Abstract
To address the problems of wall collapse, cuttings slurrying, and scaling on the inner wall of the drill pipe, which often occur in small-diameter diamond wireline core drilling, the inhibition properties of polyvinyl alcohol (PVA) was investigated, and the formulation of solids-free drilling fluid with PVA as the inhibitor were completed. PVA has the advantages of fast adsorption and easy regulation of rheological properties Firstly the inhibition effect of PVA was compared with that of common inorganic salts (sodium chloride, NaCl, potassium chloride, KCl) by bentonite dispersion test, linear swelling test, shale rolling recovery test and mud ball immersion test in this study. Then, the inhibition mechanism of PVA was analyzed with potentiometric particle size tests, Fourier transform infrared (FT-IR) and X-ray Diffraction (XRD) measurements. Based on the outstanding inhibition performance of PVA, tackifiers and filtration reducers were preferred through the compatibility test. And finally, the effects of various contaminants on the comprehensive performance of the formulated solids-free drilling fluids were evaluated. The results showed that PVA exhibited better inhibition of clay hydration and dispersion in shale recovery and linear swelling compared to NaCl and KCl, which was particularly evident in the mud ball immersion test. FT-IR and XRD tests revealed that the inorganic salts were used to replace the cations with larger radius and high degree of hydration in the clay layer by ion exchange ti achieve the effect of clay de-watering by reducing the spacing of the clay interlayer and the electrostatic repulsion between the particles. However, PVA is strongly adsorbed on the clay surface in the form of hydrogen bonds due to its unique multi-hydroxyl chain structure, forming a hydrophobic barrier to prevent water molecules from entering the clay layer, thus inhibiting the hydration and swelling of the clay. Using PVA as an inhibitor, compounded with xanthan gum, sulfonated lignite resin and sulfonated gilsonite (FT-1), the solids-free drilling fluid is promising for use in diamond wireline core drilling in complex formations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, with the increase in world energy demand and the continuous development of ore search technology, the depth of drilling holes has been increasing, and the possibility of encountering various complex strata as well as the occurrence of in-hole complications has also been gradually increasing. Diamond wireline coring drill has become the mainstream of core boring technology as its advantages of shortening the drilling time, reducing the labor intensity of workers, improving the coring rate, lowering the cost, etc., and has played a great role in the world’s geological ore search and exploration (Zeng 1980; Lu et al. 2012). It means that during the drilling process, when the core fills the core pipe, instead of lifting the drill pipe to retrieve the core, the core stored in the core pipe is lifted out of the borehole by using the drill pipe as a channel with ropes and special salvage tools. Since this method does not require lifting the drill pipe out of the hole, the time spent lifting and lowering the drilling tools is greatly reduced. Statistically, the time spent lifting and lowering drilling tools accounts for approximately 30–40% of the total production time. In order to increase pure drilling time and improve drilling efficiency, wireline coring is one of the most effective coring drilling methods (Hu et al. 2022; Guo et al. 2023).
However, unlike traditional drilling coring techniques, wireline coring has a remarkable feature that the outer annular space gap between the outer wall of the drill pipe and the hole wall (1.5–3 mm) as well as the inner annular space gap between the inner wall of the drill pipe and the core pipe (0.5–1.0 mm) are both very small. This peculiarity is accompanied by a series of complex in-hole problems such as hole wall destabilization, formation leakage, etc., which puts higher requirements on drilling fluid technology (Zhang et al. 2021; Tang et al. 2022; Wang et al. 2024). Especially when drilling water-sensitive formations, due to the characteristics of small diameter core holes, mudstone is extremely easy to absorb water, swell and disperse, and the resulting viscous particles will adhere to the surface of the drilling tools, coupled with the small gaps between the drill pipe and the hole wall, and between the drill bit and the bottom of the well, which will cause an increase in the rotational resistance to drilling, and ultimately lead to the phenomena of poor circulation of drilling fluids and holding the pump. This requires the drilling fluid to be characterized by low viscosity, few solids, strong inhibition and good lubrication. Therefore, solids-free or low-solids drilling fluids have begun to receive attention from researchers. Commonly used solids-free drilling fluids are mainly composed of one or more synthetic or natural polymers, such as polyacrylamide (PAM), partially hydrolyzed polyacrylamide (HPAM), xanthan gum (XC), vegetable gum, etc. However, due to the lack of solid particles, it is difficult for drilling fluids to form mud skins with a certain strength on the well wall when drilling mechanically unstable formations such as fractures and loose layers (Li et al. 2017; Sheth et al. 2020). Therefore, the inhibition performance of drilling fluids without solid content has a very important role in wireline core drilling.
Although inorganic salts like potassium chloride (KCl) and calcium chloride (CaCl2) have been widely researched and applied because of their low price, easy accessibility, and obvious effect of inhibiting clay dispersion, they also suffer from problems such as difficulty in controlling the amount of filtration loss, serious corrosion of drilling tools by charged ions K+, and shortening of the service life of the drilling tools (Berry et al. 2008; Ma et al. 2022). Subsequently, treatments such as silicate and polyvinyl alcohol have been appreciated by researchers for their outstanding anti-collapse effects (API 2006; API 2009; Fan et al. 2022; Duan 2022; Rana et al. 2022). Unfortunately, the application of silicate drilling fluids requires strict control of environmental alkalinity values. Once the pH value falls below 11, silicate molecules condense internally, leading to uncontrolled rheology of the drilling fluid (Huang et al. 2016; Li 2023). Although the appropriate addition of bentonite to form a low-solid system can alleviate the problem of runaway rheology, the inclusion of submicron clay particles not only reduces the mechanical drilling speed, but also increases the risk of scaling on the inner wall of the drill pipe (Muhammed et al. 2021).
Polyvinyl alcohol (PVA) is a linear, water-soluble, non-ionic carbon chain polymer derived from the alcoholysis of poly (vinyl acetate), which has the advantage of being inexpensive and readily available. In addition, it is odorless, non-toxic, and completely biodegradable. Previous studies have mainly focused on the film-forming properties of PVA, which some scholars believe is key to maintaining well wall stability and does have a good particulate effect in drilling and constructing wells in fractured-prone and collapse-prone formations (McDonald 2012; Zhao et al. 2014; Li et al. 2021; Sharma et al. 2022). However, few researchers have explored the inhibition mechanism of PVA on clay particles, and the advantage of PVA as a relatively small molecular weight polymer that can quickly react with clay particles due to competitive adsorption to encapsulate clay particles in drilling fluid treatment has rarely been mentioned.
