Abstract
During the past years, the usage of new oil recovery methods known as enhanced oil recovery methods is increasing because of energy consumption rate enhancement and reservoir pressure depletion. Unfortunately, since most of the investigations were focused on crude oil, it is hard to find a generalized pattern of interfacial tension (IFT) and wettability change for different crude oils because of its complicated composition. So, it is necessary to examine the effect of specific fractions of crude oil especially resin and asphaltene fractions on the IFT and wettability alteration using systematic investigations. Although a limited number of investigations examined the interactions between these specific fractions and salts, there are no systematic reports respecting the possible interactions between asphaltene and resin fractions in the presence of alkaline and surfactant. So, in the first stage, the impact of dissolving asphaltene (0–9 wt%) in the toluene was investigated on the IFT reduction which revealed a decrease in IFT value from 34.8 to 23.3 mN/m as the asphaltene concentration was increased. Further experiments showed that the presence of MgCl2 and NaCl with a maximum concentration of 5000 ppm led to a reduction in IFT to a minimum value of 18.3 and 17.3 mN/m for NaCl and MgCl2, respectively, which means the higher impact of MgCl2 on the IFT reduction. After that, the selected optimum concentrations of MgCl2 and NaCl (5000 ppm) were used in the rest of the experiments in which the effect of resin fraction and other chemicals including sodium dodecyl benzenesulfonate (SDBS) and NaOH concentrations was examined on the IFT reduction and rock wettability. According to the obtained results, it was possible to reach the minimum IFT value of 0.08 mN/m, which is several orders lower than the original IFT value of the binary system without the chemicals using the optimum chemical formulation obtained by mixing proper concentrations of SDBS, NaOH, MgCl2, and NaCl. Moreover, the obtained optimum formulations were used through core flooding experiments which revealed the possibility of increasing the oil recovery to a maximum value of 10.1% based on the original oil in place.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Unfortunately, only a low portion of the oils in the reservoir can be extracted using the natural pressure of the reservoirs which is not more than 30 percent of the original oil in place (OOIP). This level of oil production is not enough to satisfy the ongoing consumption considering the energy demand and industrial developments. Respecting this fact and the unfortunate shortcoming of available oil reserves, it is required to use new and innovative methods to increase the amount of extracted oil from the trapped oil generally named enhanced oil recovery (EOR) or improved oil recovery (IOR) methods (Cheraghian 2015; Abhishek et al. 2015). In general, it is possible to categorize the EOR processes into chemicals (chemical EOR known as CEOR) (Amirsadat et al. 2017; Barahoei et al. 2016; Hosseini et al. 2020; Najimi et al. 2019; Zabihi et al. 2020a, b; Zeinolabedini Hezave et al. 2014), thermal-based methods, injection of low-salinity and smart water, carbon dioxide or carbonated water injection, etc. (Lashkarbolooki et al. 2019; Rajaei et al. 2013; Zabihi et al. 2020a, b). Between the available EOR processes, chemical EOR methods dealing with an injection of chemical agents such as alkali, surfactant, and polymer (Fang et al. 2022; Zhong et al. 2021) are gaining attention since it is possible to modify the capillary number by manipulating the surface wettability and reducing the interfacial tension (IFT) (Najimi et al. 2020; Nowrouzi et al. 2019a, b, 2020a, b, 2021a, b, c, 2022a, b).
Among the possible chemical methods, alkaline and surfactant injection under low salinity conditions is an innovative and applicable method that can extract a considerable amount of trapped oil if a proper chemical formulation is obtained. According to the previously performed studies, it is hard to activate multiple and proper mechanisms using only one chemical or method for more recovery of the trapped oil. So, during the past decade, a new approach that uses different EOR methods together gained increasing attention commonly known as the hybrid method. One of the possible combinations is the application of alkaline-surfactant solution prepared using low-salinity water to activate multiple mechanisms. So, a variety of chemicals are used in CEOR, namely surface-active chemicals (Sheng 2015), ion-engineered water (Rezaeian et al. 2020), alkalis and polymers (Fakher et al. 2019; Agi et al. 2018), and nanoparticles (NPs) (Olayiwola and Dejam 2019). Among the possible chemical compounds, surface-active agents are highly interesting and applicable in a wide variety of industries since they can manipulate the water/oil interface and the wettability of rock surface (Zulkifli et al. 2020).
Surfactants can be categorized as non-ionic, anionic, cationic, and zwitterionic (Kume et al. 2008) according to the hydrophilic head charge. The surfactant molecules have a chain structure comprising its hydrophobic tail (Azam et al. 2013; Burnham et al. 2013). Moreover, Gbadamosi et al. (2019) reported that the tail group of a surfactant is often formed of a short polymer chain, a long chain of hydrocarbon, etc., which directly affects the amphiphilic nature of the surfactant (see Fig. 1).

Source: Modified from Soleimani Zohr Shiri et al. (2019)
Surfactants types regarding the headgroup charge: a non-ionic, b cationic, c anionic, and d zwitterionic (amphoteric) surfactants.
