Abstract
To separate oil–water mixtures especially in oil field operations, new energy-efficient methods are urgently required. Conventional separation techniques using demulsifiers for separation of oil–water mixtures or even use of membranes usually suffered from high cost and energy consumption, composition dependency of demulsifiers and fouling or inability of a single membrane to separate all types of oil–water mixtures. This research aimed to synthesize tungsten oxide-coated stainless steel mesh using the hydrothermal method, with a focus on evaluating its effectiveness in oil–water separation. The coating procedure was carried out using hydrothermal techniques, with an emphasis on investigating the impact of precursor concentration, pH levels, reaction temperature and duration, on the separation efficiency of the optimal coating solution. The hydrothermally coated stainless steel mesh was created within a polytetrafluoroethylene reaction vessel, submerged in a 150 ml aqueous solution containing 0.0094 mol of sodium tungstate di-hydrate at pH 3.0, achieved through the addition of hydrochloric acid. Additionally, 1 g of oxalic acid, acting as a chelating agent, was introduced. Subsequently, the mesh underwent a 4 h reaction at 220 °C and was subsequently annealed for 30 min in a 350 °C furnace. Remarkably, the resultant mesh exhibited an exceptional water separation flux of 9870 ± 15 L/hr/m2 when exposed to 1:1 v/v oil–water mixtures. This performance significantly outperformed previous filters designed for similar oil–water separation tasks. The mesh efficiently facilitated the passage of water through the oil–water mixture, achieving an efficiency rate exceeding 98 ± 1%. To gauge its wetting behavior, the hydrophilic/underwater oleophobic filter underwent static contact angle measurements. The filter's wetting mechanism was primarily attributed to its hierarchical surface structure, which enhanced surface hydrophilicity and roughness. Analytical techniques such as XRD, FTIR, and FE-SEM were employed to scrutinize the fabricated filter's composition. These analyses confirmed the successful creation of a nanostructured WO3 coating on both sides of the stainless steel mesh. Moreover, the utilization of commercially available chemicals and straightforward fabrication techniques underscores the promising potential of this approach for large-scale applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the oil and gas industry the process of crude oil desalination is required to separate dispersed phase of brine from oil phase. Water pollution caused by miscible and immiscible oil, on the other hand, is a major issue that almost every country must deal with, and disposal of oil-polluted water into the surroundings without filtration and purifying is a significant trouble. The spillage of natural mixtures particularly crude oil family has caused serious contamination in seawater. Oil–water separation, in this way, has forever been a major problem. Oil–water emulsion separation under simultaneous effects of electric field dehydration and flow field characteristics inside dehydrator has been simulated using numerical simulation and laboratory experiments (Wang et al. 2023). Scientists look forward to new methods different from the traditional techniques such as gravity separation (Peterson et al. 2003), coagulation (Bennett and Williams 2004) and flocculation (Bratby 1980), chemical oxidation (Schrank et al. 2004), electrochemical (Ma and Wang 2006) and biological treatment (Rosal et al. 2010) for efficient and low cost oil–water separation. The idea of oil–water separation using interfacial theories and wetting behaviors of these two phases on the coating surface of adsorbing or separating mediums is being investigated (Xue et al. 2011). For oil–water separation, surfaces have been developed that have special wettability especially when oil spills have occurred. These surfaces also have been applied for oil–water treatment and fuel purification (Chu et al. 2015; Jiang et al. 2015). Recently, interfacial separation materials with wettability governed by their chemical composition and surface geometrical structure have attracted special attentions. Wettability is an interaction between molecules of different substances. Wetting conditions for oil and water phases on a solid surface is quite different. Pressure difference across a membrane (or filter) causes one of the two phases (oil and water) wet the membrane or filter and preferentially pass through it. Two main needed factors for such a separation are pore size and intrusion pressure (Qiu et al. 2020). Inspired from fish scale and its anti-fouling property superhydrophilic and underwater superoleophobic membranes have been made that allows water permeates but repels oil and in this way oil–water mixture separates. The novel materials with opposite surface tension toward oil and water absorb and pass one phase while repel the other phase. A TiO2 nanofibers coated stainless steel mesh as a novel membrane was created (Gunatilake and Bandara 2017) by spray deposition of hydrothermally synthetized nanofibers on