The membrane filtration properties of PVA are demonstrated by its ability to effectively reduce water loss (İşçi et al. 2006; Needaa et al. 2016; Deville et al. 2017; Tan et al. 2023), but the rheological properties of PVA have been less well described. It is worth noting that maintaining good rheological properties in the absence of solid phase clay in the drilling fluid system is also a challenge for wireline coring fluids. İşçi and Turutoglu (2011) determined that polyvinyl alcohol has a stabilizing effect on the dispersion by observing the change in rheological properties of bentonite and seafoam mixtures in the presence of PVA. By comparing the effects of PVA and methyl cellulose on the rheological properties of bentonite slurries, Balaga and Kulkarni (2022) concluded that both substances were effective in improving the dynamic shear of slurries. These studies proved the effect of PVA on fluid flow patterns. However, in the actual construction of core drilling, the problem of insufficient rock-carrying capacity may still exist when dealing with well collapses due to the small addition of PVA. Therefore, it is necessary to find auxiliary treatment agents compatible with PVA to solve the problem of rheological performance regulation of solids-free drilling fluids.
In order to give full play to the advantages of solids-free drilling fluids in deep wireline core drilling, this study, on the one hand, macroscopically evaluated the inhibitory effects of PVA on bentonite hydration and dispersion through shale rolling recovery test, linear swelling test and mud ball immersion test, and compared them with commonly used chloride salts. On the other hand, the inhibition mechanism of PVA on the hydration and swelling of clay particles was revealed microscopically by combining Fourier transform infrared spectroscopy and X ray diffraction measurement. Then, on the basis of PVA as an inhibitor and XC as a flow regulator, a strongly inhibited solids-free drilling fluid with good rheological and filtration properties was identified through compatibility tests, which not only stabilizes the well wall, but also carries the rock cuttings smoothly and copes with a variety of pollutants. This research work provides an alternative solids-free drilling fluid for loose, water-sensitive, water-soluble and other complex formations encountered in deep exploration and scientific drilling construction, and is expected to be popularized and applied in similar deep-hole wireline core drilling, as well as providing theoretical references for the study of inhibitors.
Experimental study
Materials
NaCl, KCl, NaOH, all of which were analytically reagents and purchased from Sinopharm Chemical Reagents Co., Ltd., China. Potassium chloride (KCl) is a common and inexpensive commercially available swelling inhibitor, so it was introduced as a control group for swelling inhibition performance. Bentonite was mainly composed of montmorillonite, supplied from Tianjin Tianyu Bentonite Co. Ltd., China. Xanthan gum (XC) was provided by Xi’an Huibang Biological Engineering Co., Ltd., China. Sulfonated lignite resin (SPNH), sulfonated lignite (SMC), sulfomethyl phenol formaldehyde resin (SMP), sulfonated gilsonite (FT-1) were obtained from Shangdong Deshunyuan Petroleum Technology Co. Ltd., China. Sodium carboxymethyl cellulose (CMC) and polyanionic cellulose (PAC) were purchased by Pingxiang Lianke Chemical Co.
Polyvinyl alcohol (PVA) is a synthetic copolymer made by polymerizing vinyl acetate and partial hydrolyzing the acetate groups [–(CH2CHOH)n–(CHOCOCH3)m–] in the resulting polymer. The chemical and physical properties of commercial polyvinyl alcohols vary depending on their degree of polymerization (Table 1). Grade #0588, #1788, #2088, #2488 PVA were supplied by Shanghai Jiaying Industrial Development Co., China.
The main instruments used in the test include: ZNS-4 Multiple Medium-pressure Filtration Tester, ZNN-D6 Six-Speed Rotational Viscometer, GJSS-B12K Inverter High Speed Mixer, XGRL-4A Hot Rolling Furnace, all purchased from Qingdao Haitongda Specialized Instrument Co., Ltd. JA5003 Electronic Analytical Balance, produced by Shanghai Precision Instrumentation Co., Ltd. Zetasizer Nano ZS 3000 Potential-Particle Analyzer, manufactured by Malvern Instruments Ltd., UK. Nicolet 6700 Fourier Transform Infrared Spectrometer, manufactured by Thermo Fisher Scientific Co., Ltd., USA. X Pert PRO MPD diffractometer, manufactured by PANalytical B.V., Netherlands.
Preparation of mud balls
Bentonite powder and distilled water were mixed well at a mass ratio of 2:1, and kneaded by hand for 5 min to form balls. The mass of one ball was 20 g to minimize testing errors. The kneaded balls of clay were placed in an oven and heated at 40 ℃ for 24 h.
Preparation of various slurries
Base slurry
8 g of bentonite was poured into 400 mL of deionized water, and then stirred with a GJSS-B12K inverter high-speed mixer at 8000 rpm for 30 min to prepare the basic mud (2 wt%) without additives, named as the base slurry. Increasing the bentonite content (e. g. 24 g, 36 g, 48 g) resulted in bentonite slurries with different concentrations (6 wt%, 9 wt%, 12 wt%, mass of dry powder of agent/volume of water). The high concentration slurries were prepared to facilitate the observation of significant phenomena in the experimental testing of bentonite dispersion inhibition.
Inhibitor solution (PVA)
The solution consists of two preparations, deionized water and bentonite. Bentonite in varying amounts (4 g, 8 g, 12 g and 16 g) was slowly poured into 400 mL of deionized water and then stirred at 6000 rpm for 30 min to prepare PVA solutions at concentrations of 1 wt%, 2 wt%, 3 wt%, and 4 wt%, respectively.
Inorganic salt solution
Prepared as the PVA solution, 400 mL of inorganic salt solution containing 4 g, 8 g, 12 g or 16 g of NaCl/KCl (1 wt%, 2 wt% 3 wt% and 4 wt%), respectively, were prepared.
Test muds
After 24 h of full hydration, the base slurry was stirred at 6000 rpm for 20 min and then tested for rheological properties. In addition, an amount of powder (NaCl, KCl, PVA) was slowly added to the base slurry, and the rotational speed was set to 3000 rpm for 20 min to allow for competitive dissolution of the inhibitors.
Methods
For drilling fluid inhibitors, the most commonly used inhibition evaluation methods are mud ball immersion test, shale rolling recovery test and linear swelling test. Although each method has certain shortcomings, such as the consistency of manually rolled mud balls is not well controlled in mud ball immersion test, the result of linear swelling test is greatly affected by the type of clay, and the shale rolling recovery test measures the inhibitor’s ability to inhibit the hydration and dispersion of drilling cuttings, these performance evaluation methods reflect the strength of inhibitory ability and the trend of change on a macro scale, which is sufficient to conduct a comparative study on the inhibition effect of PVA and commonly used inorganic salts. Based on this, it is proposed to explain the inhibition mechanism of PVA by combining microscopic analysis and performance evaluation.
