Abstract
Using chemical methods in enhanced oil recovery (EOR) processes is limited since the mechanisms, interactions, and synergisms combined with heterogeneities and network complexities besides the incompatibilities of different chemicals are encountered in the chemical EOR methods with some uncertainties. Also, since using only one chemical, namely surfactant, alkali, and polymer, has a bounded effect on the oil recovery, it is highly required to combine different chemical-based methods to achieve ultimate oil recovery. Unfortunately, since most of the developed surfactants cannot tolerate harsh salinity and temperature conditions, it is highly essential to tailor efficient and stable surfactants for those conditions. Moreover, since crude oil is comprised of thousands of different compounds which are different from one crude oil to the other crude oil, using a specific fraction of oils such as asphaltene and resin has high potential to provide more applicable and generalized results. In the light of this fact, the current investigation is designed and performed for the first time to combine different methods for better synergies for higher oil production using a new class of surfactant (1-tetradecyl-3-methylimidazolium chloride ([C14mim][Cl])), titanium oxide nanoparticles (TiO2–NPs), and alkali (Ca(OH)2) concomitant with NaCl and KCl with concentrations of 50,000–200,000 ppm. The point is that, instead of using crude oil with many compounds, only resin and asphaltene fractions extracted from a heavy acidic crude oil are used as the model oil (8 wt%). The measurements revealed the reducing effect of asphaltene and resin fraction on the interfacial tension (IFT), while the presence of NaCl and KCl makes this trend more complicated. The measurements also revealed an undeniable effect of Ca(OH)2 on the IFT reduction, especially in the presence of NaCl (concentration of 200,000 ppm), no matter using resinous or ASO. Moreover, the IFT measurements revealed the significant effect of used 1-tetradecyl-3-methyl imidazolium chloride ([C14mim][Cl]) on the IFT reduction with minimum values of 0.12 mN/m and 0.32 mN/m for 200,000 ppm of NaCl + Ca(OH)2 of 1500 ppm and 200,000 ppm KCl and Ca(OH)2 of 1000 ppm, respectively. Besides, the measurement revealed that the addition of TiO2 nanoparticles (TiO2–NPs) in the range of 0–100 ppm reduces the IFT to 0.069 mN/m and 0.08 mN/m, respectively. On the other side, the contact angle (CA) measurements and Amott wettability index calculation revealed the better impact of NaCl-based chemical formulation along with the used TiO2–NPs on the wettability alteration toward strongly water-wet conditions than the KCl-based chemical formulations. In the last stage, the performed core flooding experiments using forced imbibition and spontaneous imbibition concepts reveal that the obtained chemical formulations are capable to change the wettability of the rocks toward mixed conditions, while the forced imbibition tests (conventional core flooding experiments) revealed excellent effect of IFT reduction for more oil production with a maximum value of 15.3% based on the original oil in place (OOIP).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unfortunately, only a few amounts of oil in reservoirs can be produced via the natural pressure of the reservoirs which is not more than twenty or thirty percent of the original oil in place (OOIP). Since the current production level could not meet the requirements of energy demand and the oil reserves are limited, it is required to use new and innovative methods to increase the amount of extracted oil from the trapped oil generally using newly proposed methods known as enhanced oil recovery (EOR) or improved oil recovery (IOR) methods (Cheraghian 2015; Abhishek et al. 2015). There are several classifications for EOR processes; the most important ones are chemicals (Amirsadat et al. 2017; Barahoei et al. 2016; Hosseini et al. 2020; Najimi et al. 2019a; Zabihi et al. 2020a; Zeinolabedini Hezave et al. 2014), thermal methods, low-salinity water injection, smart water injection, and carbon dioxide injection (Lashkarbolooki et al. 2019; Rajaei et al. 2013; Zabihi et al. 2020b). Among these EOR methods, chemical injection is a highly attractive method due to its significant effects on oil recovery by manipulating the capillary number mostly due to wettability alteration and IFT reduction (Najimi et al. 2020).
Among the possible chemical methods, alkaline and surfactant injection under low-salinity conditions are an innovative and applicable method that can extract a considerable amount of trapped oil if a proper chemical formulation is obtained. But, the point is that although it is well established that using low-salinity or smart water conditions is applicable for higher oil production especially if they are combined with the chemicals, the salinity is normally above 40,000 ppm in real and usual cases. So, it is highly required to investigate the interactions of different chemicals with high-salinity conditions. In detail, according to the previously performed studies, it is hard to activate multiple and proper mechanisms using only one chemical or method for more trapped oil recovery. During the past decade, a new approach that uses different EOR methods together gained increasing attention commonly known as the hybrid method. One of the possible combinations is the application of alkaline–surfactant solution prepared using low-salinity water to activate multiple mechanisms.
Besides the surfactant molecules, it is possible to use alkali to produce in situ surfactants which can act as sacrifices preventing the adsorption of expensive chemicals such as surfactant molecules. With respect to these facts, Saha et al. (2018) examined the probable synergy between wettability alteration and IFT using saponification of alkali chemicals through core flooding experiments such as sodium hydroxide (NaOH) for different crude oils of light and medium types. They reported that IFT reduction is vital to extract the residual trapped oil through emulsification and wettability reversal. Their obtained results revealed an undeniable effect of NaOH concentration on the oil recovery as the concentration of alkali increases.
Moreover, several researchers investigated the effect of different chemicals on the oil recovery through the ASP flooding using sandstone and carbonates (Sun et al. 2018; Wang et al. 2022; Zhong et al. 2020). For example, Sun et al. (2018) investigated the crude oil composition effect on different chemicals including alkaline, polymer, and surfactant under a combination of alkaline–surfactant–polymer (ASP) slug consisting of NaOH, and partially hydrolyzed polyacrylamide (HPAM), etc. The used crude oil by Sun et al. (2018) was provided from one of the Chinese oilfields. Their results demonstrated that the IFT of the oil/ASP slug was lower than that obtained for Lamadian oil due to the formation of the active components dissolved into the oil phase consequently leaving the oil–water interface unoccupied.
Also, Cai et al. (1996) examined the IFT of different alkane/brine systems and hydrocarbon/brine systems under different pressures and temperatures. According to the results obtained by Cai et al. (1996), there was a linear relationship between the IFT and pressure with a considerable impact of salts without considering the salts type.
In total, examining the previously published works shows that unfortunately there is a lack of information regarding the IFT between alkaline water and an oleic phase compared with the surfactants and oleic phase especially considering any specific fraction of crude oil such as aromatics, saturates, or asphaltenic and resinous fractions. In detail, although it is well established that the presence of alkali has an undeniable effect on IFT reduction and higher oil recovery, the impact of different ions on IFT reduction is poorly understood. In detail, it is highly required to perform investigations to find the optimum alkali concentration regarding the chemistry of oil for higher oil recovery, especially for the high acidic crude oil. Moreover, Nasr-El-Din and Taylor (1992) found that using Neodol 25-3s + Triton X-100 solution modified by alkaline in contact with the crude oil from Lloydminster oilfield is not always efficient for IFT reduction, especially using high salt concentrations.
In order to study the impact of surfactant solutions modified by alkaline on the IFT of crude oil, Taylor et al. (1990) measured the IFT values and reported that the IFT values have two different variation trend of reducing and increasing. Rudin et al. (1994) studied the dynamic IFT variation of acidic oils and aqueous solutions modified by surfactant and alkaline. They reported that the IFT variation was directly correlated to the formation of the micelles due to acid ionization that existed in the crude oil in contact with the surfactant leading to a significant reduction in IFT value. On the other side, they reported that the presence of surfactant molecules retards the ionization of acidic contents by reducing the mass transfer rate between the oil/water interface causing the necessity of more time to reach the low IFT condition. Also, Zhao et al. (2018) showed that dynamic IFT between flooding systems and acidic oil can reach and maintain ultra-low values at low surfactant concentrations for optimal alkalinity and salinity.
So, the current investigation is aimed to investigate the synergy between the different chemicals of alkali (Ca(OH)2) (0–2000 ppm), surfactant, namely 1-tetradecyl-3-methyl imidazolium chloride (C14mim][Cl]) (0–1000 ppm), different monovalent salts of NaCl and KCl under high-salinity conditions (50,000 ppm to 200,000 ppm), and titanium oxide nanoparticles (TiO2–NPs) as an effective NPs in the range of 0–100 ppm for IFT reduction and wettability alteration.
The other point is that due to the complex chemistry of crude oil (combination of thousands of chemicals), it is hard to find a generalized conclusion regarding the effect of chemicals and different crude oils. It seems that using only one fraction of crude oil such as saturates, aromatics, resins, or asphaltene fractions for experimentations provides a better to extract a more generalized correlation between the different chemicals and specific fractions of crude oil. In light of this fact, the current investigation is aimed to examine the effect of different chemicals in contact with the specific fractions of crude oil, namely resin and asphaltene fractions with concentrations close to the values that existed in the original heavy crude oil. In detail, the effect of chemicals on crude oil is highly sensitive to the composition of the crude oil, especially the alkalis which are highly interactive with the acidic contents of the crude oil (Overton et al. 2016).