stainless steel mesh; separation efficiency using the created filter was between 90 and 99%. In another research Cu2s coated copper mesh with curled plate-like structure was created in a low cost electrochemical anodization manner that showed superhydrophilicity and underwater superoleophobicity with separation efficiency more than 97% (Pi et al. 2016). For effective oil–water separation, superhydrophilic–superoleophobic (water removal) and superhydrophobic–superoleophilic (oil removal) filters have been developed. The latter causes the oil phase to spread, absorb, or filter through the filter, whereas the aqueous phase is pushed away, thus separating the oil phase from the oil–water mixture. Nevertheless, the point is oleophilic materials can easily become damaged or even blocked up (particularly for high viscosity oils), showing poor recyclability and recoverability. Conversely, it is possible to get around these drawbacks by using water-removing filters. A new approach to oil–water separation is made possible by underwater superoleophobic surfaces with low adhesion to oil phase, that protects the surface from oil fouling (Zhang et al. 2013). There are many important applications for superhydrophilic surfaces such as self-cleaning, anti-scattering of mirrors and similar matters (Russell 2002; Sun et al. 2005; Feng and Jiang 2006). Different underwater superoleophobic plates like TiO2 slim film (Zhang et al. 2013; Jo and Kim 2016), copper oxide covered mesh (Cheng et al. 2013), zinc oxide covered mesh (Yan et al. 2016), copper sulfide coated copper mesh (Pi et al. 2016), bismuth vanadate coated mesh (Song et al. 2017), nickel nanoparticle decorated copper mesh (Luo et al. 2016) and diatomite coated mesh (Li et al. 2017) have been researched and might be developed. In these and also similar researches porous surfaces have been utilized as substrate. Polymer membranes/metallic meshes have been usually used for oil–water mixture filtration; while, inorganic/polymer sponges have been used as adsorbent.
Recently, numerous investigations have been done on special wettable porous materials (Sun et al. 2005; Yao et al. 2011; Xue et al. 2014). Surface wettability is mainly affected by two agents, first, chemical composition of the surface and second, its morphology (Feng and Jiang 2006; Feng et al. 2008; Xue et al. 2012). Every liquid has its own wettability on a surface that depends on not only the surface energy of that solid surface but also to liquid surface tension, solid surface roughness and finally interfacial energy (Dalawai et al. 2020).
Metallic meshes such as copper and stainless steel are commonly used as the filtration substrate for oil–water separation. These substrates need to be modified to achieve desirable wettability properties. One conventional approach is to coat the surface of mesh with nanostructures to make it superwet.
Creation of superhydrophilic and submerged superoleophobic filters depends on the covering of nano/micro-progressive covering of profoundly molar materials on a reasonable substrate. For successful oil water separation, a filter with profoundly harsh surface is expected to accomplish stable superhydrophilicity. Superhydrophilicity denotes the extreme water-loving behavior of specifically designed surfaces (Wang et al. 1997). Structures with pores ranging from a few nanometers to few microns exhibit superhydrophilicity due to capillary effect that causes water to rapidly infiltrate into three-dimensional porous network. It induces complete wetting of water on the surface of the porous materials (Tettey et al. 2011).
A comparison of stainless steel mesh based superhydrophilic/underwater superoleophobic filters studied in recent years for oil–water separation has been presented in Table 1. It can be seen that almost all of the works have very good efficiency however; some of them suffer from complicated process of creation, expensive materials, non-uniform surface coating and other problems.
Among different inorganic materials, WO3 has been suggested as one of the best substance for fabricating superhydrophilic–underwater superoleophobic surface due to its water-loving property (Azimirad et al. 2007). This property can be enhanced by enhancement of surface roughness (Wang et al. 2018). In the present study, fabrication of tungsten oxide (WO3) coated stainless steel mesh via direct hydrothermal coating of the mesh in a 150 ml stainless steel autoclave container is presented.
In fact, this is the result of a chain reaction that its mechanism is out of the present discussion.
Recently, tungsten oxide has gained much attention for its electrochromic, photocatalytic, photoluminescent and gas sensing properties. In a previous study, hydrothermally grown nanostructured WO3 film was applied on glass plates to make them electrochromic (Jiao et al. 2010). Various methods have been proposed for creation of WO3 thin film including pulsed laser deposition, electrosynthesis, sol–gel and hydrothermal methods that among them the hydrothermal method seems to be the best approach due to its less energy consumption and also easiness of scale-up.