Mud ball immersion test
Mud balls of uniform size and shape were immersed in the inhibitor solution and the changes in size and surface of the mud balls were observed after a period of time. To obtain reliable test results, at least three mud ball immersion tests were performed in each inhibitor solution.
Shale rolling recovery test
Shale cuttings with sizes between 1700 and 3350 \(\mathrm{\mu m}\) were dried at 120 ℃ for 4 h. Then, 350 mL of a solution containing NaCl, KCl, or PVA was mixed with about 20 g of the dried cuttings and then subjected to hot rolling at 150 ℃ for 16 h. After cooling, the cuttings were carefully rinsed and filtrated by a 380 \(\mathrm{\mu m}\) sieve, then dried to constant weight at 90 ℃, and weighed. The hot rolling recovery rate was calculated as follows:
where M is the recovered mass in grams, and R is the rolling recovery rate in %.
Linear swelling test
The linear swelling height of bentonite was investigated using a NP-01 normal temperature and pressure linear swell meter. The bottom of the pressure tank was lined with filter paper, and a total of 10 g bentonite was poured into the mold and then compressed at 10 MPa for 5 min using a pressurizer. After soaking the bentonite core with 120 mL of the test solution, the tank was mounted on a swelling meter, which was calibrated to show a value of 0.00. Finally, a computer was utilized to record the change in swell height over time.
Rheology and API filtration tests
The rheological properties for the fluid system were obtained using a ZNN-D6 six-speed rotational viscometer. The readings at 300 rpm and 600 rpm could be marked as Ф300 and Ф600, respectively. The apparent viscosity (AV), flow index (n) and consistency coefficient (K) were calculated using the following formulas:
In the case of polymers, their internal structure may change under the action of a certain flow field, thus causing a change in fluid viscosity. Such fluids are known as generalized Newtonian fluids. Representative models include the Bingham model, the power law model, and the Carson model.
The Bingham model applies to plastic fluids, i.e., fluids that will flow when subjected to more than a critical shear stress, and once flow occurs, their viscosity remains constant and exhibits Newtonian properties. If the shear force of such a fluid is too low, it is unfavorable for it to carry rock chips, and also tends to cause bottom complication once pump stops. If the shear force is too high, the formation may hold leakage due to excessive flow resistance, which is obviously not applicable to the wireline coring drilling process.
Apparent viscosity is used to observe and analyze the viscosity change of bentonite slurry, as well as the degree of hydration and dispersion of clay before and after the addition of PVA, so as to evaluate the inhibition of PVA. In addition, the apparent viscosity can be used to analyse the viscosity increasing effect of tackifiers, which is one of the evaluation indexes to judge the compatibility of PVA with other treatment agents.
Although the apparent viscosity already reflects the rheological properties of drilling fluids to a certain extent, it is generally accepted that the power-law model better characterizes the flow of drilling fluids in annular space gap, therefore two important rheological parameters, flow index (n) and consistency coefficient (K), were chosen to characterize the flow pattern of drilling fluids in the wireline coring. A moderate consistency coefficient carries the rock and keeps the well bore open, while good shear dilution minimises the impact of circulating flow of drilling fluid on the stability of the well wall.
Particle distribution-zeta potential measurement
Different types of inhibitors were dissolved in water to prepare a 2 wt% test solution. Then 2 g of bentonite were poured into the test solution and shaken at 1500 rpm for 24 h to reach adsorption equilibrium. The dispersion was centrifugated at 4500 rpm for 30 min, then the supernatant was diluted with distilled water to the desired concentration range (0.05–0.5 wt%) and ultrasonically dispersed for 30 min before measurements. Finally, the particle size and zeta potential of the dispersion were measured using a Zetasizer Nano ZS 3000 Potential-Particle Analyzer.
Fourier transform infrared (FT-IR) measurement
FT-IR spectroscopy was employed to characterize the surface functional groups of montmorillonite (the main component of bentonite) treated with PVA in the optical range of 400–4000 cm−1. Prior to testing, to avoid the purity of the samples affecting the accuracy of the experimental results, the powder was washed and precipitated by anhydrous ethanol and acetone for several times, and dried, grinded and dried repeatedly. And then, around of 1 mg of the obtained bentonite was added into 100 mg of potassium bromide (KBr) and well ground to obtain a fine powder. And the fine powder was then poured into a mold which was subjected to 20 MPa for 2 min.
X-ray Diffraction measurement (XRD)
Bentonite powder (1 g) was added to 100 mL of NaCl, KCl and PVA solution at various concentrations (1–4 wt%). The solution was stirred at high speed for 30 min. After centrifugation, the bottom precipitation was washed, filtered and dried at 50 ℃ and subjected to X-ray diffraction tests on an X Pert PRO MPD diffractometer. Scans were taken from a 2 \(\theta\) angel from 3 ℃ to 20 ℃, step size 0.1, and scan time per step of 10 s.
Result and discussion
The rheological properties of PVA solution
Figure 1 represents the variation of apparent viscosity of the solution of PVA with different molecular weights at different addition. It was found that the higher the molecular weight of the polymer, the higher the viscosity of the solution at a given concentration. At 4 wt% and 5 wt% addition, the viscosity of the high polymerization #2488PVA is almost 10 times that of the low polymerization #0588PVA. This is not difficult to explain, as high molecular weight polymers, when formed into solution, need to be dissolved slowly and then stretched due to their own large molecular volume. This process binds a large amount of free liquid to the PVA molecules and also impedes the free movement of the medium, which macroscopically manifests itself in large viscosity values. It is also clearly observed that the viscosity of the solution increases with the addition of PVA. Especially when the concentration of PVA exceeds 4 wt%, the increase in the solution viscosity is significantly accelerated. The reason for this is that PVA molecules have branched chains, and when the number of chains in the solution exceeds a certain value, entanglement between the molecules occurs, and the higher the concentration, the more entangled points, which is manifested as a rapid increase in viscosity on a macroscopic level.
The shear rate-shear stress curves for different concentrations of #2488PVA solution are plotted in Fig. 2. As the concentration increases, the shear stress of the PVA solution at the same shear rate increases significantly, consistent with the change in viscosity. The PVA solution begins to flow at a very small shear stress, as shown by the curve through the origin in the graph, indicating that the solution has good pumpability. However, unlike Newtonian fluids, the shear rate of the PVA solution always varies with stress. As the additions increases to 4 wt% and 5 wt%, the relationship between shear rate and stress is not a straight line, and the viscosity decreases with increasing shear rate, indicating that the fluid can not be uniformly sheared at this point.