So, it is impossible to correctly characterize crude oil using only one specific molecular type (Demirbas and Taylan 2015). As an alternative to eliminate such a limitation, crude oil can be categorized into several main classes considering the structural similarity of different hydrocarbons. The crude oil can be characterized into four distinct chemical classes which, among them, resin and asphaltene fractions are the most important ones due to their surface active nature. Among these two fractions, both of them are vital for stable crude oil but the resin fraction is responsible to stabilize the asphaltene fraction either (Fakher et al. 2020). In detail, asphaltene and resin possess a surface active nature which directly affects the IFT and crude oil/water emulsion stability (Demirbas et al. 2015; Aske et al. 2001). The point is that it is possible to categorize the crude oil fractions into resin or asphaltene fractions using the fact the H/C ratio of resins is between 1.2 and 1.7 while this value for asphaltene fractions is between 0.9 and 1.2 although resins and asphaltenes are structurally close to each other (Ficken et al. 2002). One of the most important characteristics of the resin and asphaltene fractions is their potential to act as a surfactant to reduce interfacial tension comes from their complex configuration which is similar to head and tail combination molecules. The other noteworthy point is the effect of resin fraction on the stability of asphaltene molecules leading to better IFT reduction in theory although the real effect of these two fractions together must be thoroughly examined under different conditions especially the presence of different salts and chemicals such as surfactants and alkalis.
According to the best knowledge of the authors, there is no report regarding the effect of asphaltene and resin fractions in the presence of salts (NaCl and KCl) under high-salinity conditions up to 20,000 ppm, Ca(OH)2 as an alkali, and [C14mim][Cl] as the active surfactant from imidazolium family, and TiO2–NPs. So, the current investigation is concentrated on the possible synergy between these chemicals and resinous synthetic oil (RSO) and asphaltenic synthetic oils (ASO) (8 wt%) for the first time. In the last stage, spontaneous and forced imbibition tests are going to be performed to find the possible effect of the optimum formulations on the wettability alteration and tertiary oil recovery factor for more reliable conclusions.
Materials and methods
The performed experiments can be shown in the following flow chart for more clarification and easy stepwise investigation of the performed experimentations (see Fig. 1).
Chemicals
The required sample crude oil for resin and asphaltene extraction was kindly provided by the National Iranian South Oil Company (NISOC) from the Bangestan oilfield with a density of 0.91 g cm−3 @ 15 °C comprised of 8.3% asphaltene and 9.1% resin fractions. The required chemicals such as Ca(OH)2 with a molecular weight of 74.09 g mol-1 and CAS number of 1305–62–0 were supplied from Sigma-Aldrich with purity better than 95% and used without any further purification. Besides, the required ionic liquid ([C14mim][Cl]) and titanium oxide nanoparticles (average particle size of 23 nm using TEM) with CAS numbers 171,058–21–2 and 13,463–67–7 were supplied from Alfa Chemistry, USA, and Sigma-Aldrich with purity better than 98% and 99.5%, respectively.
Resin extraction
In general, it seems that using a specific fraction of crude oil for IFT and wettability modification studies provide several advantages. The first advantage is that more generalized correlations can be extracted since each fraction of crude oil is rather the same in contents and overall structure. So, any extracted conclusion can be used for the rest of similar fractions extracted from different crude oils to some extent while using crude oil eliminates such an advantage. The other advantage is that one can specifically investigate the synergy between different chemicals and specific fractions without distortion of other constituents gives a good and deep insight regarding the molecular interactions and possible mechanisms. Although there are several investigations regarding the interactions and possible effects of the different chemicals on the IFT and wettability of the system including crude oils, there are a very limited number of investigations dealing with specific extracted fractions of the crude oil, especially the resin and asphaltene fractions. These two fractions are more important among the different fractions of saturates and aromatics because of their complex nature and structure made of different and fused types of aromatics, branched alkyl chains, etc., bringing complex nature for these fractions. In this study, IP 143/90 (Petroleum 1985) was used step by step to extract the asphaltene fraction in the first stage and then the resin extraction was isolated from the de-asphalted crude oil.
The point is that among the different fractions of the crude oil, resin, and asphaltene fractions are natural surfactants that can have a considerable effect on the IFT reduction during the chemical EOR methods. It is highly required to investigate the sole and combinative effects of these fractions in the presence of different chemicals. Besides the effect of these fractions on the possible IFT or wettability alteration, crude oil is a combination of thousands of chemicals makes it hard to extract any reliable and generalized conclusion if the crude oil is being studied for EOR purposes. Respecting these reasons, several investigations were performed to just study the role and interactions of different chemicals in the presence of only resin and asphaltene fraction although the results are vague and no consistent results were collected by the researchers (Lashkarbolooki et al. 2014a).
Besides, since the resin and asphaltene fractions are surface active agents (Lashkarbolooki et al. 2016; Wu et al. 1998) they gain increasing attention during the past decades. In brief, using the IP 143/90 for asphaltene isolation purposes, n-heptane with a ratio of 40:1 was applied in the first place. Then, the remaining molten and de-asphalted oil was contacted with a silica gel column (Merck, 35 − 70 mesh ASTM) to extract the resin fraction using the column chromatography method (Amin et al. 2011; Soorghali et al. 2014).
After that, saturates and aromatics were washed and eliminated from the extracted fraction using 70:30 n-heptane and toluene solution. At last, an acetone/dichloromethane/toluene mixture with a ratio of 40:30:30 was used to achieve the resin fraction (Miller 1982; Yarranton et al. 2000).
Besides the isolation of resin and asphaltene fractions, these two fractions were elementally analyzed using a CHNSO analyzer (Thermo Flash EA 1112 series) to determine the C, H, N, S, and O contents. Based on the performed analysis, the resin fraction with the H/C ratio of 1.48 compared with the asphaltene H/C ratio of 1.12 revealed lower aromaticity characteristics which means a more branched structure of resin fraction than the asphaltene molecules. With respect to this finding, it is completely obvious that resin molecules have a more surface active nature than asphaltene molecules.
IFT measurement
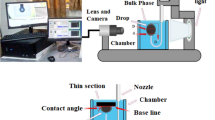
One of the most reliable and accurate methods for IFT measurement is the pendant drop or rising drop method mainly developed based on the shape of a drop if it forms at the tip of a nozzle (Yang et al. 2014). This method is mainly comprised of different sections including forming the drop at the tip of the nozzle regardless of the drop orientation (rising or pendant position which is directly correlated to the density difference of the bulk and drop phases), image capturing system which comprised of a Charge-coupled device (CCD) camera and macro lens to provide a proper image from a small drop in the bulk phase and the last section is the online image processing software. The first section uses an automatic injection pump in which the syringe and stainless steel needle are attached to it. It is possible to control the injection rate using this automatic injection system installed exactly above the cuvette. This direct installation of the needle to the syringe provides a smooth injection of fluid into the bulk phase without any time delay and provides a stable drop at the tip of the nozzle during the measurements. The second section provides suitable pictures with desired magnification and sharpness from the formed drop and then dispatches it to the online software. Finally, the software calculates the required properties of the image and then converts these measured properties to the corresponding IFT using the shape factor which is the main principle of the used software (see Fig. 2 demonstrated the used apparatus supplied by Fanavari Atiyeh Pouyandegan Exir Co., (APEX Technologies co.,), Arak, Iran).
The point must be mentioned is that not only the volume of the drop must be proper but also it is highly required to use a suitable magnification to occupy the screen as much as it is possible to ensure accurate measuring of the image properties which can guarantee the accurate calculation of IFT values (see Eq. 1):
where Δρ, g are defined as the density difference for bulk and drop, acceleration of gravity, respectively, while H is defined as the shape-dependent parameter in which it is possible to find it using shape factor (S = d/D). In detail, although the Δρ, g are easily obtainable, the H calculation is more complicated which is correlated to the reverse portion of the equatorial diameter (D) and the diameter at the distance D from the top of the drop (d) known as shape factor (Adamson and Gast 1967; Andreas et al. 2002; Stauffer 1965). The point is that the used method leading to maximum uncertainty of 0.2 mN m−1 was calculated using at least three independent measurements for each data point. In the second phase, the IFT values of the systems which were below the 0.5 mN/m were measured using the spinning drop method which was developed by Vonnegut (Vonnegut 1942) since spinning drop method is typically appropriate for IFT values below 1 mN/m, and down to ultra-low values (µN/m or less) (see Eq. 2):
where γ is the IFT in N/m, R is the radius of the drop at equator (E).
The analysis revealed that the aforementioned equation is valid for about 0.1% if the length of the drop/diameter exceeds 4. For certain calculations, the rotational speed increases to a value in which the L/D ratio exceeds 4 and even more than 4 to reach a cylinder where the hemisphere diameter at the tip of the drop comes close to the cylinder thickness as shown in Fig. 3 (Ro = 2/3 R).
