H aromatic in the range from 3,000 to 3,150 cm−1, CH3 group appears at 1,450 and 1,375 cm−1, CH2 group appears at 1,465 and 1,375 cm−1, C=C aromatic appears at 1,475 and 1,600 cm−1. Para disubstituted ring appears as one strong band from 800 to 850 cm−1.
Phosphosulphurization of the prepared Mannich bases
Prepared Mannich bases were reacted with P2S5 to prepare phosphosulphurized Mannich bases. The determined mean molecular weights of all products were found to be very near from that calculated theoretically for the chemical structures of prospected phosphosulphurized Mannich bases which can be illustrated as follows:


Elemental analysis of prepared products were found also to be in accordance with that of prospected phosphosulphurized Mannich bases. Calculated mean numbers of different elements of prepared products are given in Tables 2 and 3), support the preparation of phosphosulphurized Mannich bases of chemical structures as given here in above. Results show an increase in molecular weight of phosphosulphurized Mannich bases with increasing the molecular weight of used polyethylenepolyamine. The weight percentage of nitrogen as well as the mean number of nitrogen atoms/mole were found to be in accord with the above mentioned chemical structures of prepared phosphosulphurized Mannich bases. Also the weight percentage of phosphorus and sulphur as well as the mean number of phosphorus atoms/mole and sulphur atoms/mole were found to be in accord with the above mentioned chemical structures of prepared phosphosulphurized Mannich bases. In all products, the ratio between mean number of phosphorus and sulphur is 1:2.
Evaluation of the prepared compounds as lube oil additives
As antioxidants
Effect of Mannich bases with different ethylenepolyamines
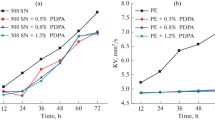
Mannich bases were prepared by using three different ethylenepolyamines. The prepared Mannich bases were added to a sample of “SAE-30” lubricating oil free from any additives and the blends obtained were subjected to severe oxidation conditions using the Indiana test methods at 165.5 °C with continuous and constant rate of stirring. Samples were taken at intervals of 24 h and up to 72 h of oxidation and tested for their oxidation stability expressed as increase in viscosity ratio (V/Vo),change in total acid number (∆T.A.N.) and optical density log (I/Io) compared with lube oil sample free from additives. Results are given in Figs. 3, 4, 5 which indicate the following:
All the prepared compounds impart better oxidation resistance properties to the lube oil compared with the undoped oil. Their efficiencies as lube oil antioxidants are very close to each other, since variations in the total acid number (∆T.A.N.), viscosity ratio (V/Vo) and optical density log (I/Io) is very small. This may be attributed to the presence of phenolic and amino groups in their structures. The mechanism of phenolic aromatic amine compounds as antioxidants were explained by phenolic and certain aromatic amine inhibitors function by donation of labile hydrogen from the groups (OH or NH) to stabilize the chain radicals; i.e., these inhibitors destroy the peroxide radicals and thus, the oxidation chain is broken. The marked efficiency of hindered phenols is attributed to their radicals being insufficiently reactive to abstract hydrogen from the hydrocarbon and initiate new oxidation chains. The presence of amine part in the structure of prepared Mannich base compounds neutralize some of the acidic products of oil oxidation. The change in total acid number (∆T.A.N.), viscosity ratio (V/Vo) and optical density log (I/Io) decrease with increasing the NH groups in the molecular of amine used. Thus, using triethylenetetramine gave better results than diethylenetriamine and ethylene diamine. In all cases, results indicate that there is a quite wide difference between the lube oil samples mixed with prepared products and that of using lube oil sample without additives.
Effect of phosphosulphurized Mannich bases reaction products
Mannich bases have been reacted with P2S5 to produce some products as lube oil additives containing sulphur and phosphorus elements. The prepared additives were added to the same lube oil sample “SAE-30” which free from additives. The lube oil samples mixed with prepared products were subjected to sever oxidation conditions using the Indiana test method of oxidation previously discussed and the results obtained are compared with those of undoped lube oil sample. Results are given in Figs. (6, 7, 8) which indicate that increasing the number of NH group enhance to some extent the oxidation properties of lube oil sample (∆T.A.N.), log (I/Io) and (V/Vo). Results also show that, using triethylenetetramine gave better results than diethylenetriamine and ethylenediamine. However, somewhat better results were obtained in case of phosphosulphurized Mannich bases due to the presence of phosphorus and sulphur. It is well known that sulphur compounds occurring naturally in mineral oils inhibit oxidation by reduction of peroxides. These compounds decompose hydroperoxide far in excess of amounts that could be accounted for by oxidation of the original inhibitor because the oxidized inhibitor also retards oxidation. It is suggested also that sulphur and phosphorus compounds react with metals to form sulphide and phosphide films that prevent the contact between metal surface and lube oil, hence prevent the catalytic action of metal on lube oil oxidation process.