Hydrothermally synthetized nanostructured WO3 coated stainless steel mesh can be installed—with proper configuration- inside the crude oil separators and also desalters so the crude oil mixtures by flow through these meshes splits to oil and water phases and the separation occurs. In this way continuous dosage of demulsifiers will be eliminated.
While for elimination of water from crude oil (containing less than 20 weight% water) in an oilfield separation process, dosage of about 25–30 ppm demulsifier with at least 2.5$/lit price is necessary, for processing 10,000 barrels of that crude oil per day, 100–120$/day should be paid for only demulsifier. The price of electrical dehydration in desalination process should also be considered. But with substitution of oil–water separation using nanostructure WO3 coated stainless steel mesh, the price of sodium tungstate di-hydrate is not more than 40$/kg that is much more than needed to construct a horizontal separation drum containing coated stainless steel meshes.
Hydrothermally synthesized tungsten trioxide WO3 coated stainless steel mesh has several potential applications due to its unique properties and characteristics. WO3 is a versatile material with semiconducting properties and various oxidation states, making it suitable for a range of applications. When coated onto a stainless steel mesh using a hydrothermal synthesis process, it can enhance the functionality and performance of the mesh in different fields. Here are some potential application areas for such a material:
Gas Sensing (Zhang et al. 2020): WO3 is known for its sensitivity to various gases, such as hydrogen, ammonia, and volatile organic compounds. Coating stainless steel mesh with WO3 can create a gas sensor capable of detecting and quantifying the presence of these gases in the environment. This could be used in environmental monitoring, industrial safety, and indoor air quality control.
Electrochromic Devices (Kumar and Subrahmanyam 2019): WO3 is an electrochromic material, meaning its optical properties can change in response to an applied voltage. Coating stainless steel mesh with WO3 can create smart windows or displays that can adjust their transparency based on external factors like sunlight or user preference.
Energy Storage (Mohan et al. 2022): WO3 has been studied for its potential in energy storage applications, particularly in pseudo capacitors and lithium-ion batteries. Coating stainless steel mesh with WO3 could enhance the performance and capacity of energy storage devices, contributing to advancements in portable electronics and renewable energy systems.
Catalysis (Ni et al. 2023): WO3 has catalytic properties that make it useful in chemical reactions. Coating stainless steel mesh with WO3 can create catalytic surfaces for reactions like water splitting, pollutant degradation, and more. These catalytic applications are important in industries such as wastewater treatment and energy production.
Photocatalysis (Fakhri and Behrouz 2015): WO3 can harness solar energy to drive chemical reactions, making it suitable for applications like water purification and air detoxification. Coating stainless steel mesh with WO3 can create efficient photo catalytic systems for environmental remediation.
Sensors and Actuators (An et al. 2020): Apart from gas sensing, WO3-coated stainless steel mesh could be employed in various other sensor types, including humidity sensors and strain sensors. It can also serve as an actuator by changing shape or dimensions in response to external stimuli, useful in micro-electro-mechanical systems robotics.
Anti-Corrosion Coatings (Arunima et al. 2022): Stainless steel mesh coated with WO3 might exhibit improved resistance to corrosion due to the protective properties of the oxide layer. This could find applications in outdoor structures, marine environments, and other situations where corrosion is a concern.
Flexible Electronics (Yao et al. 2013): WO3-coated stainless steel mesh can serve as a flexible and conductive substrate for electronic devices. This is especially relevant in wearable technology, flexible displays, and other applications where flexibility and electrical conductivity are required.
Water Splitting for Hydrogen Production (Wondimu et al. 2022): WO3 has been explored as a photo anode in water-splitting systems to produce hydrogen gas from water using solar energy. Coating stainless steel mesh with WO3 could contribute to the development of efficient and cost-effective hydrogen production technologies.
In summary, existing filter membranes with special wettability of the surface for separation of oil–water mixtures need improvement in efficiency, thermal stability, portability, good mechanical property, environmental adaptability, plasticity, working stability (in extreme conditions such as strong acid and alkali, high salinity and corrosive solutions) and finally low cost, to be applicable in industrial wastewater treatment and also offshore crude oil de-watering. Besides, large-scale productions need simple fabrication process. Oil–water emulsions separation also remains a challenge in oil–water separation by filter membranes that need to be researched.