Contact angle measurement
In the current investigation, the solid surface was prepared from outcrop carbonate rocks of the oilfield according to the performed XRD analysis, the majority of the used rock samples are made of oxygen, carbon, calcium, and magnesium which can be considered an indication of the dolomite core sample (C = 14.12%, O = 36.60%, Ca = 34.20, Mg = 11.12%, Al = 0.51%, S = 0.22%, Si = 3.21%). In the sessile drop measurement technique, if the water CA is lower than 90°, the surface is water-wet while as the contact angle is higher than 90°, the surface is oil-wet.
The captive method which is one of the sessile drop method types was used to measure the contact angle of the oil drop in the presence of an aqueous bulk phase settled beneath the rock surface submerged in the aqueous phase (see Fig. 3). In detail, the required drop suspended at the tip of the nozzle and then the submerged thin section of the rock surface moved toward the suspended drop to be attached into the rock surface very gently. At this time, the surface is moved upward to completely detach the suspended drop, and the measurement begins using online software.
Core flooding experiment (forced imbibition test)
In the current investigation, a high-pressure–high-temperature core flooding apparatus was designed and constructed for pressure up to 150 °C and 700 bar, respectively, (Fanavari Atiyeh Pouyandegan Exir Co., (APEX Technologies co.,), Arak, Iran) was used to perform the required experiments (see Fig. 4).
This equipment mainly consists of different parts including a high-pressure injection pump, high-pressure high-temperature accumulators for different liquids (three of them have a volume of 500 cc while one of them has a volume of 100 cc for easy handling of the chemical slugs), high-pressure–high-temperature hassler core holder type, confining pressure system control and monitoring, gas back pressure regulator (GBPR) monitoring and controlling unit, and data acquisition system. In this equipment, the injection of fluids is done using a high-pressure pump with desired injection rate mostly between 0.1 and 0.6 cc/min since the laminar flow in the reservoir can be achieved about 1 ft/day which is close to 0.3 cc/min. The data acquisition section is utilized to monitor the injection rate of the fluids and record the inlet and outlet pressures of the core holder which means the upstream and downstream pressure of the core. So, the current apparatus can be used as a way to perform the different injection patterns and inject different solutions under different pressures and temperatures using the flowing sequence:
-
Measuring the porosity and permeability of the core plugs
-
Saturation of the fresh core plug using desired aqueous solution (generally formation brine) injection for several pore volumes (PVs) to ensure no air existed in the core
-
Injection of crude oil under follow rate of 0.3 cc/min to saturate the core plug with crude oil and reach the irreducible water saturation (Swirr). At this point, the core reached the reservoir condition after the primary production stage which means the core is ready to be used for secondary and tertiary oil recovery stages.
-
Injection of formation brine or any aqueous solution for several pore volumes with a flow rate of 0.3 cc/min to reach the point where no oil is being produced (Swro) (secondary oil recovery stage).Injection of any chemical solution slug in the range of 0.1 to 0.5 pore volume (PV) with desired injection rate in the range of 0.1 to 0.6 cc/min chased with formation brine for several PVs for further oil production as the tertiary oil recovery stage
-
Soaking the flooded core plug with the chemical slug for a specific period of time more than 7 days to give the required chance to reach the ultimate wettability alteration. It is possible to differentiate the effect of different mechanisms such as IFT reduction from wettability alteration to some extent.
Spontaneous imbibition tests
Through the spontaneous imbibition tests, 1″ core plugs with a length of 2″ were used to find the effect of the optimum chemical formulation on the wettability alteration using the Amott index measurements kit. In detail, instead of measuring the Amott wettability index, the core plugs were firstly flooded with several PVs of formation brine and then flooded with several PVs of sample synthetic oil to a point in which no formation brine was produced. At this point, the core plugs are ready to be tested for possible wettability alteration using the optimum chemical formulations by placing the core plugs in the Amott wettability index measurements glass vessel and surrounding the core plugs with the optimum chemical formulation. After submerging the core plug into the optimum chemical formulation solutions, the system was held under steady conditions for about 60 days to see the oil production profile. The results obtained after this test can be considered as an indication to decide if the optimum chemical formulations are effective to change the wettability of the rocks toward more desired conditions which are strongly water-wet conditions or not. The point is that although changing the wettability toward water-wet conditions is desired, changing the wettability toward mixed or neutral wet is the other option leading to a high amount of trapped oil production by producing a continuous film of the oil phase.
Results and discussions
Synergy between the resin and asphaltene fractions and NaCl and KCl
In the first place, the effects of NaCl and KCl concentration in the range of 50,000 to 200,000 ppm were examined on the IFT reduction which is in the region of high-salinity conditions. A glance into the obtained results revealed that the presence of these salts under high-salinity conditions leads to a complicated trend of IFT variation. In detail, the depicted results in Fig. 6 revealed that for both examined salts, there is a shifting point for the concentration of 150,000 ppm although for NaCl this shifting point is the minimum value while for the case of KCl, this shifting point is the maximum IFT value. In detail, respecting the case of KCl, there is no clear correlation between the salt concentration and IFT although the overall trend is increasing. But in the case of NaCl salt, the IFT increases as the concentration was increased to 150,000 ppm, while the IFT is faced with a reduction as the concentration was further increased to 200,000 ppm (see Fig. 5).
The overall increasing effect of these salts on the IFT can be directly correlated to the interface saturation with the ions leading to enhancement in the repulsive forces that put the molecules far away from each other and harder packing of the molecules at the interface which means higher IFT values. The point is that although the effect of NaCl on the IFT is clear, the effect of KCl was more complicated which can come from the larger molecular radius providing higher repulsive forces and more complicated interactions between the ions and molecules gathered at the interface leading to IFT fluctuation without any clear trend (Fig. 6).
In the second stage of this investigation, the effects of different salts were examined on the synthetic oil prepared by dissolving a specific amount of resin and asphaltene fraction separately (see Fig. 7). In this way, resinous synthetic oil (RSO) and asphaltenic synthetic oil (ASO) (8% wt) were prepared and used as the model oil while the concentration of salts was ranged between 0 and 200,000 ppm to cover the high-salinity conditions. The obtained results revealed that for both prepared oils which were contacted with the different concentrations of aqueous solutions of salts, IFT was reduced for NaCl solutions while the IFT variation for KCl solution was more complicated. The point is that among the ASO and RSO, the resinous type revealed better interaction for IFT reduction since the resin fraction is more similar to the surfactant molecules than the asphaltene molecules. In general, it has been reported that the interfacial tension of a hydrocarbon increases as the salt concentration increases in the water; but as a small amount of surface active agent or surfactant is added into the aqueous solution, the interfacial tension of the system reduces with the salinity. According to this fact and since the asphaltene and resin factions are surface active fractions considering their structure, the addition of salts into the aqueous solutions leads to a reducing trend due to the positive interactions that occur between the asphaltene and resin molecules and salts. In detail, in the absence of surface active agents, the repulsive forces enhance as the salts concentration increases leading to an increase in the IFT, while the presence of surface active compounds provides the chance of ions rearrangement in the complex structures of the resin and asphaltene molecules with lower repulsive forces makes it possible to reduce the IFT value.
Similar results were reported by Kuang (2018) during the IFT measurement for the different oils and salinity in the absence and presence of surfactants. They reported that in both cases, the IFT was increased as the salt concentration was enhanced to about 20 g/L (2.2 wt%) asphaltenes (or 2 wt% 5β-cholanic acid) in toluene while increasing the asphaltene concentration to the values higher than 2.2 wt% leading to IFT reduction as the salinity was increased which showed that the asphaltenes are similar to weak ionic surfactants.
The other point is that according to the H/C analysis, the surface activity of the resin fraction is higher than the asphaltene fraction due to the more branched structure of resin and lower aromaticity gives it a higher surface activity nature. In light of this fact, it is completely acceptable to see the better-reducing trend of IFT for RSO/saline water as it is depicted in Fig. 6.
But, in the case of using KCl as an active salt, the IFT variation was so different from the trend observed for NaCl. As it is depicted in Fig. 7, a shifting point can be observed in the case of ASO which is about 100,000 ppm. In detail, as the salt concentration was increased from 0 to 100,000, IFT experienced an enhancement, although the further increase in the salt concentration leads to lower IFT values. However, in the case of RSO, as the salt concentration was increased from 0 to 50,000, IFT faced with a reduction although the further increase in the salt concentration moves the IFT value to a higher level. The point is that rather a similar trend was observed by Lashkarbolooki et al. (2014b) regarding the effect of salts on the IFT.