Besides the surfactant molecules, it is possible to use alkali to produce in situ surfactants which can act as sacrifice preventing the adsorption of expensive chemicals such as surfactant molecules. With respect to these facts, Saha et al. (2018) examined the probable relation between IFT and wettability using saponification of alkali chemicals through core flooding experiments such as sodium hydroxide (NaOH) for different crude oils of light and medium types. They reported that IFT reduction is vital to extract the residual trapped oil through emulsification and wettability reversal. Their obtained results revealed an undeniable effect of NaOH concentration on the oil recovery as the concentration of alkali increases.
Moreover, Sun et al. (2018) investigated the crude oil composition effect on different chemicals including alkaline, polymer, and surfactant under a combination of alkaline–surfactant–polymer (ASP) solution comprised of sodium hydroxide (alkali), alkylbenzene sulfonate (surfactant), and partially hydrolyzed polyacrylamide (polymer). The results obtained by Sun et al. (2018) demonstrated that the IFT for Saertu, Xingshugang oil/ASP slug was lower than that obtained for Lamadian oil which was correlated with the presence of heavy alkyl benzene sulfonate in the Lamadian oil, leading to the formation of the active components dissolved into the oil phase and consequently leaving the oil–water interface unoccupied. Also, Cai et al. (1996) examined several alkane/brine systems IFT and hydrocarbon mixture water/brine systems IFT in the ranges of 0.1 to 30 MPa and 298.15–353.15 K. According to the results obtained by Cai et al. (1996), IFT and pressure have a linear relationship to each other which is dramatically manipulated with salt concentration, while the type of salt has a slight effect.
In total, examining the previously published works shows that unfortunately there is a lack of information regarding the alkaline/oleic phase IFT compared with the surfactants and oleic phase especially considering any specific fraction of crude oil such as aromatics, saturates, or asphaltenic and resinous fractions. Although it is well established that the presence of alkali has an undeniable effect on IFT reduction and higher oil recovery, the impact of monovalent and divalent ions for IFT reduction is poorly understood. If the alkali concentration is suitably selected using the oil chemistry, it is possible to reach a desired oil recovery factor by injection of only an alkali solution, especially if the crude oil sample is highly acidic and heavy. Nasr-El-Din and Taylor (1992) examined the Neodol 25–3 s and Triton X-100/enhanced alkaline/Lloydminster crude oil IFT value which revealed that it is incorrect to expect IFT reduction for all the solutions prepared by synthetic surfactants under high salt tolerance.
In order to study the impact of surfactant solutions modified by alkaline on the IFT of crude oil, Taylor et al. (1990) measured the IFT values using a spinning drop technique. They showed that for such systems, the dynamic IFT behavior included minima which vary in both magnitude and interfacial age at which they occur. These minima were followed by a sharp increase in the IFT.
Rudin et al. (1994) investigated the IFT behavior of alkaline solution modified by surfactant/acidic oils. They found that the main mechanism for IFT reduction to ultra-low values is the formation of mixed micelles (ionization of crude oil acidic contents) although the presence of surfactant molecules can act as a resistance for mass transfer leading to the more required time to reach the equilibrium in IFT values. Zhao et al. (2018) also reported that dynamic IFT (DIFT) between aqueous solution and sample acidic has the chance to reach the ultra-low IFT values even if a low concentration of surfactant is used. Their results revealed the significant effect of salt on the adsorption of surfactant onto the interface and the possible partitioning between the aqueous and crude oil phases.
This study is firstly concentered to investigate the synergy between the different chemicals of alkali and surfactant namely sodium hydroxide and sodium dodecyl benzene sulfonate (SDBS) under different salts concentration of 0–5000 ppm which covers the low salinity conditions. So, the salinity ranged between 0 and 5000 ppm for both examined salts of NaCl and MgCl2, while the alkali concentration was changed from 0 to 2000 ppm to see whether it is possible to produce in situ surfactant using the acidic contents of the sample oil. Since the effect of alkali on IFT reduction is not high enough to reduce the IFT to a desired value, the influence of this chemical on IFT reduction was investigated as it is combined with SDBS in the range of 0–1000 ppm.