Experimental section
Materials
Sodium tungstate di-hydrate (Na2WO4–2H2O) from Central Drug House, India, concentrated HCl (35wt% ~ 37% in water) and oxalic acid di-hydrate (C2H2O4–2H2O) from Sigma-Aldrich, acetone and ethanol from Mojallali Industrial Chemical Complex Company, Iran, distilled water from a common lab-scaled plant, commercial xylene, methyl blue and 2-layer 5 micron stainless steel mesh (#316 L) were used as purchased. All the reagents except those stated, were of analytical grade.
Methods
The initial tests conducted on the fabricated filters consistently demonstrated separation efficiencies exceeding 98 ± 1%. Consequently, in alignment with the primary research goal of oil–water separation, the filtration flux was selected as the key response for the design of experiments. Within this framework, precursor concentration, pH levels, reaction time, and temperature were treated as the designated influencing factors. To facilitate this experimental design, the Box–Behnken methodology from Response Surface Methodology (RSM) was employed (please refer to Supplementary Document 1 for more details).
The process of producing WO3-coated stainless steel mesh through the hydrothermal technique commenced with the creation of a homogeneous solution by stirring 3.09 gr of Na2WO4–2H2O with 25 ml of de-ionized water. Gradual addition of aqueous HCl was executed with pH monitoring until a pH of 3.0 was attained. This solution's volume was then increased to approximately 150 ml using de-ionized water, with the addition of 1.0 gr of oxalic acid to act as a chelating agent. Subsequently, this suspension was transferred into a 150 ml Teflon chamber.
For surface cleaning and impurity removal, a 5 × 10 cm2 double-layered stainless steel mesh with a 5-micron pore size was subjected to an ultrasonic cleaning process. The mesh underwent sequential cleaning with acetone for around 10 min, followed by a switch to de-ionized water for an additional 5 min. Subsequently, the mesh was placed vertically within the chamber to ensure uniform coverage by the solution. The chamber, containing the mesh, was then positioned inside a stainless steel container and heated to 220 °C within an oven, allowing the hydrothermal reaction to proceed for 4 h.
Following the reaction period, the autoclave was gradually cooled to room temperature, at which point the filter was removed from the solution. The filter underwent successive rinsing with ethanol and de-ionized water, followed by a cooling period to reach room temperature. Subsequently, the filter was placed in a programmed furnace and sintered at 350 °C for duration of 30 min. In the concluding step, the cooled filter underwent thorough washing and rinsing with acetone and de-ionized water to eliminate any remaining impurities, including unbound or excess nanoparticles. Refer to Supplementary Image 2 for an illustrative representation of the resulting filter.
It should be noted that as all available programmable ovens in the research center had the upper working temperature of 220 °C, and the precision − 5 °C for reaction temperature due to machine depreciation was unavoidable.
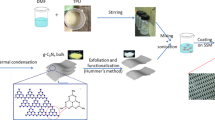
The intrinsic porous structure of the stainless steel mesh provides a great mechanical and chemical stability and makes it suitable for separation application. Hence, it was chosen as the substrate. It is also simply available with low cost. Figure 1. displays a schematic of the process utilized to create the nanostructure WO3-coated mesh.
Making a water–oil mixture
Xylene was considered as the oil phase. In order to prepare the water–oil mixture, de-ionized water was added to xylene in equal ratio without further mixing. Instead, while transferring to the separation set-up, the container is shacked vigorously for about 15 s.
Characterization
In order to identify probable creation of functional groups, Fourier transform infrared spectroscopy (FTIR) was employed. Thus, the surface structure of the prepared WO3-coated stainless steel mesh can also be determined. The surface morphology was investigated using field emission scanning electron microscopy (FE-SEM). The crystal phase of the sample was confirmed via an X-ray diffractometer (XRD).
The separation efficiency of nanostructure coated mesh was attained using Eq. (1):
where \({{\text{v}}}_{{\text{separated}}}\) and \({{\text{v}}}_{{\text{initial}}}\) are volumes of the water passed through the filter and initially in the mixture, respectively.
Contact angle measurement
The wetting characteristics of the bare stainless steel mesh and hydrothermally coated & annealed mesh were investigated by underwater contact angle measurements. In total, 5 μL of xylene was dropped on the coated stainless steel mesh. By averaging the measured angles in various locations throughout the sample that recorded by a professional camera linked to a PC, it was allowed the evaluation of the contact angle values (Mir et al. 2022). The oil underwater contact angle (contact angle) was measured on a set-up shown in Fig. 2. below.