In detail, they reported that the IFT increases as the concentration of salt in the solution increases up to 0.017 mol·kg−1 (1000 ppm) and 0.013 mol·kg−1 (1000 ppm) for NaCl and KCl, respectively. However, as the salinity increases above this concentration the IFT decreases and then increases again. Considering these obtained results by Lashkarbolooki et al. (2014b), a similar trend was obtained in the case of RSO with a shifting concentration value of 100,000 ppm although no increasing trend was observed for the salt concentration larger than 100,000 ppm in contrast to the results observed by Lashkarbolooki et al. (2014b). According to these findings, it seems that in the case of both ASO and RSO in contact with the aqueous solution modified by KCl, there is a low synergism between the KCl salt and the natural surfactants (resin and asphaltene molecules). Besides, when inorganic salts (Na+ and K+) are present in the aqueous phase, the salt ions can be surrounded by the water molecules which form a cage-like structure in the light of hydrogen-bonded. In this situation, water molecules are in contact with another phase leading to disruption of hydrogen bonding leading to a higher energy environment for the ions. As a consequence of this phenomenon, the surface excess concentration of salts moves toward negative values (Kumar 2012).
The investigations revealed that the resin and asphaltene fractions distribution is directly correlated to the salts concentrations which mean the direct effect of salts on the IFT of the systems including natural surfactants (asphaltene and resin). According to the results reported by Al-Sahhaf et al. (2005) as the NaCl and KCl concentrations were enhanced to 1000 ppm, respectively, the salt molecules moved to the oil phase in the light of activity coefficient modification. Moreover, the findings revealed that as the salt concentration increases and the cations are transferred to near the surface, the possible interaction between the natural surfactant, polar asphaltenes, and resins fractions appear leading to a level of solubility in both oleic and aqueous phases (Moeini et al. 2014). At this point, the negative surface excess concentration for salt turns to be positive type as the cations presented at the interface, which consequently moves the IFT values toward lower values based on the Gibbs adsorption isotherm. However, the IFT trend changes to an increasing pattern as the salt concentration increases to a value well above the optimum salinity where the salting-out effect is the dominant mechanism for IFT manipulation.
The natural surfactants are depleted near the interface and transferred back to the oil phase and breaking the balance of oil − water interface adsorption (Chang 2000). Consequently, the concentration of salts and natural surfactant at the interface will be depleted and Γasphaltene and Γsalt become negative, therefore the IFT increases.
Respecting these findings, the concentration of 200,000 ppm for NaCl regardless of using RSO or RSO and 50,000 ppm of KCl for ASO and 200,000 ppm of KCl for RSO was selected as the optimum concentration through the rest of the experiments.
Effect of Ca(OH)2 on the IFT of the binary systems
In the next stage of this investigation, the effect of divalent alkali namely Ca(OH)2 in the range of 0–2000 ppm was investigated on the IFT reduction using the optimum salts concentrations of 200,000 ppm for NaCl and KCl in contact with resinous synthetic oil and 200,000 and 50,000 ppm for NaCl and KCl concentrations in contact with ASO. The first finding of this section was that the systems experienced precipitation and incompatibility between the chemicals for the Ca(OH)2 concentrations of higher than 1500 ppm for NaCl and 1000 ppm for KCl regardless of using ASO or resinous synthetic oils. In other words, there is a limitation regarding the dissolution of Ca(OH)2 with concentrations higher than 1500 and 1000 ppm for NaCl and KCl, respectively. This observed trend is directly correlated to two different phenomena which are the intrinsic low solubility of Ca(OH)2 in the water and the incompatibilities that exist between different chemicals. So, the limited concentration of this alkaline was used to prevent the production of milk of lime based on the threshold reported by Greenberg and Copeland (1960) which was 9.10 × 10−6 at 25 °C.
But, a closer look into the results obtained for the Ca(OH)2 concentrations lower than the precipitation threshold, revealed that the presence of Ca(OH)2 led to a significant reduction in IFT of about two times as it is depicted in Fig. 7. As it is clear, the overall trend of IFT variation is reducing trend no matter which salts or oil fractions are used. A close look into the results revealed that in the case of KCl with a concentration of 50,000 ppm in contact with ASO, a direct reducing trend for IFT is evident, while for the other examined systems of ASO in contact with 200,000 ppm NaCl solution, the system experienced a shifting point of 500 ppm of Ca(OH)2. In detail, it seems that the production of in situ surfactant in the ASO/200,000 ppm solution is not capable to well-pack in the interface and reducing the repulsive forces that exist between the ions leading to an increasing trend in IFT. However, while as the Ca(OH)2 concentration increases to values higher than 500 ppm, the produced in situ surfactant can neutralize the repulsive forces exited between the ions of the interface by occupying the distances. In this condition, the ions faced with less repulsive forces provide a better chance for the molecules especially the in situ surfactant molecules to rearrange in the interface for lower IFT values.
In the case of using RSO in the presence of 200,000 ppm NaCl and KCl, an increase in the Ca(OH)2 concentration leads to a continuous reduction in IFT although the reduction slope for KCl is sharper than the reduction slope for NaCl as the Ca(OH)2 concentration increases from 0 to 500 ppm. However, there is no significant difference between the IFT reduction slope for both salts at the higher concentrations of Ca(OH)2.
The reason for the observed trend is directly related to the presence and increase in the interfacial concentration of in situ surfactant in the interface causes IFT reduction. The interfacial concentration is directly dependent on the structural groups that existed in the surfactant molecules and their arrangement at the interface. In this way, the presence of Ca(OH)2 leads to the production of in situ surfactants in the solution and the consequent packing of these surfactant molecules in the interface leading to an increase in the interfacial concentration. At this point, the co-presence of in situ surfactants and resin and asphaltene fractions which are similar to surfactant molecules provides a positive interaction leading to a significant reduction in IFT.
Similar results were reported by Seetharaman et al. (2021) regarding the application of Ca(OH)2 as an effective alkali during the EOR processes for IFT reduction purposes. According to their findings, it was obvious that the effect of Ca(OH)2 on the IFT reduction was better than monovalent alkali (NaOH) which was related to the divalent cations of Ca(OH)2 since the anions were the same. In detail, since Ca2+ ions have a larger surface charge than sodium ions, better polarization was observed for Ca2+(Robb and Smith 1974). Similar results were reported by Lashkarbolooki et al. during IFT measurements of the systems dealing with different divalent cations (Lashkarbolooki et al. 2014b).
Moreover, Seetharaman et al. (2021) reported that for the case of alkanes, an increase in the alkali concentration reduced the IFT of the aromatics/water systems with a higher impact of Ca(OH)2 than NaOH for IFT reduction. Moreover, comparing the influence of using a similar concentration of Ca(OH)2 and NaOH with the value of 100 ppm showed better efficiency of Ca(OH)2 than NaOH which justifies the better role of divalent alkali for IFT reduction compared with the monovalent alkali.
The findings of the current investigation are in contrast to the results reported, Zhao et al. (2018) reported that the effect of alkali concentration on the IFT for aqueous solution/crude oil is the same as the IFT variation was observed for paraffin/water system which means the similar effect of NaOH on crude oil and normal alkanes. The point is that if the acidic or other constituents exist in crude oil, in situ surfactant production due to interactions between active species and NaOH is inevitable. However, the effect of this surfactant on IFT reduction is not well understood.
Zhao et al. (2018) surprisingly reported that the aqueous solution of NaCl was acting similarly to NaOH for IFT variation and surface active compounds packing at the interface. According to these findings, one can conclude that as the NaOH concentration increases, the electric double layer (EDL) of hydrophilic groups in asphaltene molecules and the produced in situ surfactant molecules can be compressed to become smaller and the electrostatic interactions between hydrophilic groups weakened which leading to unpreventable accumulation of the surface active molecules in the interface and IFT reduction.
Moreover, Zhao et al. (2018) reported that according to the aforementioned effect of NaOH on the accumulation of surface active agent in the interface and possible salting-out effect, IFT can show a dual trend behavior which is decreasing in the first stages and then change to increasing trend as the NaOH concentration increases. They also reported that as the NaOH concentration goes beyond a threshold, surfactant molecules were driven into the oil phase by the salting-out effect, and this process resulted in a decrease of IFT for water-soluble p-S12-5 and p-S14-5. On the other hand, for oil-soluble p-S18-5, the above process had a slight effect on the effective distribution in the oil phase. The effective distribution of surfactant in the oil phase played an important role in stabilizing the interface and reducing IFT. They also claimed that there was no clear evidence regarding the effect of NaOH on the IFT reduction considering the synergistic behavior between the surfactant and active species (formed in situ surfactants) in the crude oil. To sum up and based on the findings, it is possible to summarize the effect of Ca(OH)2 on the IFT reduction as below:
In the first stage, the ionic strength of the water phase increases as the Ca(OH)2 existed in the solution due to the polarity characteristics of the water phase leading to the EDL compression of hydrophilic groups. In the light of this compression, the accessible area for the molecules to be occupied reduces at the interface which means a higher interfacial concentration of surface active agents leading to lower IFT values. In the next stage, as the Ca(OH)2 concentration increases, the EDL is further compacted leading to weaker electrostatic interactions between hydrophilic groups and shortened electrical effect range and moving the water molecules toward the interface because of the existing gap between loose polar groups. On the other side, it is possible to drive the surfactant molecules into the oil phase under the light of the salting-out effect appears at a higher Ca(OH)2 concentration which directly increases the mutual attraction of lipophilic chains and the close-packed lipophilic groups at the interface.