Moreover, the effect of chemicals on crude oil is highly sensitive to the composition of the crude oil, especially the alkalis which are highly interactive with the acidic contents of the crude oil (Overton et al. 2016). So, it is impossible to use one specific molecular type for characterizing the crude oil (Demirbas and Taylan 2015). As a way out for such a limitation, it is possible to use a specific fraction of crude oil instead of crude oil for investigations (Azam et al. 2013; Demirbas 2016). Among the four main fractions of crude oil, resin, and asphaltene fractions are the most attractive fractions due to their surface activity that comes from their structure. These two fractions are important and attractive since it is proven that these fractions are vital for stable crude oil although the resin fraction is mainly responsible to keep the asphaltene molecules stabilized in the crude oil (Fakher et al. 2020). Besides, it is well accepted that the resin and asphaltene fractions are highly effective on the IFT reduction due to their structure which is highly similar to the surfactant molecules. Moreover, among these two fractions, resin fractions which include polar molecules with heteroatoms such as nitrogen, oxygen, or sulfur are more effective on the IFT reduction (Demirbas et al. 2015). In general, it is impossible to dissolve the resin fraction in liquid propane, while light alkanes such as pentane and heptane are good candidates to use as solvents for resin fraction. In the previously published works, only a limited number of studies investigated the properties of resin fraction (Aske et al. 2001). In general, it is possible to categorize the crude oil fractions into resin or asphaltene fractions using the fact that the H/C ratio of resins is between 1.2 and 1.7, while this value for asphaltene fractions is between 0.9 and 1.2 although resins are structurally similar to asphaltenes (Ficken et al. 2002). In light of this fact, it seems that using a specific fraction of crude oil for IFT and wettability modification studies provide several advantages. The first advantage is that more generalized correlations can be extracted since each fraction of crude oil is rather the same in contents and overall structure. So, any extracted conclusion can be used for the rest of similar fractions extracted from different crude oils to some extent, while using crude oil eliminates such an advantage. The other advantage is that one can specifically investigate the synergy between different chemicals and specific fractions without distortion of other constituents gives a good and deep insight regarding the molecular interactions and possible mechanisms. Although there are several investigations regarding the interactions and possible effects of the different chemicals on the IFT and wettability of the system including crude oils, there are a very limited number of investigations dealing with specific extracted fractions of the crude oil, especially the resin and asphaltene fractions. These two fractions are more important among the different fractions of saturates and aromatics because of their complex nature and structure made of a different and fused type of aromatics, branched alkyl chains, etc., bringing complex nature for these fractions. One of the most important characteristics of the resin and asphaltene fractions is their potential to act as a surfactant to reduce interfacial tension. The other noteworthy point is the effect of resin fraction on the stability of asphaltene molecules leading to better IFT reduction in theory although the real effect of these two fractions together must be thoroughly examined under different conditions especially the presence of different salts and chemicals such as surfactants and alkalis.
Since there is no report regarding the effects of asphaltene and resin fractions in the presence of salts (NaCl and MgCl2), NaOH as an alkali, and SDBS as the active surfactant, the current investigation is concentrated on the interactions that existed between these chemicals considering wettability alteration and IFT reduction mechanisms.
Materials and methods
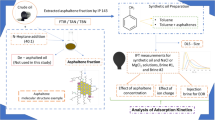
The performed experiments can be shown as the following flowchart which can clarify the stepwise stages of this investigation (see Fig. 2).
Crude oil, alklai, salts, and surfactant
The required sample crude oil for resin extraction was kindly provided by the Iranian Offshore Oil Company (IOOC) which is a subsidiary of the National Iranian Oil Company (NIOC) from Dorood oilfield with a density of 0.86 g cm−3 @ 15 °C comprised of 8.9% asphaltene and 7.6% resin fractions. This oilfield is the largest Iranian offshore oil field (25 km and 5 km long and wide, respectively) located in the Persian Gulf which has 88 wells 47 of which are production wells (Chehrazi et al. 2013). The formation of this oilfield is a combination of Asmari, Surmeh, Yamama, and Manifa whose Asmari section has an APIo of 23–25, while the APIo of Surmeh, Yamama, and Manifa is 29.5, 35, and 31, respectively (Setudehnia 1978).
The current oilfield has 2 gas injection wells and 12 water injection wells, which means that this oilfield is a good candidate for the water-based EOR processes. On the other side, the maximum production of 139.74 thousand bpd of crude oil and condensate was the old record of this reservoir which will lose its economic production rate in 2041. The required chemicals such as SDBS with a molecular weight of 348.48 g mol−1 and CAS number of 25155-30-0, NaOH, MgCl2, n-heptane, and NaCl were supplied from Sigma-Aldrich with purity better than 95% and used without any further purification.
Resin extraction procedure
In this study, IP 143/90 (Petroleum 1985) was used step by step to extract the asphaltene fraction in the first stage and then the resin extraction was isolated from the de-asphalted crude oil. The point is that among the different fractions of the crude oil, resin, and asphaltene fractions is a natural surfactant that can have a considerable effect on the IFT reduction during the chemical EOR methods. It is highly required to investigate the sole and combinative effect of these fractions in the presence of different chemicals. Besides the effect of these fractions on the possible IFT or wettability alteration, crude oil is a combination of thousands of chemicals that makes it hard to extract any reliable and generalized conclusion if the crude oil is being studied for EOR purposes. Respecting these reasons, several investigations were performed to study the role of different chemicals in the presence of only resin and asphaltene fractions (Lashkarbolooki et al. 2014, 2016; Wu et al. 1998), leading to no generalized and consistent results. In brief, using the IP 143/90 for asphaltene isolation purposes, n-heptane with a ratio of 40:1 was applied in the first place. Then, the remaining molten and de-asphalted oil was contacted with a silica gel column (Merck, 35 − 70 mesh ASTM) to extract the resin fraction using the column chromatography method (Amin et al. 2011; Soorghali et al. 2014).