Results and discussion
Wettability performance of nanostructure WO3-coated stainless steel mesh
The wettability of bare stainless steel mesh and coated stainless steel mesh was examined using oil underwater contact angle measurements. As can be seen from Table 2, Xylene underwater contact angle on both coated stainless steel mesh has been increased.
The oil underwater contact angles for all coated stainless steel meshes that fabricated according to the designed experiments, increased considerably relative to the bare stainless steel mesh as shown in Fig. 3.
It should be noted that Measuring contact angles with a high level of precision usually requires high-tech contact angle goniometers that can perform a great number of automated measurements per drop, thus reducing the error on each returned average value but such goniometers were not available especially for university researchers. So the precision of ± 5° in contact angle measurement & reading using above mentioned system was unavoidable.
Characterization of nanostructure WO3-coated stainless steel mesh
FTIR spectroscopy relates peak at 3289.8 and 1646.80 cm−1 in Fig. 4. To the functional groups O–H stretching vibrations and bending modes of the adsorbed water (W–OH), respectively, that have been created on the nanostructure WO3-coated mesh. At the same time, peaks at 925.59 and 675.73 cm−1 has been related to bending W=O and W–O–W, respectively.
The crystalline nature of WO3-coated stainless steel mesh stainless steel mesh was analyzed by XRD that its graphical view utilizing the High Score plus 3.05 from PANanalytical B.V. is shown in Fig. 5. The sample with the highest flux according to the designed experiments exhibited good crystallinity. Details of XRD pattern related to nanostructure WO3 coating is shown in Table 3 below.
FE-SEM images of the top and cross of nanostructure WO3-coated stainless steel mesh was indicated in Fig. 6a–e. These images show the fully coverage of stainless steel mesh with WO3 nanosphere-like morphology. It confirm that the nanospheres successfully have been assembled on the stainless steel mesh wires (Zheng et al. 2011; Yao et al. 2013; Li et al. 2015a, b). The sphere-like WO3 nanostructures are visible in Fig. 6d. This morphology increases the surface roughness. Figure 6a demonstrates that the pore diameter of the dual layer stainless steel mesh is about 25–35 m. The cross section of SEM images reveals that created film has an average thickness of about 1.08–3.51 m. The average size of nanospheres as shown in Fig. 6e is in the range of 27–44 nm.
Oil–water separation
In order to examine the separation ability of the nanostructure WO3-coated stainless steel mesh, the water–oil (here xylene) mixture (1:1 v/v) was prepared by mixing 25 ml of xylene and 25 ml of water. Only three to four particles of methyl blue was added to the mixture in order to characterize the aqueous phase. The nanostructure WO3-coated stainless steel mesh (~ 4.91 cm2 area) was first wetted with de-ionized water just before separation process begins and mounted between two glass tubes using a brass union shown in Fig. 7.
While shaking the mixture container vigorously for at least 15 s, it was poured into the upper tube of the set-up and immediately the separation started only under the gravity. In the lower tube the separated water was collected.
In Fig. 7. One can see that only a portion of the liquid in blue color has been collected in the lower tube, and the other portion—a colorless liquid—has remained in the upper tube. Oleophobicity of the filter in the brass union caused aqueous phase died with methyl blue (that only solves in aqueous phase) to pass through the filter but blocked against oil phase.
Furthermore, de-ionized water was employed as a rinsing agent for the mesh coated with WO3 nanospheres. This rinsing step was repeated after each separation cycle to ensure the removal of any oil residues from the mesh surface. Notably, the water flow flux through the filter reached an impressive 9870 ± 15 L M−2 h−1, coupled with a separation efficiency of 98 ± 1%. The unique underwater oleophobic properties of the filter allowed the oil phase to remain above the filter, with the separation process being solely driven by gravity, without the need for external forces. The achievement of a 98 ± 1% efficiency stands as clear evidence of the near-complete and successful separation of water from the mixture as it traverses the filter (please refer to Supplementary Movies 3 and 4 for visual demonstrations).
Consequently, the development of this new nanostructure WO3-covered stainless steel mesh, achieved with minimal expenses and a straightforward manufacturing procedure, holds the potential for widespread applications. The promising outcomes underscore its suitability for various scenarios and industries.
Filter sturdiness and anti-fouling capacity
Durability is one of the crucial and required specs of the filter to be industrially applicable. The anti-fouling capacity of nanostructure WO3-covered stainless steel mesh was investigated through consistent separation of water/xylene blends (1:1 v/v) for 50 cycles. It is noticeable that after every cycle, the oil is eliminated from the surface pores exclusively by washing it with water that affirms self-cleaning ability of the surface. Flux-efficiency-cycle diagram is shown in Fig. 8.