At present, it is generally believed that the power-law model is closer to the flow characteristics of the actual drilling fluid in the lower shear rate range in the annulus, therefore, this paper adopts two important parameters of the power law fluid—the flow index (n) and consistency coefficient (K) to describe the flow law of the solution with different addition of PVA, and the results are shown in Table 2. It was found that the n value basically stayed around 1 when the addition amount was in the range of 1–3 wt%, presenting a Newtonian flow pattern. When the additive amount of PVA was 4 wt% and 5 wt%, the n value decreased to 0.95 and 0.87, respectively, and the solution showed some pseudoplastic features. This is basically consistent with the flow characteristics on the shear stress-shear rate curve.
In general, PVA has a relatively small influence on the rheological properties of the fluid. In order to meet the requirements of the viscosity range and the strong inhibition performance of the drilling fluid, 1 wt% #2488 PVA was selected as the basic inhibitor for the wire-line coring drilling fluid formulation.
Inhibition of bentonite dispersion test
The bentonite slurry inhibition test is the simplest method for evaluating the inhibition performance of an inhibitor. By this method, the maximum amount of bentonite that can be treated by a given amount of inhibitor can be determined. If the inhibitor’s inhibition performance is strong, the clay particles will be less hydrated and dispersed, and the rheological properties of the drilling fluid will be maintained to the maximum extent possible when drilling encounters water-sensitive formations, thus protecting the well stability. From this perspective, different amounts of bentonite were weighed and placed in water, 1 wt% PVA solution, 1 wt% NaCl solution and 1 wt% KCl solution, respectively, and the apparent viscosity of the different solutions were measured after rolling at 80 ℃ for 16 h to analyze the inhibition of bentonite dispersion by NaCl, KCl, and PVA, and the results are shown in Fig. 3.
The apparent viscosity of the slurry gradually increased with increasing bentonite concentration. In contrast, at the same bentonite addition, the KCl solution showed the smallest increase in viscosity, the slurry without additives (deionized water) had the largest increase in viscosity, while the NaCl and PVA solutions exhibited significant increases in viscosity. It is worth noting that at 6 wt%, 9 wt%, 12 wt% and 15 wt% of bentonite, the dynamic shear stress of the PVA solution is almost zero, while the dynamic shear stress of the NaCl solution is 0.5 Pa, 1.25 Pa, 2 Pa, 4 Pa, 6.5 Pa, respectively, indicating that the inhibition effect of PVA on bentonite is better than that of NaCl.
Figure 4 shows the dispersion of bentonite in PVA solution. It is observed that the bentonite is in a flocculated state and deposited at the bottom of the beaker. This is conducive for inhibiting hydrated dispersion in water-sensitive formations and purifying surface drilling fluids.
Linear swelling test
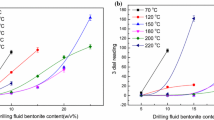
The heights of linear swelling of bentonite in different concentrations of KCl, NaCl and PVA solutions as well as deionized water are presented in Fig. 5. PVA inhibited the strongest inhibition of shale swelling, followed by KCl, and NaCl was slightly better than deionized water. Bentonite is a silicate mineral with a large amount of negative charge in the interlayer. The negatively charged clay repel each other in water, which leads to clay swell.
All curves show that the swelling height of bentonite in PVA solution is lower than that in other solutions. After 24 h of immersion, the swelling heights of bentonite in 1 wt% PVA and KCl solutions were 1.97 mm and 3.25 mm, respectively, which indicates that PVA has good inhibition performance. Interestingly, the swelling heights of the clay cores in low concentration salt solutions (1 wt% KCl and 1 wt% NaCl) were surprisingly larger than in distilled water, which contradicts the conclusion that inorganic salts have inhibition ability. The reason for this phenomenon is that the results of the linear swelling test are strongly influenced by the type of clay. The bentonite selected for this test was provided by Tianjin Tianyu Bentonite Co., Ltd. In China and was analyzed as pure calcium-based bentonite in the linear swelling experiment, but the bentonite may have been mixed with some sodium-based bentonite during the production process without being taken into account, which led to the test results being different from the expected results. Therefore, it is necessary to make as many comparisons as possible when selecting test samples to avoid test errors caused by the samples themselves. Nevertheless, it was found that the swelling height of the bentonite cores decreased by 16.3%, 28.5% and 34.5%, respectively, as the concentration of PVA increased from 1 to 4 wt%. At 4 wt% PVA and KCl solutions, the swelling height of the bentonite core decreased to 1.33 mm and 2.59 mm after 24 h, respectively. The inhibition ability of PVA was stronger compared to the commonly used inorganic salts, with an overall ranking of PVA > KCl > NaCl.
Shale rolling recovery test
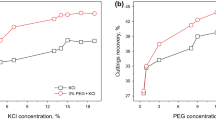
The inhibition performance of PVA was evaluated by comparing the rolling recovery rates of shale cuttings in different solutions as shown in Fig. 6.
The cuttings recoveries were found to be less than 30% for 1 wt% NaCl solution and distilled water, and only about 40% for 1 wt% KCl. All others solutions had recoveries above 60%. PVA solutions had the highest recoveries, especially #2488 PVA, at 78%, which was much higher than the inorganic salt and deionized water. The higher the degree of polymerization, the higher the recovery and the stronger the inhibition. From the appearance of the particles, the drill cuttings recovered with deionized water and salt solutions were very fine, while the cuttings recovered with the PVA solution basically maintained their original shapes, indicating that PVA has a strong ability to stabilize the cuttings and prevent them from dispersing as a result of hydration during upward return in the well.
Figure 7 shows the recoveries obtained by varying the amount of PVA. The results indicate that the higher the concentration of PVA, the higher the shale recovery. Considering the effectiveness and cost, a range of 1–2 wt% is sufficient for full inhibition.
Mud ball immersion test
The mud balls were immersed in water, NaCl solution, KCl solution and PVA solution, respectively, and the changes in the appearance of the mud balls with the immersion time were observed, and the results are shown in Fig. 8.
The cracks and volume of the mud balls increased with the increase in time. After 24 h of immersion, the mud balls in deionized water collapsed quickly, half of them collapsed rapidly after 30 min, and after 6 h, the whole spilt into two pieces with longitudinal and transversal cracks appearing on the surface. This suggests that bentonite is highly susceptible to be hydrated and dispersed. The mud balls immersed in 4 wt% NaCl disintegrated on the surface after 1 day, and the cracks were bigger and deeper, with a tendency to split into two halves. When immersed in 4 wt% KCl, tiny cracks began to appear on the surface of the mud ball, and the number of cracks increased after 24 h. However, the mud balls immersed in 1 wt% PVA did not show any obvious cracks. The above results show that both KCl and PVA have strong advantages in inhibiting bentonite dispersion, but the inhibition ability of PVA is better than that of KCl.