Furthermore, since the calculated zeta potential value was positive it can be concluded that the Ca2+ ions occupied the interface of the hydrocarbon-water system. Moreover, Wang et al. (1998) used rotating disk method to study the Ca(OH)2 dissolution mechanism considering the surface occupation by OH− ions in the preliminary phases while as the reaction progressed the OH− ions are replaced by the Ca2+ ions freely existed in the solution, As a consequence of this substitution, the interface charge variation toward positive values will occur which revealed the close correlation between the surface adsorption of OH− ions and the nature of the cation associated with it. The other point that Wang et al. (1998) mentioned is that the IFT reduction was related to the existing impurities coming from the undissolved calcium gathered in the interface leading to lower IFT values.
Effect of [C14mim][Cl] on the IFT of the Alkali/salt/synthetic oil
In the next stage of this investigation, the effect of [C14mim][Cl] was examined on the IFT reduction of the optimum chemical solution obtained (1500 ppm Ca(OH)2 + 200,000 ppm NaCl/ASO or RSO and 1000 ppm Ca(OH)2 + 50,000 ppm KCl/ASO and 1000 ppm Ca(OH)2 + 200,000 ppm KCl/RSO). The obtained results revealed that the presence of IL in the range of 100 to 1000 ppm leads to a linear reduction in IFT value to the minimum value of 2.1, 0.84, 1.3, and 0.94 mN/m for 1500 ppm Ca(OH)2 + 200,000 ppm NaCl/ASO or 1500 ppm Ca(OH)2 + 200,000 ppm NaCl/RSO, 1000 ppm Ca(OH)2 + 50,000 ppm KCl/ASO and 1000 ppm Ca(OH)2 + 200,000 ppm KCl/RSO, respectively. This IFT reduction which is about 10 times reduction for 1000 ppm Ca(OH)2 + 200,000 ppm KCl/RSO is depicted in Fig. 8. A glance into Fig. 9 revealed that the presence of IL has a higher effect on IFT reduction in the RSO systems (50 times for NaCl and 31 times for KCl) than ASO which means better synergy between the IL and resin molecules.
In other words, it seems that the bulky structure of IL provides the chance for resin molecules to move toward the interface and be trapped in the bulky structure of the ionic liquids (ILs) leading to lower IFT value. In detail, according to the H/C ratio analysis which revealed the higher aromaticity of the asphaltene molecules than the resin molecules it can be concluded that more branches existed in the resin molecules than the asphaltene molecules providing a higher chance for the resin molecules to bond and interconnect with the bulky structure of the ILs in the interface consequently leading to more IFT reduction. Moreover, the more polarity of the resin molecules than the asphaltene molecules boosts the adsorption of resin molecules into the IL structure leading to stronger bonding and more effective reduction in IFT values than the systems dealing with asphaltene molecules.
Similar results were reported by Hezave et al. (2013a, 2013b, 2013c) and Najimi et al. (2019b) by examining different families of ILs including 1-dodecyl 3-methyl imidazolium chloride 1-octyl 3-methyl imidazolium chloride, 1-dodecyl 3-methyl pyridinium chloride, and 1-octyl 3-methyl pyridinium chloride. They reported that all of the examined ILs were effective for IFT reduction especially in the presence of salinity compared with the conventional surfactants. They also reported that the efficiency of the used ILs was reduced as the temperature was increased since it directly affects the nitrogen atoms in the imidazolium and pyridinium-based ILs. According to the obtained results, it can be concluded that the ILs are applicable for IFT reduction of the systems dealing with harsh salinity and temperature conditions.
Moreover, Shaktivel and his coworkers (2014) carried out similar experiments using imidazolium-based ILs with different anions such as 1-butyl-3- methyl imidazolium chloride ([C4mim][Cl]), 1-butyl-3-methylimadazolium bromide ([C4mim][Br]), etc. in the presence of NaCl to see the possible synergy between these parameters using the Wilhelmy plate method. They reported that although the IL adsorption at the interface between crude oil and water is a crucial parameter, IL concentration is the other effective parameter on the crude oil/water IFT (Sakthivel et al. 2015). In detail, their measurements revealed the IFT reduction from 36.24 to 25.86 mN/m as the [C4mim][Cl] concentration was increased from 0 to 50 ppm at 288.15 K and IFT reduction from 36.24 to 23.26 mN/m for 1-octyl-3 methyl imidazolium chloride [C8mim][Cl] under similar concentration enhancement. The further experiments performed by Hashmi and Firoozabadi (2013) and Sakthivel et al. (2015) revealed that the longer the alkyl chain length is used, the more IFT reduction is the result even under lower concentrations especially if the NaCl existed in the aqueous solution.
Effect of TiO2–NPs on the IFT of the Alkali/surfactant/salt/synthetic oil
In the third stage of this investigation, the effect of TiO2–NPs in the range of 0–100 ppm on the IFT reduction was investigated using four selected optimum solutions of 1500 ppm Ca(OH)2 + 200,000 ppm NaCl/ASO or 1500 ppm Ca(OH)2 + 200,000 ppm NaCl/RSO, 1000 ppm Ca(OH)2 + 50,000 ppm KCl/ASO and 1000 ppm Ca(OH)2 + 200,000 ppm KCl/RSO. The point is that all of the aforementioned optimum solutions were further modified by the addition of 1000 ppm [C14mim][Cl]. The first point is that the performed compatibility analysis without IL revealed that the TiO2–NPs were precipitated in the distilled water rapidly after mixing and agitation while using 1000 ppm of [C14mim][Cl] was capable to retard the precipitation of TiO2–NPs for more than 20 days although the higher concentration of TiO2–NPs (higher than 100 ppm faced with precipitation no matter IL was used or not). So, the 1000 ppm IL was used in the current section as the optimum concentration of IL since not only was capable to stabilize the TiO2–NPs for a long period of time but also was capable to well reduce the IFT to a desired level. Similar results were reported by Kuang et al. (2018) respecting the stability of TiO2–NPs.
In detail, they reported that pure TiO2 is highly unstable and rapidly precipitated in the absence of any chemical agents specifically surfactant molecules. But, their results revealed that it is possible to prepare stable nanofluid with a lower average diameter of TiO2 aggregates to 246 nm (SEM) and 268 nm (DLS) and zeta potential of −80 mV (compared to the original value of −6 mV in the absence of chemical agent) by adding 0.1 wt% OA. However, the addition of cationic surfactant was more effective to prepare more stable nanofluids (Kuang et al. 2018).
Respecting these optimum formulations, the addition of TiO2–NPs in the range of 0–200 ppm into the four selected optimum chemical formulations was done to find the effect of these particles on the IFT reduction. The measurements revealed the significant effect of TiO2–NPs even in the low dosage for IFT reduction since the IFT values were reduced from 0.51, 0.12, 0.44, and 0.32 for 1500 ppm Ca(OH)2 + 200,000 ppm NaCl/ASO or 1500 ppm Ca(OH)2 + 200,000 ppm NaCl/RSO, 1000 ppm Ca(OH)2 + 50,000 ppm KCl/ASO and 1000 ppm Ca(OH)2 + 200,000 ppm KCl/RSO at the presence of 1000 ppm of IL to 0.17, 0.07, 0.19 and 0.08, respectively. In other words, the presence of TiO2–NPs was so effective to reduce the IFT of the system to values well close to ultra-low values leading to better oil production of trapped oil.
Similar to the results obtained in the current investigation, several investigations revealed that the application of NPs such as silicon dioxide (SiO2), aluminum oxide (Al2O3), and titanium dioxide (TiO2) are highly efficient to reduce the IFT of the systems (Ahmed et al. 2017; Dahle 2013; Esmaeilzadeh et al. 2014; Hendraningrat et al. 2013; Joonaki and Ghanaatian 2014; Li et al. 2013; Roustaei et al. 2012; Saigal et al. 2010; Zaid et al. 2014).
In general, it is well accepted that using NPs leads to an undeniable effect on the solid–fluid and fluid–fluid interfaces because their large surface area to volume ratio provides high adsorption affinity for the nanofluids consequently making it possible to change the IFT and especially wettability of the rock surfaces in the light of this affinity (Jin 2017).
Similar results were reported by Kuang et al. (2018) respecting the effect of different NPs especially TiO2–NPs in the presence of different surfactants. Their results revealed that the presence of TiO2–NPs with poly acryl amide has a lower effect on the IFT reduction than the TiO2–NPs solution modified by a cationic surfactant. In other words, their results demonstrated that using cationic surfactant with TiO2–NPs not only provides better stability for the NPs but also is more effective to reduce the IFT toward more desired conditions. The point is that although the application of prepared TiO2–NPs solution with cationic surfactant led to low IFT values, Kuang et al. (2018) claimed that this IFT reduction was mainly related to the cationic surfactant since in the case of SiO2 and TiO2 compared with the Al2O3 electrostatic attractive forces which are responsible for IFT reduction are absent, However, in the current investigation, the performed analysis revealed direct relation of the TiO2–NPs on the IFT reduction may be due to better interaction may exist between the asphaltene and resin fractions and the TiO2–NPs.