It can be seen that there is minor changes in both the efficiency and flux related to the cycle number. Both of them stayed almost constant at around 9800 L m−2 hr−1 and 98–93%, respectively. The result shows the stability of nanostructure WO3-coated stainless steel mesh against fouling by oil droplets.
Conclusions
In this study:
-
1.
A hydrophilic and underwater oleophobic nanostructure WO3-coated stainless steel mesh for separation of free water/oil mixture was successfully prepared by a simple and low cost hydrothermal process followed by sintering to stabilize coating structure.
-
2.
Close monitoring of wetting behavior of the nanostructure coated stainless steel mesh showed that underwater contact angle relative to the bare mesh has been increased considerably that by comparison of SEM images and according to FTIR and XRD graphs, is clearly attributed to the change in both surface chemistry and also hierarchical structure.
-
3.
As-prepared mesh can separate different oil–water mixtures in a single-unit operation, with more than 98 ± 1% separation efficiency and flux of 9870 ± 15 Lm−2 Hr−1. Therefore, nanostructure WO3-coated stainless steel mesh showed good ability to separate water/oil mixtures using the facile preparation method that was not found to be considered in oil–water separation researches using membranes and filters. This is a new and cost-effective application of nanostructure tungsten oxide in oil–water separation.
References
An F, Zhou AF, Feng PX (2020) Effect of tungsten oxide nanostructures on sensitivity and selectivity of pollution gases. Sensors 20(17):4801
Arunima SR, Deepa MJ, Elias L, Sha MA, Sumi VS, Riyas AH, Chacko F, Remya R, Shibli SMA (2022) Tuning of WO3 nanoparticles integration into Fe–Zn intermetallic layers of hotdip zinc coating to control corrosion. Mater Sci Eng: B 276:115539
Azimirad R, Naseri N, Akhavan O, Moshfegh AZ (2007) Hydrophilicity variation of WO3 thin films with annealing temperature. J Phys D Appl Phys 40(4):1134–1137
Bennett MA, Williams R (2004) Monitoring the operation of an oil/water separator using impedance tomography. Miner Eng 17:605–614
Bratby J (1980) Coagulation and flocculation. Croydon, uplands, England
Chen YE, Wang N, Guo F, Hou L, Jingchong L, Xu Y, Zhao Y, Jiang L (2016) A Co3O4 Nano-needles Mesh for High-efficient, High-flux Emulsion Separation. J Mater Chem A. https://doi.org/10.1039/C6TA02579J
Cheng Z, Lai H, Du Y, Fu K, Hou R, Zhang N, Sun K (2013) Underwater superoleophilic to superoleophobic wetting control on the nanostructured copper substrates. ACS Appl Mater Interfaces 5(21):11363–11370
Chu Z, Feng Y, Seeger S (2015) Oil/water separation with selective superantiwetting/superwetting surface materials. Angew Chem Int Ed 54(8):2328–2338
Dalawai SP, Saad Aly MA, Latthe SS, Xing R, Sutar RS, Nagappan S, Ha C-S, Kumar Sadasivuni K, Liu S (2020) Recent advances in durability of superhydrophobic self-cleaning technology: a critical review. Prog Org Coat 138:105381
Dong Y, Jing L, Shi L, Wang X, Guo Z, Liu W (2014) Underwater superoleophobic graphene oxide coated meshes for the separation of oil and water. Chem Commun (camb, Engl) 50(42):5586–5589
Du X, Wang Q, Wang X (2019) Underwater superoleophobic mesh with transformable micro-nano structure for ultrafast oil/water separation. Surf Coat Technol 358:806–816
Fakhri A, Behrouz S (2015) Photocatalytic properties of tungsten trioxide (WO3) nanoparticles for degradation of Lidocaine under visible and sunlight irradiation. Sol Energy 112:163–168
Feng XJ, Jiang L (2006) Design and creation of superwetting/antiwetting surfaces. Adv Mater 18(23):3063–3078
Feng L, Zhang Y, Xi J, Zhu Y, Wang N, Xia F, Jiang L (2008) Petal effect: a superhydrophobic state with high adhesive force. Langmuir 24(8):4114–4119