Discussion of the inhibition mechanism of PVA
Changes in particle size distribution of bentonite
The clay particles are separated into tiny particles and individual sheet layers by hydration (Jia et al. 2019). The particle size distribution of the particles reflects their degree of hydration and the inhibition performance of the inhibitor (Murtaza et al. 2022). The larger the clay particles in the dispersion, the better the inhibition performance of the swelling inhibitor (Li et al. 2019).
The particle size distribution curves of the base slurry (2 wt% prehydrated bentonite slurry) treated with 1 wt% and 4 wt% inhibitors are presented in Fig. 9. First, both the chloride salt and the PVA caused the bentonite particles to become larger, which alters the particle size distribution in the system. The differential distribution curve was shifted to the right and the range of the particle size cumulative distribution curve became wider. Secondly, in brine, the bentonite particles flocculated due to the compression of the diffuse bilayer of bentonite by sodium and potassium ions, manifested as an increase in particle size.
The particle size was larger in the PVA solution compared to the brine and increased with increasing PVA concentration. The cumulative distribution curve shifted to the right with increasing PVA concentration, which implied that the proportion of large particles increased. The results indicate that the molecular chains of PVA can wrap around fine clay particles and bridge them through hydrogen bonds, thus increasing the particle size of bentonite particles. Therefore, PVA can inhibit the hydration and dispersion of clay minerals.
Reduction of electric potential of clay particles
To a 1 wt% pre-hydrated bentonite dispersion, different concentrations of inhibitors (NaCl/KCl/PVA) were added and the pH of the system was adjusted to 9. After stirring for 30 min, the system was allowed to stand in a closed chamber for 24 h. The Zeta potential of the suspension was measured using the Zetasizer Nano ZS-3000 Potential-Particle Meter, and the results are shown in Fig. 10.
The zeta potential reflects the electrical potential of the double layer at the interface between the particles moving in the electric field and the surrounding liquid. The charge characteristics of the clay particle surface can be characterized by the zeta potential. In the absence of PVA, the bentonite dispersion exhibited a zeta potential value of − 41.5 mV, indicating a dispersed system. Increasing the concentration of the inhibitor resulted in a decrease in the zeta potential of the bentonite dispersion. The effects of the various inhibitors on the zeta potential of the clay particles varied, with the addition of KCl and NaCl causing the ionized K+ and Na+ in solution to act as counter ions into the adsorbed layer of the montmorillonite diffusion bilayer, leading to a decrease in the number of ions in the diffuse layer, a reduction in the thickness of the bilayer, and thus reducing the electric potential. Sodium salts are relatively ineffective. None of the chloride salts were able to reverse the electrical properties of the clay particles and the Zeta potential of the clay particles remained negative.
With the increase of PVA adsorption, a zeta potential of only about − 10 mV was observed for the clay particles in the base slurry containing 3 wt% PVA. It is evident that PVA is able to reduce the electrostatic repulsion and surface hydration short-range repulsion of the clay by reducing the negative charge of the clay particles, thus inhibiting the hydration and swelling of the clay. Although PVA also fails to reverse the electrical properties of the clay particles, it does have relatively significant inhibition properties compared to conventional salts. It is worth noting that PVA’s change in the charge of the clay particles is relatively gentle and does not cause an abrupt change in electrical properties, which facilitates its compatibility with other treatment agents.
Hydrogen bonding
The FTIR analysis of bentonite, PVA and bentonite/PVA composites were analyzed as shown in Fig. 11. The pristine bentonite spectra exhibited typical characteristic absorption peaks of clay minerals. The peak corresponding to the structural hydroxyl stretching at 3626 cm–1 was attributed to bentonite (Fig. 11c). The hydrogen vibration indicated by a broad peak centered at 3426 cm–1 was assigned to O–H stretch. A sharp peak arousal from Si–O stretching at 1036 cm–1 and other sharp peaks arousal from Si–O bending at 519 cm–1 and 467 cm–1 were also observed in the FTIR spectrum of bentonite.
In the bentonite/PVA spectra, the stretching vibration of O–H of the montmorillonite (bentonite) shifted to 3622–3618 cm−1, with the band moved to a lower frequency of about 10 cm–1, and this shift might be attributed to the formation of hydrogen bonding between the H+ of the montmorillonite and the O–H of PVA (Sabzevari et al. 2022). The O–H of the water molecules hardly vibrates, implying that the interlayer water of the clay minerals did not change significantly, i.e., it was not replaced by PVA. In addition, the Si–O stretching frequencies broadened and peaks appearing at 1040 cm–1 in the montmorillonite spectrum moved to 1042 cm–1, suggesting the formation of hydrogen bonding between the Si–O of the clay minerals and the OH of the PVA (Li et al. 2018; Sun et al. 2021). These results suggest that PVA molecules are firmly bounded and interacted with each other near the surface of clay particles.
Compression of clay hydration layer spacing
Different concentrations (1–4 wt%) of NaCl, KCl and PVA were taken and added into the bentonite suspension (2 wt%), stirred thoroughly for 30 min and then left closed for 24 h. The appropriate amount of the mixture was then centrifuged and after pouring off the upper clear liquid, the lower precipitate was taken directly for the determination of the interlayer spacing by X-ray diffraction analysis, and the results are shown in Fig. 12.
After compete hydration of the bentonite, the layer spacing increased to 2.02 nm. The main reason is that the water molecules enter into the crystal layer of the mineral and exchange with the cations of the bentonite mineral, which increases the layer spacing due to the presence of sodium ions in the form of hydrated ions of larger sizes between the crystal layers.
Figure 12a shows that the spacing between the montmorillonite layers decreased with the addition of NaCl, but did not change much with increasing concentration. This is because the Na+ in NaCl is the same ion as that in the montmorillonite layers, and when the addition of NaCl is sufficient to form a concentration difference between the montmorillonite interlayer and the solution, some of the water molecules are repelled out of the interlayer by the difference in chemical potential, which leads to a decrease in the montmorillonite interlayer spacing.
In addition, it is clearly observed that the montmorillonite interlayer spacing gradually decreased with the increase of KCl concentration (curve b). The layer spacing decreased to 1.49 nm at a KCl concentration of 3 wt%, and to 1.45 nm when the concentration continued to increase to 4 wt%. The reason for this is that the diameter of K+ coincides with that of the hexagonal oxygen ring at the bottom of the tetrahedron of the clay silica, and the smaller hydration energy of the K+ makes it easy to insert into the clay crystal layer. Therefore, Ca2+ and Na+, which have larger hydration radii and hydration energies, replace K+. The K+ entering the hexagonal oxygen ring is not easy to separate from the clay wafer after firmly connected, which will inhibit the hydration and swelling of shale. For the bentonite used in this experiment, KCl had good inhibition at a concentration of about 3 wt%.