After that, saturates and aromatics were washed and eliminated from the extracted fraction using 70:30 n-heptane and toluene solution. At last, an acetone/dichloromethane/toluene mixture with a ratio of 40:30:30 was used to achieve the resin fraction (Miller 1982; Yarranton et al. 2000).
Besides the isolation of resin and asphaltene fraction, these two fractions were elementally analyzed using a CHNSO analyzer (Thermo Flash EA 1112 series) to determine the C, H, N, S, and O contents. Based on the performed analysis, the resin fraction with the H/C ratio of 1.39 compared with the asphaltene H/C ratio of 1.17 revealed lower aromaticity characteristics, which means a more branched structure of resin fraction than the asphaltene molecules. With respect to this finding, it is completely obvious that resin molecules have a more surface-active nature than asphaltene molecules.
Interfacial tension measurement procedure
One of the most reliable and accurate methods for IFT measurement is the pendant drop or rising drop method mainly developed based on the shape of a drop if it forms at the tip of a nozzle (Yang et al. 2014) (see Fig. 3 apparatus supplied from Fanavari Atiyeh Pouyandegan Exir Co., (APEX Technologies Co., Arak, Iran)). This method is mainly comprised of different sections: a) forming the drop at the tip of the nozzle, b) an image capturing system comprised of a CCD camera and macro lens to provide a proper image from a small drop in the bulk phase, and c) the online image processing software. A brief description of the used equipment was previously explained in detail (Abbood et al. 2022). The point is that during the IFT measurements, not only the volume of the drop must be proper but also a suitable magnification must be applied in a way that the image occupies the maximum area of the screen to guarantee the accuracy of IFT measurement (see Eq. 1):
where Δρ, g, and H are the difference between the densities of bulk and drop phases, acceleration of gravity, and the shape-dependent parameter, respectively. The H value and shape factor are dependent on each other, i.e., S = d/D, where D is the equatorial diameter and d is the diameter at the distance D from the top of the drop.
With the assistance of Eq. (1), an operator can calculate the IFT using the drop shape analysis concept using the captured images. Besides, using this equipment and the sessile drop concept one can calculate the CA (Adamson and Gast 1967; Andreas et al. 2002; Stauffer 1965). The point is that each reported IFT data point was the average of at least three independent measurements used for calculating the average value. According to the obtained results and calculated averages, the maximum uncertainty of about ± 0.2 mN m−1 can be calculated. The noteworthy point is that this low value of uncertainty was impossible to be shown in the IFT plots because of a broad range of measured IFT values in each IFT plot.
In the second stage, the spinning drop technique was used to measure the IFT value of solutions that are below 0.5 mN/m. The spinning drop method was originally developed by Vonnegut (1942) based on drop deformation to a very long drop under centrifugal force. At higher rotation speeds, the droplet formed is merely a cylinder and R/L → ∞, in which case Eq. (2) (Vonnegut 1942) can be used to calculate the interfacial tension:
where γ is the IFT in N/m and R is the radius of the drop at equator (E) as indicated in Fig. 4 with unit of m.
This formula has been shown to be valid within 0.1% if the length of the drop exceeds four times its diameter. In practice, a more elongated drop is used which really looks like a cylinder, but it is worth remarking that the hemisphere at the tip of the drop has not had the same radius as the cylinder at the center as indicated in Fig. 4 (Ro = 2/3 R). This is due to the fact that the centrifugal acceleration is not constant but increases with the distance from the axis. ∆ρ is the difference between densities of bulk and drop phases with a unit of Kg/m3, and ω is the angular velocity with a unit of rad/s. According to the above formula, low tension will be associated with a small radius, i.e., elongated drops and slow rotational velocity, whereas high tension would require a high rotational velocity and the drop might not be elongated enough to fall in the Vonnegut formula case. This method is thus appropriate to measure low tensions, typically below 1 mN/m, and down to ultra-low values (µN/m or less) found in surfactant–oil–water systems containing microemulsions.
Contact angle measurement
Knowing the wettability of a system is one of the most important characteristics of several industries from enhanced oil recovery processes to medical. In this way, during the past decades, several methods are proposed to measure the wettability of the systems; one of the most important methods is contact angle measurement. Among the different possible methods for measuring the contact angle values, using the sessile drop is the most widely used and accurate method which provides the chance of static CA measurement for the operator with an acceptable level of accuracy using an online software (see Fig. 5). If the water CA is lower than 90°, the surface is said to be hydrophilic, and if the contact angle is higher than 90°, the surface is hydrophobic. Respecting this fact, using the water CA as a quick and non-destructive method for surface chemistry control is one of the first choices for the researchers.