As detergents/dispersants
The different prepared Mannich base additives were added to lube oil samples in a concentration of 2 wt%, using the spot test method. Results are given in Table 4 for Mannich base additives with different polyethylenepolyamines A1, A2 and A3, show clearly that the prepared additives have moderate to very good dispersancy power (60–80 %) for the sludge and solid particles formed during lube oil oxidation compared with lube oil only. Compounds A1- A3 at the beginning of oxidation (after 24 h) show somewhat moderate dispersancy power for lube oil as indicated from the data given in Table 4. After 48 h, their efficiencies as dispersant become clear. The efficiencies of these compounds are nearly the same, but compound A3 which prepared from triethylenetetramine, gives excellent dispersancy power.
The experimental data show that addition of such Mannich base compounds disperse solid particles into the oil and thus prevent their agglomeration and precipitation on metallic parts of engines causing their damage. It is clear from data which given in Table 4 that increasing the NH groups in the structures of the prepared compounds, increase their capacity in dispersing sludge and solid particles into the lube oil samples used. This may be explained by the fact that the NH groups form hydrogen bonds with polar groups of the oxidation products as alcohols, aldehydes, ketones, acids… etc.
Results given in Table 5 for the phosphosulphurized Mannich base additives with different polyethylenepolyamines B1, B2 and B3 indicate the following:
After 24 h of oxidation, all additives give good dispersancy power, this may be attributed to the fact that the solid particles produced through this period of oxidation are very little and thus easily dispersed in the lube oil.
After 48 h of oxidation, there is very little difference between their efficiencies. The difference is very clear when comparing their efficiencies with that of the lube oil sample without additives.
After 72 h, as the quantity of oxidation products becomes greater, the difference between the efficiency of these compounds becomes clear. Data given in Table 5 indicate also that increasing the number of the effective NH groups in the structure of the prepared additives increases their capacity in dispersing sludge and solid particles into the lube oil samples used. Results show also that additive B3 prepared from triethylenetetramine, gives the best efficiency as lube oil dispersant compared with the other additives.
Conclusions
The conclusions of the study are:
-
1.
The Mannich base obtained by using triethylenetetramine gave good results than the others.
-
2.
Phosphosulphurized Mannich base prepared from triethylenetetramine gave also good results than others.
-
3.
Phosphosulphurized Mannich bases impart good properties of Mannich bases as lube oil antioxidants.
-
4.
The prepared additives were evaluated as lube oil antioxidants as well as lube oil detergents/dispersants and found to be good lube oil additives.
References
Abdel-Azim A-AA, Nassar AM, Ahmed NS, Kamal RS (2009) Multifunctional additives viscosity index improvers, pour point depressants and dispersants for lube oil. Petrol Sci Technol 27:20–32
Nassar AM, Ahmed NS, Abdel Aziz KI, El-Kafrawy AF, Abdel-Azim A-AA (2006) Synthesis and evaluation of detergent/dispersant additives from polyisobutylene succinimdes. Int J Polym Mater 55:703–713
Gatis VA, Bergstrom RF, Wendt LA (1955) Society of automative engineers (SAE), Na 572
Hus SM (2004) Molecular basis of lubrication. Tribol Int 37:553
Lamp GG, Loance CM, Gaynor JW (1941) Indiana stirring oxidation test for lubricating oil. Ind Eng Chem Anal Ed 13:317–321
Najman M, Kasrai M, Bancroft GM, Davidson R (2006) Combination of ashless antiwear additives with metallic detergents. Tribol Int 39:342
Ahmed NS, Nassar AM, Abdel-Azim A-AA (2008) Synthesis and evaluation of some detergent/dispersant additives for lube oil. Int J Polym Mater 57:114–124
Pirro DM, Wessol AA (2001) Lubrication fundamentals, vol 3. Marcel Dekker Inc., New York, p 37
Rundnick LR (2003) Lubricant additives: chemistry and applications. Marcel Dekker, New York, p 1293
Santos JCO, Santos VJF, Souza AG, Sobrinho EV, Fernandes VJ Jr, Silva AJN (2004) Thermoanalytical and rheological characterization of automotive mineral lubricants after thermal degradation. Fuel 83:2393–2399
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kamal, R.S., Ahmed, N.S. & Nasser, A.M. Study the efficiency of some compounds as lubricating oil additives. Appl Petrochem Res 3, 1–8 (2013). https://doi.org/10.1007/s13203-012-0020-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-012-0020-8