When the nonionic compound PVA encounters clay particles, multiple hydroxyl groups on its molecular chain will form hydrogen bonds with the polar groups on the surface of the crystalline layer of the clay particles and adsorb onto the surface of the clay particles. It was found that the increase in the montmorillonite layer spacing was insignificant at a concentration of 1 wt% of PVA, and the layer spacing remained almost constant as the concentration was further increased to 4 wt%.
Unlike NaCl and KCl, which compress the layer spacing to exclude water molecules, PVA is a low molecular weight nonionic polymer that can replace water molecules in competitive adsorption on the clay surface due to its low molecular weight, fast adsorption speed, and strong cementation. When the number of PVA molecules is large enough, it can form a hydrophobic cover layer on the clay surface, which is macroscopically expressed as a "membrane" structure, thus preventing water molecules from intruding into the inner layer of clay crystals, and achieving the effect of inhibiting hydration and dispersion (the mechanism is shown in the Fig. 13).
Optimization of solids-free drilling fluid systems
Evaluation of PVA’s compatibility
-
1.
Compatibility with tackifiers
XC, HV-CMC, PAM, and HV-PAC were selected as tackifiers and the rheological parameters of a 1 wt% PVA solution (base solution for the solids-free drilling fluid system) modified with 0.2 wt% tackifier were measured, and the results are shown in Fig. 14. All types of treatments increased the viscosity of the base solution, with XC having the most significant viscosity increasing effect. Fitting the rheological parameters revealed that the n-values of the four agents were found to be 0.69, 0.98, 0.95, and 0.99, respectively, which indicated that the PVA solution with XC exhibited obvious pseudoplastic characteristics. In order to further investigate the effect of XC addition on the rheological properties, 0.05 wt%, 0.1 wt%, 0.15 wt% and 0.2 wt% of XC were added to the PVA solution, and the fitting results are shown in Fig. 15. The n values of 1, 0.95, 0.84, and 0.69 indicate that the shear shinning characteristics increase with the increase of the XC addition. Unlike the linear molecular structure of PAM, HV-CMC, and HV-PAC, XC is a double helix structure with strong structural viscosity, and thus has a good potential for application in drilling fluids, especially in diamond wireline core drilling (Mohamed et al. 2009; Emmanuel et al. 2020).
-
2.
Compatibility with filtration loss reducers
The funnel viscosity of the 1 wt% PVA + 0.2 wt% XC solution was 28 s, and the medium-pressure filtration loss was 18 mL. For core drilling, this viscosity value meets the requirements for use, but the large filtration loss is not conducive to well stabilization in water-sensitive formations. To further reduce the filtration loss, a non-viscosity filtration loss reducer was selected for formulation optimization. As shown in Fig. 16, the AV and API filtration loss were measured for 1 wt% PVA + 0.2 wt% XC modified by 2 wt% SPNH, SMP, FT-1 or SMC. FT-1 had the best loss reduction, SMP had the worst loss reduction, there was little difference in loss reduction between SPNH and SMC. In terms of viscosity, SMP had the greatest effect on viscosity, followed by SPNH and FT-1, while SMC had the least effect on viscosity. The combined results of rheological and filtration properties showed that SMP was the least compatible with PVA and was not suitable for use as a filtration loss reducer in this formulation.
Considering that FT-1 can seal micropores and microfractures in the formation, it is a good anti-collapsing agent and therefore more suitable to be used as a treatment agent for controlling the filtration loss. SPNH, as an anti-temperature anti-salt loss reducer, can improve the ability of drilling fluids to cope with the contamination of high mineralization. Therefore, the formulation of the solids-free drilling fluid was determined as: 1 wt% PVA + 0.2 wt% XC + 1 wt% FT-1 + 1 wt% SPNH.
In the process of deep hole wireline coring, the wall protection principle of the solids-free anti-collapse drilling fluid is shown in Fig. 17. On the one hand, FT-1 and SPNH contain non-water-soluble components (black particles) of different sizes, which effectively fill the micropores and microfractures of the formation rock under the effect of differential pressure. On the other hand, the molecular chains of PVA and XC contain a large number of adsorption groups, which are quickly and efficiently adsorbed on the rock surface to avoid further filtrate intrusion. Therefore, the drilling fluid can form a layer of "mud skin" with low permeability in the near-wall zone, thus playing a good role in protecting the wall.
Evaluation of anti-pollution performance of the solids-free drilling fluid
In order to fully characterize the anti-pollution performance of the formulated drilling fluid, 1 wt% PVA + 0.2 wt% XC + 1 wt% FT-1 + 1 wt% SPNH was used as the formulation of the solids-free drilling fluid, and different concentrations of NaCl, calcium chloride (CaCl2) and Ca-based bentonite were added respectively to determine the changes in the performance, and the results are shown in Table 3.
The addition of CaCl2 and Ca-based bentonite led to a decrease and increase in K value, respectively, while NaCl caused a decrease and then an increase in K value. The hydration and dispersion of Ca-based bentonite increased the solid-phase particles in the slurry, and although the hydration was partially inhibited by PVA, the high concentration inevitably led to an increase in viscosity. Because XC contains a large number of anionic groups, the presence of a small amount of NaCl reduces the electrostatic repulsion between the ions, causing the polymer molecular chain to contract, which is manifested as a decrease in the solution viscosity. However, when the amount of NaCl increases, the C–O bonds in the molecular chains of XC are compounded with Na+ in the solution, which enhances the intermolecular interactions, forms molecular aggregation and increases the viscosity of the solution. Because of the strong electrostatic shielding effect of CaCl2, the increase of salt amount obviously reduces the viscosity of the formulated system.
The n-values of the formulations for different contaminants in Table 3 ranged from 0.63 to 0.84, indicating that the drilling fluids maintained good shear thinning properties. In terms of filtration loss, all formulations were consistently within 10 mL, indicating good compatibility between the treatments. Overall, the performance of the formulated drilling fluid remained within the appropriate range under contaminated conditions of up to 35 wt% NaCl, 4 wt% CaCl2 and 12 wt% Ca-based bentonite, suggesting that the formulated drilling fluid is highly resistant to contamination and has good potential for application in a variety of complex formations.
Conclusions and prospects
Conclusions
-
1.