Core flooding procedure
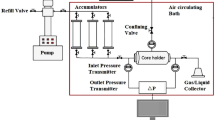
In the current investigation, a high-pressure–high-temperature core flooding apparatus was designed and constructed for pressure up to 150 °C and 700 bar, respectively (Fanavari Atiyeh Pouyandegan Exir Co., (APEX Technologies Co., Arak, Iran), and used to perform the required experiments (see Fig. 6).
This equipment mainly consists of different parts including a high-pressure injection pump, high-pressure high-temperature accumulators for different liquids (three of them have a volume of 500 cc, while one of them has a volume of 100 cc for easy handling of the chemical slugs), high-pressure–high-temperature hassler core holder type, confining pressure system control and monitoring, gas back pressure regulator (GBPR) monitoring and controlling unit, and data acquisition system. In this equipment, the injection of fluids is done using a high-pressure pump with desired injection rate mostly between 0.1 and 0.6 cc/min since the laminar flow of the fluids in the reservoir is about 1 ft/day which is close to 0.3 cc/min. So, the current apparatus can be used as a way to perform the different injection patterns, and inject different solutions under different pressures and temperatures using the following sequence:
-
Measuring the porosity and permeability of the core plugs.
-
Saturation of the fresh core plug using formation brine injection for several pore volumes (PVs) to ensure no air existed in the core.
-
Injection of crude oil under a flow rate of 0.3 cc/min to saturate the core plug with crude oil and reach the irreducible water saturation (Swirr). At this point, the core reaches the reservoir condition after the primary production stage, which means the core is ready to be used for secondary and tertiary oil recovery stages.
-
Injection of formation brine or any aqueous solution for several pore volumes with a flow rate of 0.3 cc/min to reach the point where no oil is being produced (Swro) (secondary oil recovery stage). Injection of any chemical solution slug in the range of 0.1 to 0.5 PV with desired injection rate in the range of 0.1 to 0.6 cc/min chased with formation brine for several PVs for further oil production as the tertiary oil recovery stage.
-
Soaking the flooded core plug with the chemical slug for a specific period of time more than 7 days to give the required chance to reach the ultimate wettability alteration. It is possible to differentiate the effect of different mechanisms such as IFT reduction from wettability alteration to some extent.
Results and discussion
Effect of asphaltene fraction on the Interfacial tension
In the first series of the current study, the influence of asphaltene concentration on the interfacial tension was examined by ranging it between 0 and 9 wt% dissolved in toluene to prepare the synthetic asphaltenic oil which is going to be contacted with distilled water in the first place. The findings, which are depicted in Fig. 7, revealed that the presence of asphaltene has a considerable impact on the IFT by reducing the initial IFT value of toluene/distilled water from 34.8 to 23.3 mN/m, which is about a 33% reduction in IFT. The reason is that asphaltenes have a stronger influence at the interface due to the presence of aromatic and polycyclic aromatic hydrocarbons in their structure leading to easier irreversible adhesion of asphaltene molecules into nanoaggregates. As a consequence of this adhesion, viscoelastic interfacial films will form which can stabilize the oil/water emulsions, which means a reduction in IFT value (Jian et al. 2016; Lashkarbolooki and Ayatollahi 2016; Zhang et al. 2016).
So, considering the water-in-oil interaction, asphaltenes move from the bulk phase medium to the interface where the asphaltene molecules can rearrange in a way that a reduction in IFT occurs till the system reaches equilibrium and the IFT remains constant at equilibrium value (Cagna et al. 2018). The asphaltene adsorption into the interface is a diffusion-controlled phenomenon that principally occurs in a short time regime, which can be turned to non-diffusion-controlled kinetics with respect to the characteristics of crude oil including oil viscosity and mass fraction of asphaltenes (Rane et al. 2012). Moreover, besides the aforementioned mechanism for the adsorption of the asphaltene molecules into the interface, a three-step mechanism proposed to justify the influence of asphaltene respecting the IFT reduction as follows:
-
(1)
Occurrence of initial short-term diffusion-controlled process.
-
(2)
Adsorption reduction due to hindrance of steric compartments compared with the adsorption of asphaltenes.
-
(3)
A long-term process with the formation of adsorbed sublayers above the interface (Zhang et al. 2018).
A similar trend was reported by Alves et al. (2022) that in the first place, there is a fast asphaltene migration from the bulk to the interface leading to a considerable reduction in IFT and then the interfacial tension reaches a plateau under IFT decay rate pattern, although they report a weak version of this trend for resin.