Effect of optimum chemical formulation on the wettability alteration using spontaneous imbibition
In this section, the effects of four optimum chemical formulations including NaCl/KCl, Ca(OH)2, resin/asphaltene fraction, and TiO2–NPs were examined using Amott wettability index measurements. The reason behind using the Amott wettability index instead of contact angle measurements is that the Amott wettability index provides a better understanding regarding the effect of chemicals on the wettability alteration than the contact angle since contact angle is position dependent, while the Amott wettability index measurements provide more generalized understanding and conditions regarding the effect of chemicals on the wettability. In this way, although the contact angle measurements for the optimum chemical formulation were performed in the current investigation, the Amott wettability index measurements were also performed for better conclusions regarding the effect of the used chemical formulations on the wettability alteration of the rocks.
Moreover, the Amott wettability index measurements can provide this chance for the operator to find if the used chemicals were capable to move the wettability toward mixed-wet conditions which are highly desired in the EOR processes. The point is that the mixed-wet condition is highly desired since it is claimed that the trapped oil can be entirely drained if the wettability of the rock is mixed-wet which can provide a continuous film of oil drops leading to the entire production of trapped oil to reach the zero oil saturation although it would be a time-consuming phenomenon.
The performed contact angle measurements revealed that the used chemical formulation of 1500 ppm Ca(OH)2 + 200,000 ppm NaCl/ASO or 1500 ppm Ca(OH)2 + 200,000 ppm NaCl/RSO, 1000 ppm Ca(OH)2 + 50,000 ppm KCl/ASO and 1000 ppm Ca(OH)2 + 200,000 ppm KCl/RSO at the presence of 1000 ppm of IL and 100 ppm TiO2–NPs, were capable to change the wettability of the rock surface toward strongly water-wet conditions with contact angle values of 18.6°, 23.3°, 33.2°, and 21.3°, respectively. However, the original contact angle was 112.2° (ASO), and 125.6° (RSO). Besides the contact angle measurements, the Amott wettability index measurements revealed that the system is completely moved toward the water-wet conditions as the contact angle measurements showed (see Table 1).
The other point is that the measured produced oil volume during the spontaneous imbibition stage revealed the continuous production of oil during the spontaneous imbibition stage even after 3 months of placing the flooded core inside the Amott cell regardless of the optimum chemical formulations used. The noteworthy point is that except in the early days of oil production, the oil production was stopped for about 23 days and then the oil production continuously started to produce even after 2 months (see Fig. 9). It is possible to consider this period of oil production as an indication of an alteration in the wettability of rock toward mixed-wet condition which is highly desired through the tertiary oil production processes.
Not only the researchers reported the effect of ILs on IFT reduction, they also reported the effect of ILs on changing the rock wettability. For example, Bin Dahbag et al. (2015) performed several wettability alteration experiments using different chemicals, salinities, and even different thermodynamic operating parameters such as temperature.
After finding the optimum formulation, they performed several core flooding experiments using Berea sandstone cores to find the effect of ILs along with the optimum chemical formulation on the IFT reduction and wettability alteration that appears through the tertiary oil recovery. Their results revealed that using tetra-alkyl-ammonium sulfate between nine examined ILs leading to the best tertiary oil recovery comes from its effect on the IFT reduction and wettability alteration (Bin Dahbag et al. 2015).
In the past three decades, the main concentration of the investigations was dedicated to the imidazolium-based ILs with chloride counter-ions while the ILs with pyridinium, ammonium, and phosphonium cations are limitedly used. In the light of this fact, Rodriguez-Palmeiro et al. (2015) studied synthesized 1-dodecyl-3- methylimidazolium cation and acetate anion [C12mim] [OAc] to study the dynamic interfacial tension (DIFT) at different temperatures, water qualities, and alkalis. Their results revealed that the application of 1-dodecyl-3- methyl imidazolium cation and acetate anion ([C12 mim] [OAc]) led to better IFT reduction compared with the ILs tailored with chloride, bromide, and iodide as the counter-ions (Rodriguez-Palmeiro et al. 2015).
Core flooding experiments (forced imbibition tests)
In the last stage of this investigation, the effect of these four obtained optimum chemical formulations was examined on the tertiary oil recovery through core flooding experiments. The performed experiments (see Table 2) revealed the successfulness of all of the used chemical formulations for tertiary oil recovery purposes. In detail, the obtained results revealed that it is possible to increase the oil recovery factor to a maximum value of 15.3% based on the OOIP using 1500 ppm Ca(OH)2 + 200,000 ppm NaCl + 1000 ppm IL + 100 ppm TiO2–NPs in contact with RSO. A close look into the results revealed that the used optimum chemical formulations that were more efficient if they are contact with RSO. The reason behind this finding is directly correlated to the lower IFT values of these systems which are 0.07 and 0.08 for solutions 2 and 4. The point is that although the contact angle measurements and Amott wettability index measurements revealed a rather better effect of solutions 1 and 3 in contact with ASO for wettability alteration toward strongly water-wet conditions, the core flooding experiments is a fast process that provides a better chance for IFT reduction to impact the oil recovery than the wettability alteration which is a time-consuming process. In this way, it can be concluded that the IFT reduction is a dominant mechanism during the tertiary oil recovery than the wettability alteration. According to this fact, it seems that if the system flooded with the optimum chemical formulations and then shut down for a while, not only the IFT reduction but also the wettability alteration would meet its highest level of impact consequently leading to the higher oil recovery than those values obtained at the current stage.
The point that must be mentioned is that the results revealed better interactions between RSO and optimum chemical formulations for higher oil recovery. The reason behind this finding can be attributed to the performed H/C analysis which revealed the higher surface activity of the resin fraction compared with the asphaltene fraction due to the more branched structure of resin and lower aromaticity gives it a higher surface activity nature. In other words, although the chemical formulations lead to IFT reduction and wettability alteration, the surfactant nature of resin provides a better chance for the chemicals to be adsorbed in t interface and provides better oil recovery capability as it appears in the formation of an oil bank by mobilizing the trapped in the pores and throats.
Conclusions
The current investigation is aimed to propose a new class of enhanced oil recovery (EOR) methods known as hybrid methods based on the combination of NaCl and KCl with concentrations of 50,000–200000 ppm, Ca(OH)2, 1-tetradecyl 3-methyl imidazolium chloride ([C14mim][Cl]), titanium oxide (TiO2–NPs) in the range of 0–2000 ppm, 0–1000 ppm, 0–200 ppm, respectively for interfacial tension (IFT) reduction and wettability alteration. The point is that, instead of using crude oil with many compounds, only resin and asphaltene fractions are extracted from a heavy acidic crude oil and used as the model oil with a concentration of 8 wt% (close to the resin and asphaltene contents of the original oil with asphaltene and resin contents of 8.3 wt% and 9.1 wt%, respectively). The measurement revealed that:
-
Asphaltene and resin fractions have a reducing influence on the IFT due to their surface activity making them natural surfactants. Moreover, the contact angle measurements revealed that the aging process using these two fractions moved the rock surface toward the strongly oil-wet conditions of 112.2° and 125.6° for asphaltene and resin fractions.
-
The measurements revealed that NaCl reduced the IFT for both asphaltenic synthetic oil (ASO) and resinous synthetic oil (RSO), while the effect of KCl concentration is increasing for ASO and both increasing/decreasing effects for resinous synthetic oil with shifting concentration of 100,000 ppm.
-
Besides, the measurements revealed a higher effect of Ca(OH)2 on the IFT reduction for the solutions prepared by NaCl with a concentration of 200,000 ppm regardless of using RSO or ASO than the solutions prepared by 50,000 and 200,000 ppm of KCl.
-
IFT measurements revealed the significant effect of used [C14mim][Cl] on the IFT reduction with minimum values of 0.12 mN/m and 0.32 mN/m for 200,000 ppm of NaCl + Ca(OH)2 of 1500 ppm and 200,000 ppm KCl and Ca(OH)2 of 1000 ppm, respectively.
-
Addition of TiO2–NPs with concentrations in the range of 0–100 ppm reduced the IFT to 0.069 mN/m and 0.08 mN/m, respectively.
-
The measurements revealed the higher impact of TiO2–NPs on the KCl solution especially for the systems dealing with RSO.
-
The measured contact angle values revealed the superior effect of NaCl-based chemical formulation along with the used TiO2–NPs on the wettability alteration toward strongly water-wet conditions than the KCl-based chemical formulations.
-
The performed core flooding experiments revealed the excellent effect of IFT reduction for more oil production with a maximum value of 15.3% based on the original oil in place (OOIP) using the optimum chemical formulation.