Gunatilake UB, Bandara J (2017) Efficient removal of oil from oil contaminated water by superhydrophilic and underwater superoleophobic nano/micro structured TiO2 nanofibers coated mesh. Chemosphere 171:134–141
Hou K, Zeng Y, Zhou C, Chen J, Wen X, Xu S, Cheng J, Lin Y, Pi P (2017) Durable underwater superoleophobic PDDA/halloysite nanotubes decorated stainless steel mesh for efficient oil–water separation. Appl Surf Sci 416:344–352
Jiang T, Guo Z, Liu W (2015) Biomimetic superoleophobic surfaces: focusing on their fabrication and applications. J Mater Chem A 3(5):1811–1827
Jiao Z, Sun XW, Wang J, Ke L, Demir HV (2010) Hydrothermally grown nanostructured WO3films and their electrochromic characteristics. J Phys D Appl Phys 43(28):285501
Jing L, Mingming L, Shi L, G. zhiguang, (2015) Stable underwater superoleophobic conductive polymer coated meshes for high-efficiency oil-water separation. RSC Adv 5(42):33077–33082
Jo S, Kim Y (2016) Superhydrophilic–underwater superoleophobic TiO2-coated mesh for separation of oil from oily seawater/wastewater. Korean J Chem Eng 33:3203–3206
Kumar KU, Subrahmanyam A (2019) Electrochromic properties of tungsten oxide (WO3) thin films on lexan (polycarbonate) substrates prepared with neon as sputter gas. Mater Res Express 6(6):065502
Li J, Liu X, Cui J, Sun J (2015a) Hydrothermal synthesis of self-assembled hierarchical tungsten oxides hollow spheres and their gas sensing properties. ACS Appl Mater Interfaces 7(19):10108–10114
Li J, Yan L, Li W, Li J, Zha F, Lei Z (2015b) Superhydrophilic–underwater superoleophobic ZnO-based coated mesh for highly efficient oil and water separation. Mater Lett 153:62–65
Li J, Yan L, Hu W, Li D, Zha F, Lei Z (2016) Facile fabrication of underwater superoleophobic TiO2 coated mesh for highly efficient oil/water separation. Colloids Surf, A 489:441–446
Li J, Guan P, Zhang Y, Xiang B, Tang X, She H (2017) A diatomite coated mesh with switchable wettability for on-demand oil/water separation and methylene blue adsorption. Sep Purif Technol 174:275–281
Liu J, Li P, Chen L, Feng Y, He W, Yan X, Lü X (2016) Superhydrophilic and underwater superoleophobic modified chitosan-coated mesh for oil/water separation. Surf Coat Technol 307:171–176
Luo Z-Y, Chen K-X, Wang Y-Q, Wang J-H, Mo D-C, Lyu S-S (2016) Superhydrophilic nickel nanoparticles with core-shell structure to decorate copper mesh for efficient oil/water separation. J Phys Chem C 120(23):12685–12692
Ma H, Wang B (2006) Electrochemical pilot-scale plant for oil field produced wastewater by M/C/Fe electrodes for injection. J Hazard Mater 132(2–3):237–243
Mir S, Rashidi A, Naderifar A, Alaei M (2022) A novel and facile preparation of superhydrophilic/superoleophobic nanofilter using carbon nitride nanosheet for W/O emulsion separation. Sep Purif Technol 284:120279
Mohan VV, Anjana PM, Rakhi RB (2022) One pot synthesis of tungsten oxide nanomaterial and application in the field of flexible symmetric supercapacitor energy storage device. Mater Today: Proc 62:848–851
Ni Z, Wang Q, Guo Y, Liu H, Zhang Q (2023) Research progress of tungsten oxide-based catalysts in photocatalytic reactions. Catalysts 13(3):579
Peterson CH, Rice SD, Short JW, Esler D, Bodkin JL, Ballachey BE, Irons DB (2003) Long-term ecosystem response to the Exxon Valdez oil spill. Science 302(5653):2082–2086
Pi P, Hou K, Zhou C, Wen X, Xu S, Cheng J, Wang S (2016) A novel superhydrophilic-underwater superoleophobic Cu2S coated copper mesh for efficient oil-water separation. Mater Lett 182:68–71
Qiu L, Sun Y, Guo Z (2020) Designing novel superwetting surfaces for high-efficiency oil–water separation: design principles, opportunities, trends and challenges. J Mater Chem A 8(33):16831–16853