They also reported that distinguished differences between the IFT of resin and asphaltene systems come from the difference existed between their molecular sizes but also it is correlated with chemical characteristics. In detail, the presence of oxygenated groups and heteroatoms directly affects the activity of crude oil fractions in the interface. A similar report was also reported by Zhang and his coworkers (2016) on the relation between the IFT for asphaltenes and resin solutions, which confirmed the better effect of resin fraction for IFT reduction due to the higher content of oxygenated compounds. So, it seems that not only the content of oxygenated compounds in the resins is a crucial parameter but also the smaller size of resin molecules boosts the interfacial activity of the resin fractions, leading to better IFT reduction capability of this fraction. To sum up, it can be concluded that it seems that molecules with bigger sizes, such as leading to a faster equilibration and a minor reduction of the IFT on the other side. However, it is possible to reach slower equilibration and a major reduction in IFT if the molecular size is smaller, which means that IFT reduction has an inverse relation with the molecular size of the asphaltenes and resins molecules.
Moreover, Lashkarblooki and Ayatollahi (2018) reported that there is a specific trend between the influence of asphaltene fractions as a function of H/C ratio and salt concentration on the IFT behavior of crude oils. They reported that the IFT was reduced regardless of using distilled water or formation brine as the aqueous solution as the H/C ratio of asphaltene fraction was decreased. The point is that they claimed that there is an evident effect between the H/C ratio of asphaltene and IFT although a contrary relation was observed for the resin fraction.
Effect of mono and divalent salts on the Interfacial tension
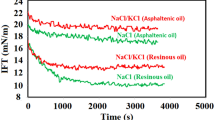
The effect of mono- and divalent chloride salts including NaCl and MgCl2 was examined on the IFT reduction, while the concentration of asphaltene fraction ranged between 3 and 9 wt%. The salts concentrations were changed between 0 and 5000 ppm, which covers the low salinity (LoSal) conditions due to its unique advantages (see Fig. 8).
The obtained results demonstrated that the presence of salts has a positive effect on the IFT reduction considering the overall effect of asphaltene fraction on the IFT reduction. In other words, the measurements revealed that for all the examined asphaltene concentrations of 3–9 wt% regardless of the used salts, the IFT experienced a reduction. Besides, the findings revealed that both of used sodium chloride and magnesium chloride salts led to better IFT reduction of the synthetic oil/aqueous solution. The reason is the positive and synergistic effect of salts for easier packing of the asphaltene molecules at the interface and providing an effective film of asphaltene molecules which can act as the surface-active agents consequently reducing the IFT of the binary system. Similarly, Zhao et al. (2018) reported that the salting out effect and an increase in the concentration of surfactant molecules in the interface are the most effective parameters on the reducing effect of NaCl on the IFT. Considering the effect of electrolyte concentration on IFT, Chan and Shah (1980) claimed that this is the “salting out” effect reduce the IFT in the interface as a function of active substances concentration.
They claimed that the surfactant molecules can be transferred into the oil phase via the “salting-out” phenomenon as the salt concentration increases. In other words, as the salt concentration reaches a desired value, surfactant molecules can be re-distributed into the oil and water phases to a point that the concentrations became equal on both sides. At this time, the affinities between surfactant molecules at the interface and between the two phases of oil and water are also equal which can move the system toward high interfacial concentration and IFT reduction as a consequence. The other point they mentioned is that during IFT measurement using the spinning drop technique, the volumes of the oil and water phases are significantly different since only a few microliters of oil drop are contacted with at least 2 cc of the bulk phase inside the rotating chamber. Therefore, the surfactant concentration in the aqueous phase remains unchanged which means unreal situation of IFT examination.
Effect of NaOH (alkali) on the interfacial tension reduction
It is well accepted that the application of surfactants is not the only way to the IFT reduction. In general, the nature of the oil reservoirs is acidic which usually contains natural surface-active constituents. So, it seems that mixing the acidic oil with an alkaline will form an in situ surfactant that will reduce the IFT between oil/water. This process commonly known as “alkaline flooding” is one of the useful economically efficient EOR techniques without using costly chemicals such as surfactants. In light of this fact, a series of experiments were designed and performed to find the possible effect of NaOH on the formation of in situ surfactant formed by a reaction between the acidic contents of the asphaltene fraction and NaOH, which leads to the formation of a stable film in the fluid/fluid interface and consequently reduces the IFT. In this way, the NaOH concentration ranged between 0 and 2000 ppm in the current phase of this investigation, while the concentration of MgCl2 and NaCl was held constant at 5000 ppm (the optimum values were obtained from the previous section), which covers the low salinity conditions (see Fig. 9).