Abbreviations
- D :
-
Equatorial diameter, m
- d :
-
Diameter at the distance D from the top of the drop, m
- g :
-
Acceleration of gravity, m s−2
- H :
-
Shape-dependent parameter
- L :
-
Drop length
- R and R m :
-
Radius of the drop at equator
- R o :
-
Radius of the drop at edge
- γ :
-
Interfacial tension (N/m)
- Δ:
-
Difference between two parameters
- ρ :
-
Density, kg m−3
- ω :
-
Rotational speed (rad/s)
- ASP:
-
Alkaline–surfactant–polymer
- Al2O3 :
-
Aluminum oxide
- ASO:
-
Asphaltenic synthetic oil
- CA:
-
Contact angle
- CCD:
-
Charge-coupled device
- EDL:
-
Electrical double layer
- EOR:
-
Enhanced oil recovery
- Fe2O3 :
-
Iron oxide
- GBPR:
-
Gas back-pressure regulator
- GC:
-
Gas chromatography
- IOR:
-
Improved oil recovery
- IFT:
-
Interfacial tension
- ILs:
-
Ionic liquids
- NaOH:
-
Sodium hydroxide
- NISOC:
-
National Iranian South Oil Company
- NPs:
-
Nanoparticle
- OOIP:
-
Original oil in place
- HPAM:
-
Partially hydrolyzed polyacrylamide
- ppm:
-
Part per million
- RSO:
-
Resinous synthetic oil
- SiO2 :
-
Silicon oxide
- SiO2–NPs:
-
Silicon oxide nanoparticles
- PV:
-
Pore volume
- TiO2 :
-
Titanium oxide
- wt%:
-
Percentage by weight
- [C18mim][Cl]:
-
1-Octadecyl-3-methyl imidazolium chloride
- [C12mim][Cl]:
-
1-Dodecyl-3-methyl imidazolium chloride
- [C14mim][Cl]]:
-
1-Tetradecyl-3-methyl imidazolium chloride
- [C4mim][Cl]:
-
1-Butyl-3- methylimadazolium chloride
- [C4mim][Br]:
-
1-Butyl-3-methylimadazolium bromide
- [C8mim][Cl]:
-
1-Octyl-3 methyl imidazolium chloride
- [C12 mim] [OAc]:
-
1-Dodecyl-3-methylimidazolium acetate
References
Abhishek R, Kumar GS, Sapru R (2015) Wettability alteration in carbonate reservoirs using nanofluids. Pet Sci Technol 33(7):794–801
Adamson AW, Gast AP (1967) Physical chemistry of surfaces, vol 150. Interscience Publishers, New York
Ahmed A et al (2017) Investigation of dispersion stability and IFT reduction using surface modified nanoparticle: enhanced oil recovery. J Appl Environ Biol Sci 7(4S):56–62
Al-Sahhaf T, Elkamel A, Suttar Ahmed A, Khan A (2005) The influence of temperature, pressure, salinity, and surfactant concentration on the interfacial tension of the n-octane-water system. Chem Eng Commun 192(5):667–684
Amin JS et al (2011) Investigating the effect of different asphaltene structures on surface topography and wettability alteration. Appl Surf Sci 257(20):8341–8349
Amirsadat SA, Moradi B, Hezave AZ, Najimi S, Farsangi MH (2017) Investigating the effect of nano-silica on efficiency of the foam in enhanced oil recovery. Korean J Chem Eng 34(12):3119–3124
Andreas J, Hauser E, Tucker W (2002) Boundary tension by pendant drops1. J Phys Chem 42(8):1001–1019
Aske N, Kallevik H, Sjöblom J (2001) Determination of saturate, aromatic, resin, and asphaltenic (SARA) components in crude oils by means of infrared and near-infrared spectroscopy. Energy Fuels 15(5):1304–1312
Barahoei M, Hezave ZA, Sabbaghi S, Ayatollahi S (2016) Copper oxide nano-fluid stabilized by ionic liquid for enhancing thermal conductivity of reservoir formation: applicable for thermal enhanced oil recovery processes. Chem Ind Chem Eng Q 22(2):211–225
Bin Dahbag M, AlQuraishi A, Benzagouta M (2015) Efficiency of ionic liquids for chemical enhanced oil recovery. J Pet Explor Prod Technol 5:353–361
Cai B-Y, Yang J-T, Guo T-M (1996) Interfacial tension of hydrocarbon+ water/brine systems under high pressure. J Chem Eng Data 41(3):493–496
Chang R (2000) Physical chemistry for the chemical and biological sciences. University Science Books, Melville
Cheraghian G (2015) Effects of nanoparticles on wettability: a review on applications of nanotechnology in the enhanced oil recovery. Int J Nano Dimens 6(5):443–452. https://doi.org/10.7508/ijnd.2015.05.001
Dahle G (2013) The effect of nanoparticles on oil/water interfacial tension. NTNU, Trondheim
Demirbas A, Taylan O (2015) Recovery of gasoline-range hydrocarbons from petroleum basic plastic wastes. Pet Sci Technol 33(23–24):1883–1889
Demirbas A, Alidrisi H, Balubaid M (2015) API gravity, sulfur content, and desulfurization of crude oil. Pet Sci Technol 33(1):93–101
Esmaeilzadeh P, Hosseinpour N, Bahramian A, Fakhroueian Z, Arya S (2014) Effect of ZrO2 nanoparticles on the interfacial behavior of surfactant solutions at air–water and n-heptane–water interfaces. Fluid Phase Equilib 361:289–295
Fakher S, Ahdaya M, Elturki M, Imqam A (2020) Critical review of asphaltene properties and factors impacting its stability in crude oil. J Pet Explor Prod Technol 10(3):1183–1200
Ficken KJ, Wooller MJ, Swain D, Street-Perrott FA, Eglinton G (2002) Reconstruction of a subalpine grass-dominated ecosystem, Lake Rutundu, Mount Kenya: a novel multi-proxy approach. Palaeogeogr Palaeoclimatol Palaeoecol 177(1–2):137–149
Greenberg S, Copeland LE (1960) The thermodynamic functions for the solution of calcium hydroxide in water. J Phys Chem 64(8):1057–1059
Hashmi SM, Firoozabadi A (2013) Self-assembly of resins and asphaltenes facilitates asphaltene dissolution by an organic acid. J Colloid Interface Sci 394:115–123
Hendraningrat L, Li S, Torsæter O (2013) A coreflood investigation of nanofluid enhanced oil recovery. J Pet Sci Eng 111:128–138
Hezave AZ, Dorostkar S, Ayatollahi S, Nabipour M, Hemmateenejad B (2013a) Dynamic interfacial tension behavior between heavy crude oil and ionic liquid solution (1-dodecyl-3-methylimidazolium chloride ([C12mim][Cl]+ distilled or saline water/heavy crude oil)) as a new surfactant. J Mol Liq 187:83–89
Hezave AZ, Dorostkar S, Ayatollahi S, Nabipour M, Hemmateenejad B (2013b) Effect of different families (imidazolium and pyridinium) of ionic liquids-based surfactants on interfacial tension of water/crude oil system. Fluid Phase Equilib 360:139–145
Hezave AZ, Dorostkar S, Ayatollahi S, Nabipour M, Hemmateenejad B (2013c) Investigating the effect of ionic liquid (1-dodecyl-3-methylimidazolium chloride ([C12mim][Cl])) on the water/oil interfacial tension as a novel surfactant. Colloids Surf A 421:63–71
Hosseini S, Sabet M, Zeinolabedini Hezave A, Ayoub MA, Elraies KA (2020) Effect of combination of cationic surfactant and salts on wettability alteration of carbonate rock. Energy Sources Part A Recover Util Environ Effects 177:1–17. https://doi.org/10.1080/15567036.2020.1778141
Institute of Petroleum (1985) IP standards for petroleum and Its products: methods for analysis and testing. The University of Michigan, Ann Arbor
Jin F (2017) Principles of enhanced oil recovery. Physics of petroleum reservoirs. Springer, Cham, pp 465–506
Joonaki E, Ghanaatian S (2014) The application of nanofluids for enhanced oil recovery: effects on interfacial tension and coreflooding process. Pet Sci Technol 32(21):2599–2607
Kuang W, Saraji S, Piri M (2018) A systematic experimental investigation on the synergistic effects of aqueous nanofluids on interfacial properties and their implications for enhanced oil recovery. Fuel 220:849–870
Kumar B (2012) Effect of salinity on the interfacial tension of model and crude oil systems. Graduate Studies University of Calgary, Calgary
Lashkarbolooki M, Ayatollahi S, Riazi M (2014a) Effect of salinity, resin, and asphaltene on the surface properties of acidic crude oil/smart water/rock system. Energy Fuels 28(11):6820–6829
Lashkarbolooki M, Ayatollahi S, Riazi M (2014b) The impacts of aqueous ions on interfacial tension and wettability of an asphaltenic–acidic crude oil reservoir during smart water injection. J Chem Eng Data 59(11):3624–3634
Lashkarbolooki M, Riazi M, Ayatollahi S, Hezave AZ (2016) Synergy effects of ions, resin, and asphaltene on interfacial tension of acidic crude oil and low–high salinity brines. Fuel 165:75–85
Lashkarbolooki M, Hezave AZ, Ayatollahi S (2019) The role of CO2 and ion type in the dynamic interfacial tension of acidic crude oil/carbonated brine. Pet Sci 16(4):850–858
Li S, Hendraningrat L, Torsaeter O (2013) Improved oil recovery by hydrophilic silica nanoparticles suspension: 2 phase flow experimental studies. In: IPTC 2013: international petroleum technology conference, EAGE Publications BV, p cp-350–00212