Rosal R, Rodriguez A, Perdigon-Melon JA, Petre A, Garcia-Calvo E, Gomez MJ, Aguera A, Fernandez-Alba AR (2010) Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Res 44(2):578–588
Russell TP (2002) Surface-responsive materials. Science 297(5583):964–967
Schrank SG, Jose HJ, Moreira RF, Schroder HF (2004) Comparison of different advanced oxidation process to reduce toxicity and mineralisation of tannery wastewater. Water Sci Technol 50(5):329–334
Song S, Yang H, Zhou C, Cheng J, Jiang Z, Lu Z, Miao J (2017) Underwater superoleophobic mesh based on BiVO4 nanoparticles with sunlight-driven self-cleaning property for oil/water separation. Chem Eng J 320:342–351
Sun T, Feng L, Gao X, Jiang L (2005) Bioinspired surfaces with special wettability. Acc Chem Res 38(8):644–652
Tettey K, Dafinone M, Lee DH (2011) Progress in superhydrophilic surface development. Mater Express 1:89–104
Wang R, Hashimoto K, Fujishima A, Chikuni M, Kojima E, Kitamura A, Shimohigoshi M, Watanabe T (1997) Light-induced amphiphilic surfaces. Nature 388(6641):431–432
Wang B, Chen C, Liu H, Xia B, Fan Y, Chen T (2018) WO3/TiO2 superhydrophilic and underwater superoleophobic membrane for effective separation of oil-in-water emulsions. Thin Solid Films 665:9–16
Wang Z, Qi X, Zhuang Y, Wang Q, Sun X (2023) Effect of flow field and electric field coupling on oil-water emulsion separation. Desalin Water Treat 283:79–96
Wondimu TH, Bayeh AW, Kabtamu DM, Xu Q, Leung P, Shah AA (2022) Recent progress on tungsten oxide-based materials for the hydrogen and oxygen evolution reactions. Int J Hydrogen Energy 47(47):20378–20397
Xue Z, Wang S, Lin L, Chen L, Liu M, Feng L, Jiang L (2011) A novel superhydrophilic and underwater superoleophobic hydrogel-coated mesh for oil/water separation. Adv Mater 23(37):4270–4273
Xue Z, Liu M, Jiang L (2012) Recent developments in polymeric superoleophobic surfaces. J Polym Sci Part b Polym Phys 50(17):1209–1224
Xue Z, Cao Y, Liu N, Feng Y, Jiang L (2014) Special wettable materials for oil/water separation. J Mater Chem A 2(8):2445–2460
Yan L, Li J, Li W, Zha F, Feng H, Hu D (2016) A photo-induced ZnO coated mesh for on-demand oil/water separation based on switchable wettability. Mater Lett 163:247–249
Yao X, Song Y, Jiang L (2011) Applications of bio-inspired special wettable surfaces. Adv Mater (deerfield Beach, Fla.) 23(6):719–734
Yao YR, Ma R, Song XC (2013) Hydrothermal synthesis of tungsten oxide nanoparticles. Appl Mech Mater 268–270:176–179
Zeng J, Guo Z (2014) Superhydrophilic and underwater superoleophobic MFI zeolite-coated film for oil/water separation. Colloids Surf, A 444:283–288
Zhang L, Zhong Y, Cha D, Wang P (2013) A self-cleaning underwater superoleophobic mesh for oil-water separation. Sci Rep 3(1):2326
Zhang D, Cao Y, Wu J, Zhang X (2020) Tungsten trioxide nanoparticles decorated tungsten disulfide nanoheterojunction for highly sensitive ethanol gas sensing application. Appl Surf Sci 503:144063
Zheng H, Ou JZ, Strano MS, Kaner RB, Mitchell A, Kalantar-zadeh K (2011) Nanostructured tungsten oxide—properties, synthesis, and applications. Adv Func Mater 21(12):2175–2196
Acknowledgements
The authors gratefully thank the Department of Chemical Engineering, Iran University of Science and Technology (IUST), and also Iran Research Institute of Petroleum Industry (RIPI) for their financial support.
Funding
There was no funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nouri, M., Sadeghi, M.T., Rashidi, A. et al. Hydrothermally synthetized WO3 coated stainless steel mesh for oil–water separation purposes. J Petrol Explor Prod Technol 14, 1247–1258 (2024). https://doi.org/10.1007/s13202-023-01741-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-023-01741-z