The rheological properties of PVA solutions are positively correlated with the addition amount. The higher the degree of polymerization or amount of addition, the higher the apparent viscosity of PVA solution. According to the shear rate-shear stress curve found that 1–3 wt% of PVA solution showed a Newtonian rheological state (n - 1), with the continued increase in the amount of addition (4 wt%, 5 wt%), the solution exhibited some pseudoplasticity characteristics (n = 0.95, 0.87). The viscosity of the low concentration PVA solution basically meets the requirements of rock-carrying, and the small value of static shear stress makes the drilling fluid have good pumpability. Overall, the rheological characteristics of PVA solution are more suitable for the lower shear rate environment of wireline core drilling annulus.
-
2.
The inhibition effect of PVA is significantly stronger than that of chloride salts. Compared with KCl and NaCl, at the same addition amount (1 wt%), the linear swelling of bentonite in PVA solution was significantly lower (only 1.97 mm), and the recovery of shale was higher (less than 40% for both water and chlorinated salts, but as high as 78% for PVA), and the surface morphology of the mud balls was basically intact, and no obvious cracks were observed in the immersion experiments. The strong inhibition effect of PVA was fully verified by different experimental methods in this study.
-
3.
Combined with the zeta potential test, particle size distribution test, FITR analysis and XRD experiments, the inhibition mechanism of PVA was revealed. K+ in KCl inhibits the hydration of the clay by displacing the cations with larger hydration radius and hydration energies. The hydroxyl group of PVA adsorbed on the surface of the clay to form a strong hydrogen bond, and the long chain molecules lapped each other to form a dense hydrophobic barrier, thus preventing the entry of water molecules and achieving the effect of inhibiting the hydration and swelling of bentonite. This study analyzes the inhibition mechanism of PVA by comparing it with commonly used inorganic salts, which provides a certain theoretical basis for the development of inhibitors and drilling fluid systems.
-
4.
Based on the good inhibition performance and easily adjustable rheological property of PVA, a solids-free drilling fluid with strong inhibition ability was developed through compatibility experiments. XC enhanced the shear force of PVA solution, which was beneficial to carry the drill cuttings, while SPNH and FT-1 effectively reduced the filtration loss of the solution. The resulting drilling fluid has high adhesion to clay minerals, strong resistance to contamination, stable well wall, and is suitable for water-sensitive, loose and other complex formations, and is expected to be popularized and applied in deep exploration, scientific drilling and other coring construction.
Study limitations and future research directions
This study only compares the inhibition effect of PVA and two common chlorinated salts, and the selection range of inhibitors is too small to provide a strong basis for the strong inhibition effect of PVA.
The next step is to plan to introduce several types of inhibitors, such as formate, other inorganic salts (potassium silicate), polymer-based inhibitors (e.g., potassium polyacrylate, polyhexadecanol), and amine-based inhibitors for comparison with PVA. Combining organic salts with PVA can also be attempted, which can effectively improve the inhibition effect of the drilling fluid while having less impact on the overall performance of the drilling fluid.
In addition, a novel evaluation method can be established by quantitatively determining the degree of clay hydration, such as the values of free water, capillary condensate, osmotic hydration water and surface hydration water in clay before and after inhibition, so as to evaluate the inhibition ability more objectively and accurately.
Abbreviations
- AV :
-
Apparent viscosity, mPa·s
- CaCl2 :
-
Calcium chloride
- CMC:
-
Sodium carboxymethyl cellulose
- F L :
-
Filtration loss, mL
- FT-1:
-
Sulfonated gilsonite
- K :
-
Consistency coefficient, Pa·sn
- KCl:
-
Potassium chloride
- M :
-
The recycling mass, g
- n :
-
Flow index
- NaCl:
-
Sodium chloride
- PAC:
-
Polyanionic cellulose
- PVA:
-
Polyvinyl alcohol
- R :
-
Rolling recovery rate, %
- SPNH:
-
Sulfonated lignite resin
- SMC:
-
Sulfonated lignite resin
- SMP:
-
Sulfomethyl phenol formaldehyde resin
- wt%:
-
Mass of dry powder of the agent/volume of water
- XC:
-
Xanthan gum
References
API Recommended Practice 13B-1 (2009) Recommended Practice for Field Testing Water-Based Drilling Fluids. Washington, DC, American Petroleum Institute
API Recommended Practice 13I (2006) Recommended Practice for Standard Procedure for Laboratory Testing Drilling Fluids. Washington, DC, American Petroleum Institute
Balaga DK, Kulkarni SD (2022) A review of synthetic polymers as filtration control additives for water-based drilling fluids for high-temperature applications. J Petrol Sci Eng 215:110712. https://doi.org/10.1016/j.petrol.2022.110712
Berry SL, Boles JL, Brannon HD, Beall BB (2008) Performance evaluation of ionic liquids as a clay stabilizer and shale inhibitor. Lafayette, Louisiana, USA SPE International Symposium and Exhibition on Formation Damage Control. https://doi.org/10.2118/112540-MS
Deville JP, Livanec PW, Zhou H (2017) Polyvinyl alcohol-based shale inhibitor. US: 2017/0218251A1, 2017-08-03
Duan HY (2022) Study on strong inhibition solid free drilling fluid system. Xi’an Shiyou University, Xi’an
Emmanuel UA, Godpower CE, Ghasem GN (2020) Enhancing the performance of xanthan gum in water-based mud systems using an environmentally friendly biopolymer. J Petrol Explor Prod Technol 10:1933–1948. https://doi.org/10.1007/s13202-020-00837-0
Fan LY, Wang R, He RJ, Zhang RY, Zhang Q, Zhou Q, Zhou Y (2022) In-situ modification of polyurethane foams by Ionic polyacrylamide for highly-efficient emulsion separation. Mater Rep 36(19):212–217
Guo XS, Fan N, Liu YH, Liu XL, Wang ZK, Xie XT, Jia YG (2023) Deep seabed mining: frontiers in engineering geology and environment. Int J Sci Technol 2:1–31