The obtained results revealed that regardless of the used salts, an increase in the NaOH concentration from 0 to 2000 ppm led to IFT reduction from 29.4 mN/m (3 wt% asphaltene concentration, NaCl concentration of 5000 ppm with no NaOH) to 7.8 mN/m (9 wt% asphaltene fraction, and NaOH concentration of 2000 ppm and NaCl concentration of 5000 ppm). Moreover, the results revealed that the IFT reduced from 25.6 mN/m for the asphaltenic synthetic oil of 3 wt% and MgCl2 concentration of 5000 ppm, while no NaOH existed in the aqueous solution to a value of 5.7 mN/m for the asphaltenic synthetic oil of 9 wt%, 5000 ppm MgCl2 and NaOH concentration of 2000 ppm. The reason behind this fact is related to the concentration of the in situ surfactant in the interface, leading to IFT reduction. It is accepted that it is possible to reduce the IFT by increasing the surfactant concentration in the interface. The value of interfacial concentration is directly related to the area in the interface occupied by the surfactant molecules. In the shadow of this definition, the smaller the effective cross-sectional area accessible for the surfactant molecules, the greater its interfacial concentration means. So, it can be concluded that the structure of surfactant molecules and their packing in the interface directly impact the interfacial concentration. Although the findings of the current investigation revealed the direct synergism between the NaOH, acidic contents of the asphaltenic synthetic oil and the asphaltene molecules, Zhao et al. (2018) reported a similar trend for the effect of NaOH on the normal paraffin solutions. Even if the acidic or other constituents exist in crude oil production in situ form active species via NaOH reaction, it is not completely clear that these active constituents can decrease oil/water IFT. The point regarding their conclusion is that in contrast to the current investigation, they used normal paraffin molecules for their investigations, while in the current work, asphaltene molecules were extracted from the crude oil and then used to prepare the synthetic oil which was far from the normal paraffin type oil. According to this fact, it can be concluded that the effect of NaOH on the IFT is directly correlated with the both acidic contents of the crude oil and the structure of the aromaticity of the crude oil, which means the structure of the crude oil. Zhao et al. (2018) also claimed that both NaOH and NaCl dissolved in aqueous solutions introduce similar effects on the interfacial concentration and molecules packing at the interface.
According to these findings, one can conclude that as the NaOH concentration increases, the electric double layer of hydrophilic groups in asphaltene molecules and the in situ surfactant molecules can be compressed. As a consequence of this compression, the electrostatic interactions between hydrophilic groups weakened, leading to unpreventable accumulation of the surface-active molecules in the interface and IFT reduction, which resulted in an increase in the interfacial concentration. Continuing to increase the concentration of NaOH, the electrical double layer was further compressed; water molecules could incorporate into the interface through loose hydrophilic groups.
Moreover, Zhao et al. (2018) reported that according to the aforementioned effect of NaOH on the accumulation of surface-active agent in the interface and possible salting out effect, IFT can show a dual-trend behavior which is decreasing in the first stages and then change to an increasing trend as the NaOH concentration increases. They also reported that as the NaOH concentration goes beyond a threshold, para-dimethyl alkylbenzene sulfonate molecules were driven into the oil phase by the salting-out effect, and this process resulted in IFT reduction for p-S12-5 and p-S14-5 which are water-soluble chemicals. On the other hand, for oil-soluble p-S18-5, the above process had a slight effect on the effective distribution in the oil phase. The distribution of surfactant molecules in an effective pattern can guarantee interface stabilization and proper IFT reduction. They also claimed that there was no clear evidence regarding the effect of NaOH on the IFT reduction considering the synergistic behavior between para-dimethyl alkylbenzene sulfonate molecules and active species (formed in situ surfactants) in the crude oil.
To sum up and based on the findings, it is possible to summarize the effect of NaOH on the IFT reduction as follows:
-
(a)
The ionic strength of the water phase increases as the NaOH existed in the solution due to the polarity characteristics of the water phase, leading to the electrical double-layer compression of hydrophilic groups.
-
(b)
In light of this compression, the accessible area for the molecules to be occupied reduces at the interface, which means a higher interfacial concentration of surface-active agents, leading to lower IFT values.
-
(c)
After that, an increase in the NaOH concentration leading to further compression of EDL reduced the strength of electrostatic interactions between hydrophilic groups and shortened the electrical effect range. At this point, the IFt values increase since the water molecules incorporate into the interface via the gap between loose polar groups.
-
(d)
On the other side, the salting-out phenomenon can move the surfactant molecules toward the oil phase if the NaOH concentration goes beyond a threshold, which consequently leads to better packing of lipophilic groups at the interface. In light of this phenomenon, IFT reduces for a water-soluble surfactant system. In contrast to the water-soluble surfactants, the oil-soluble surfactant slightly is influenced by the electrolytic concentration considering the close packing of longer chains because of greater van der Waals attraction between longer chains. Therefore, IFT reduction stopped at a higher NaOH concentration for an oil-soluble surfactant.
Investigation on several studies revealed that the optimum concentration for alkali is about 0.1 wt% to reach the lowest IFT which is a low alkaline concentration. But, using a low alkaline concentration leads to a limited effect of alkali through the system since under a low concentration of alkali, the alkaline bank may not be capable of propagating through the reservoir due to the high adsorption and consumption of alkali. So, it is required to use higher alkali concentrations for practical application to ensure the ultimate effect of used alkali as a sacrifice and primary IFT reducer chemical. Respecting this fact, the optimum NaOH concentration for the rest of the experiments was considered as 2000 ppm although the IFT reduction for 1000 ppm of NaOH was close to the IFT values obtained for 2000 ppm.