Miller R (1982) Hydrocarbon class fractionation with bonded-phase liquid chromatography. Anal Chem 54(11):1742–1746
Moeini F, Hemmati-Sarapardeh A, Ghazanfari M-H, Masihi M, Ayatollahi S (2014) Toward mechanistic understanding of heavy crude oil/brine interfacial tension: the roles of salinity, temperature and pressure. Fluid Phase Equilib 375:191–200
Najimi S et al (2019a) Investigating the effect of [C8Py][Cl] and [C18Py][Cl] ionic liquids on the water/oil interfacial tension by considering Taguchi method. J Pet Explor Prod Technol 9(4):2933–2941
Najimi S et al (2019b) Investigating the effect of [C8Py][Cl] and [C18Py][Cl] ionic liquids on the water/oil interfacial tension by considering Taguchi method. J Pet Explor Prod Technol 9:2933–2941
Najimi S, Nowrouzi I, Khaksar Manshad A, Mohammadi AH (2020) Experimental study of the performances of commercial surfactants in reducing interfacial tension and wettability alteration in the process of chemical water injection into carbonate reservoirs. J Pet Explor Prod Technol 10(4):1551–1563
Nasr-El-Din HA, Taylor KC (1992) Dynamic interfacial tension of crude oil/alkali/surfactant systems. Colloids Surf 66(1):23–37
Overton EB et al (2016) Chemical composition of Macondo and other crude oils and compositional alterations during oil spills. Oceanography 29(3):50–63
Rajaei H, Hezave AZ, Lashkarbolooki M, Esmaeilzadeh F (2013) Representing experimental solubility of phenylephrine hydrochloride in supercritical carbon dioxide and modeling solute solubility using semi-empirical correlations. J Supercrit Fluids 75:181–186
Robb ID, Smith R (1974) Nuclear spin-lattice relaxation times of alkali metal counterions at micellar interfaces. J Chem Soc Faraday Trans Phys Chem Condens Phases 70:287–292
Rodriguez-Palmeiro I, Rodriguez-Escontrela I, Rodríguez O, Arce A, Soto A (2015) Characterization and interfacial properties of the surfactant ionic liquid 1-dodecyl-3-methyl imidazolium acetate for enhanced oil recovery. RSC Adv 5(47):37392–37398
Roustaei A, Moghadasi J, Iran A, Bagherzadeh H, Shahrabadi A (2012) An experimental investigation of polysilicon nanoparticles’ recovery efficiencies through changes in interfacial tension and wettability alteration. In: SPE international oilfield nanotechnology conference and exhibition, OnePetro. https://doi.org/10.2118/156976-MS
Rudin J, Bernard C, Wasan DT (1994) Effect of added surfactant on interfacial tension and spontaneous emulsification in alkali/acidic oil systems. Ind Eng Chem Res 33(5):1150–1158
Saha R, Uppaluri RV, Tiwari P (2018) Influence of emulsification, interfacial tension, wettability alteration and saponification on residual oil recovery by alkali flooding. J Ind Eng Chem 59:286–296
Saigal T, Dong H, Matyjaszewski K, Tilton RD (2010) Pickering emulsions stabilized by nanoparticles with thermally responsive grafted polymer brushes. Langmuir 26(19):15200–15209
Sakthivel S, Velusamy S, Gardas RL, Sangwai JS (2014) Experimental investigation on the effect of aliphatic ionic liquids on the solubility of heavy crude oil using UV–visible, fourier transform-infrared, and 13C NMR spectroscopy. Energy Fuels 28(9):6151–6162
Sakthivel S, Velusamy S, Gardas RL, Sangwai JS (2015) Use of aromatic ionic liquids in the reduction of surface phenomena of crude oil–water system and their synergism with brine. Ind Eng Chem Res 54(3):968–978
Seetharaman GR, Jadhav RM, Sangwai JS (2021) Effect of monovalent and divalent alkali [NaOH and Ca(OH)2] on the interfacial tension of pure hydrocarbon-water systems relevant for enhanced oil recovery. J Pet Sci Eng 197:107892
Soorghali F, Zolghadr A, Ayatollahi S (2014) Effect of resins on asphaltene deposition and the changes of surface properties at different pressures: a microstructure study. Energy Fuels 28(4):2415–2421
Stauffer CE (1965) The measurement of surface tension by the pendant drop technique. J Phys Chem 69(6):1933–1938
Sun Z, Kang X, Lu X, Li Q, Jiang W (2018) Effects of crude oil composition on the ASP flooding: a case from Saertu, Xingshugang and Lamadian Oilfield in Daqing. Colloids Surf A 555:586–594
Taylor KC, Hawkins BF, Islam MR (1990) Dynamic interfacial tension in surfactant enhanced alkaline flooding. J Can Pet Technol. https://doi.org/10.2118/90-01-05
Vonnegut B (1942) Rotating bubble method for the determination of surface and interfacial tensions. Rev Sci Instrum 13(1):6–9
Wang J, Keener TC, Li G, Khang S-J (1998) The dissolution rate of Ca(OH)2 in aqueous solutions. Chem Eng Commun 169(1):167–184
Wang Z, Xu Y, Gan Y, Han X, Liu W, Xin H (2022) Micromechanism of partially hydrolyzed polyacrylamide molecule agglomeration morphology and its impact on the stability of crude oil−water interfacial film. J Pet Sci Eng 214:110492
Wu J, Prausnitz J, Firoozabadi A (1998) Molecular-thermodynamic framework for asphaltene-oil equilibria. AIChE J 44(11):2568–2568
Yang Z et al (2014) Interfacial tension of CO2 and organic liquid under high pressure and temperature. Chin J Chem Eng 22(11–12):1302–1306
Yarranton HW, Alboudwarej H, Jakher R (2000) Investigation of asphaltene association with vapor pressure osmometry and interfacial tension measurements. Ind Eng Chem Res 39(8):2916–2924
Zabihi S, Faraji D, Rahnama Y, Zeinolabedini Hezave A, Ayatollahi S (2020a) Relative permeability measurement in carbonate rocks, the effects of conventional surfactants vs. Ionic liquid-based surfactants. J Dispers Sci Technol 41(12):1797–1811
Zabihi S et al (2020b) Experimental solubility measurements of fenoprofen in supercritical carbon dioxide. J Chem Eng Data 65(4):1425–1434
Zaid HM, Ahmad Latiff NR, Yahya N (2014) The effect of zinc oxide and aluminum oxide nanoparticles on interfacial tension and viscosity of nanofluids for enhanced oil recovery. Adv Mater Res 1024:56–59
Zeinolabedini Hezave A, Dorostkar S, Ayatollahi S, Nabipour M, Hemmateenejad B (2014) Mechanistic investigation on dynamic interfacial tension between crude oil and ionic liquid using mass transfer concept. J Dispers Sci Technol 35(10):1483–1491
Zhao C et al (2018) The effect of NaOH on lowering interfacial tension of oil/alkylbenzene sulfonates solution. RSC Adv 8(11):6169–6177
Zhong H, Yang T, Yin H, Lu J, Zhang K, Fu C (2020) Role of alkali type in chemical loss and asp-flooding enhanced oil recovery in sandstone formations. SPE Res Eval Eng 23(02):431–445
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statements
The current manuscript is only submitted to the journal of “Journal of Petroleum Exploration and Production Technology” and would not be submitted to the other journal when the decision regarding this submission completed. The current manuscript is original and performed by the authors of the manuscript in a clear way to enlighten concerns existed in the area of enhanced oil recovery based on chemical injection. Besides, it is not published elsewhere in any form or language (partially or in full) and is an expansion of our previous researches. It is not extracted from one study and it is published from a series of studies performed regarding the EOR concerns. The obtained results and used procedure in the current manuscript are clearly stated and discussed make it easy for the readers to follow the current work. No data, text, or theories by others are presented as if they were the author’s own (‘plagiarism’). Proper acknowledgements are brought regarding the other works (this includes material that is closely copied (near verbatim), summarized and/or paraphrased), quotation marks (to indicate words taken from another source) are used for verbatim copying of material, and permissions secured for material that is copyrighted. There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mehdipour, R., Hosseini, S. Interactions between Ca(OH)2, imidazolium ionic liquid, and titanium oxide nanoparticles using resinous and asphaltenic synthetic oils under high-salinity conditions. J Petrol Explor Prod Technol 14, 203–220 (2024). https://doi.org/10.1007/s13202-023-01692-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-023-01692-5