Hu YQ, Xie J, Xue SN, Xu M, Fu CH, Liu ZQ, Ma SM, Sun SQ, Wang CL (2022) Research and application of thermal insulation effect of natural gas hydrate freezing corer based on the wireline-coring principle. Pet Sci 19(3):1291–1304. https://doi.org/10.1016/j.petsci.2021.11.019
Huang WA, Lan Q, Qiu ZS, Zhang Y, Zhong HY, Feng GT (2016) Colloidal properties and clay inhibition of sodium silicate in solution and montmorillonite suspension. SILICON 8(1):111–122. https://doi.org/10.1007/s12633-015-9351-2
Jia H, Huang P, Wang QX, Han YG, Wang SY, Zhang F, Pan W, Lv KL (2019) Investigation of inhibition mechanism of three deep eutectic solvents as potential shale inhibitors in water-based drilling fluids. Fuel 244:403–411. https://doi.org/10.1016/j.fuel.2019.02.018
İşçi S, Ünlü CH, Atici O, Gungor N (2006) Rheology and structure of aqueous bentonite-polyvinyl alcohol dispersions. Bull Mater Sci 29(5):449–456
İşçi S, Turutoglu SI (2011) Stabilization of the mixture of bentonite and sepiolite as a water-based drilling fluid. J Petrol Sci Eng 75:1–5. https://doi.org/10.1016/j.petrol.2010.11.021
Li X, Jiang G, Yang L, Wang K, Shi H, Li G, Wu X (2019) Application of gelatin quaternary ammonium salt as an environmentally friendly shale inhibitor for waterbased drilling fluids. Energy Fuels 33(9):9342–9350. https://doi.org/10.1021/acs.energyfuels.9b01798
Li SH, Ma ZY, Deng XM, Duan ZQ, Han YY, Liu XY, Kang X (2017) Research and application of high-molecular polymers solid-free drilling fluid in niutoushan Deep Drilling Hole CUSD3. Explor Eng (rock Soil Drill Tunn) 44(01):29–32
Li ZJ, Chen JX, Zhao G, Xiang HT, Liu K (2021) Effect and mechanism of microbial solid-free drilling fluid for borehole wall enhancement. J Petrol Sci Eng 208(D):109705. https://doi.org/10.1016/j.petrol.2021.109705
Li X, Jiang G, Yang L, Peng S (2018) Study of gelatin as biodegradable shale hydration inhibitor. Colloids Surf A 539:192–200. https://doi.org/10.1016/j.colsurfa.2017.12.020
Li Y (2023) Study of the film-forming and plugging properties and wellbore stabilization mechanism of lithium silicate-based drilling fluids. Jilin University, China
Lu YB, Wu Y, Chen Y (2012) Application of the wire-line coring technique to large-diameter deep drilling. Geol Explor 48(6):1221–1228
Ma JY, Xia B, An YX (2022) Advanced developments in low-toxic and environmentally friendly shale inhibitor: a review. J Petrol Sci Eng 208:109578. https://doi.org/10.1016/j.petrol.2021.109578
McDonald MJ (2012) A novel potassium silicate for use in drilling fluids targeting unconventional hydrocarbons// Paper 162180-MS presented at the SPE Canadian Unconventional Resources Conference, 30 October-1 November 2012, Calgary, Alberta, Canada
Mohamed N, Nadji MM, Jean PC, Aicha B (2009) Investigation of combined effects of xanthan gum, sodium dodecyl sulphate, and salt on some physicochemical properties of their mixtures using a response surface method. J Dispers Sci Technol 30(9):1333–1341
Muhammed NS, Olayiwola T, Elkatatny S (2021) A review on clay chemistry, characterization and shale inhibitors for water-based drilling fluids. J Petrol Sci Eng 206:109043. https://doi.org/10.1016/j.petrol.2021.109043
Murtaza M, Ahmad HM, Zhou X, Al-Shehri D, Mahmoud M, Kamal MS (2022) Okra mucilage as environment friendly and non-toxic shale swelling inhibitor in water based drilling fluids. Fuel 320:123868. https://doi.org/10.1016/j.fuel.2022.123868
Needaa AM, Peyman P, Hamoud AH, Jamil A (2016) Controlling bentonite-based drilling mud properties using sepiolite nanoparticles. Pet Explor Dev 43(4):717–723
Rana A, Murtaza M, Saleh TA, Kamal MS, Mahmoud M (2022) An efficient, cost-effective, and green natural extract in water-based drilling muds for clay swelling inhibition. J Petrol Sci Eng 214:110332. https://doi.org/10.1016/j.petrol.2022.110332
Sabzevari AG, Sabahi H, Nikbakht M, McInnes SJ (2022) Development and characteristics of layered EGCG/Montmorillonite hybrid: an oral controlled-release formulation of EGCG. J Drug Deliv Sci Technol 76:103750. https://doi.org/10.1016/j.jddst.2022.103750
Sharma T, Joshi A, Jain A, Chaturvedi KR (2022) Enhanced oil recovery and CO2 sequestration potential of Bi-polymer polyvinylpyrrolidone-polyvinyl alcohol. J Petrol Sci Eng 211:110167. https://doi.org/10.1016/j.petrol.2022.110167
Sheth T, Seshadri S, Prileszky T, Helgeson ME (2020) Multiple nanoemulsions. Nat Rev Mater 5(2020):214–228
Sun JS, Wang ZL, Liu JP, Lv KH, Zhang F, Shao ZH, Dong XD, Dai ZW, Zhang XF (2021) Notoginsenoside as an environmentally friendly shale inhibitor in water-based drilling fluid. Pet Sci 19(2):608–618. https://doi.org/10.1016/j.petsci.2021.11.017
Tan HJ, He W, Ye Y, Chen Y, Zheng XH (2023) Influence of polyvinyl alcohol coated porous Al2O3 ceramic waste particles on cement properties in geothermal applications. Constr Build Mater 376(2):131046
Tang Y, Li ZL, Wang GR, He YF, He Y, Wei JF (2022) Study on shear fracture performance of subsea test tree under emergency conditions in the deepwater oil and gas completion testing. J Petrol Sci Eng 213:110399. https://doi.org/10.1016/j.petrol.2022.110399
Wang JL, Chen C, Hu, YJ, Wan BY, Xiao BB, Xu ZG, Hu SQ, Yu MF (2024) Scheme design and flow performance analysis of double-bits combination wire-line coring technology for seafloor drill. Marine Georesources and Geotechnology, early access. https://doi.org/10.1080/1064119X.2024.2309283
Zeng XX (1980) The characteristics of the diamond drilling and the demand for drilling mud. J Cent South Univ (sci Technol) 01:55–64
Zhao X, Qiu ZS, Huang WA, Xu JF, Jie S (2014) Multifunctional properties of polyglycol in deepwater drilling fluids. Chem Technol Fuels Oils 50(4):362–362
Zhang K, Meng ZP, Liu SM, Hao HJ, Chen T (2021) Laboratory investigation on pore characteristics of coals with consideration of various tectonic deformations. J Nat Gas Sci Eng 91:103960. https://doi.org/10.1016/j.jngse.2021.103960
Funding
This work was supported by School-level research projects of Henan University of Economics and Law (No. 22HNCDXJ17).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence that the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, Y., Zheng, W., Zhang, G. et al. Laboratory investigation on inhibition of polyvinyl alcohol used for wireline coring drilling. J Petrol Explor Prod Technol (2024). https://doi.org/10.1007/s13202-024-01819-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13202-024-01819-2