Synergies between resin and asphaltene on the Interfacial tension reduction
In the next stage of this study, the interactions between asphaltene and resin fraction in the range of 0–9 wt% of asphaltene and 1–5 wt% of resin were examined, while NaCl and MgCl2 concentrations individually remained constant at 5000 ppm and NaOH concentration was kept constant at 2000 ppm. As the measured IFT values demonstrated (see Fig. 10), the presence of resin fraction (1–5 wt%) has a linear effect on the IFT reduction regardless of the used salts (NaCl or MgCl2 = 5000 ppm) which are in the range of low salinity conditions and pH of the system was modified by the addition of 2000 ppm NaOH.
A close investigation of the obtained results clearly demonstrated the reducing effect of resin on the IFT due to its possible effect on stabilizing the asphaltene molecules in the interface leading to evident IFT reduction. The point is that resin fraction boosts the IFT reduction due to its effect on the stabilizing asphaltene molecules and its surface-active nature. In addition, the elemental analysis revealed that the resin fraction with the H/C ratio of 1.39 compared with the asphaltene H/C ratio of 1.17 showed lower aromaticity characteristics, which means the more branched structure of resin fraction than the asphaltene molecules, which means better detergency feature of resin fraction than the asphaltene fraction. Although, regarding the performed experiments in the current investigation it is impossible to extract any clear conclusion regarding the superiority of the resin and asphaltene molecules to each other. A similar trend was reported by Zhang et al. (2016) regarding the better effect of resin molecules for the IFT reduction compared with the asphaltene fraction which was correlated with the higher content of oxygenated compounds in the resin fraction. Besides, they claimed that it is the chemical characterization of low stability asphaltenes and resins with richer oxygenated compounds in the resins than in the asphaltenes fractions that could be responsible for their interfacial activity difference concomitant with the smaller resins size leading to better efficiency of resin for IFT reduction. They also reported that using the equimass asphaltene/resin ratio led to measuring the IFT values closer to the pure asphaltene model oil, probably due to the predominant presence of asphaltenes complex structures at molecules at the interface.
They also found that a similar trend can be obtained for a rich resin content mixture that can behave like pure resin model oil. According to these findings, it seems that there is a minimum asphaltene concentration at which their effect on the IFT measurement is predominant. They even reported the effects of salts at the model oil/aqueous solution interface for IFT reduction, which means the boosting effect of salts for better efficiency of both asphaltene and resin fractions. In brief, they claimed that in the range of studied H/C ratio, resin contained a minimum H/C ratio and asphaltene consisted of a maximum H/C ratio. According to these ratios, a lower favorable interaction with H/C ratio lower than crossover was observed which boosted the performance of resin molecules as the salts were added. However, for H/C ratios higher than the crossover, not only the performance of resin molecules decreased as a function of salinity but also the effect of salinity significantly diminished. Therefore, it can be deduced that light crude oil with high resin content and low asphaltene content follows a trend similar to resin fraction (Lashkarbolooki and Ayatollahi 2018).
Besides the overall effect of the resin on the IFT reduction, it seems that there is a synergistic effect between the asphaltene and resin molecules considering the better IFT reduction as both fractions existed in the synthetic oil. It seems that as the asphaltene molecules are arranged at the interface, the resin molecules still can populate the empty sites of the interface and better stabilizes the asphaltene molecules at the interface leading to more detergency nature of these formed films which is with an ultimate thickness of three or four layers of molecules. The last point regarding the synergistic effect of resin and asphaltene fractions on the IFT reduction is that as the resin concentration increases from 1 to 3 wt%, the IFT reduces in a sharper slope than the IFT reduction, which was observed for the resin concentration enhancement from 3 to 5 wt%. The reason is that as the resin concentration increases, the empty sites are occupied by the resin molecules till the resin concentration passes a threshold where the interface is completely saturated with the different molecules. As a consequence of this phenomenon, the effect of resin fraction diminishes as it is obvious in the IFT variation slope as the resin concentration increases from 1 to 3 and 3 to 5 wt%.
Effect of sodium dodecyl benzene sulfonate (SDBS) on the interfacial tension reduction using the optimum formulation
It is well established that using surfactants leads to better oil recovery by modifying the interfacial properties and capillary number using interfacial tension and wettability of the rock. In general, in the systems containing surfactants with a single hydrophilic group, the area will be occupied by a surfactant molecule at the surface as a function of EDL size for the hydrophilic groups. Respecting these facts, the effect of sulfonate-based surfactant namely SDBS was examined on the IFT of the alkaline-low salinity solution in the presence of ASO in the range of 100–1000 ppm, while the concentrations of NaOH, NaCl, and MgCl2 were held constant at 2000, 5000 ppm, and 5000 ppm, respectively (see Fig. 11). The measurements revealed that the effect of SDBS for IFT reduction is more evident for the NaCl aqueous solution than the MgCl2 aqueous solution. The measurements showed that the IFT experienced a sharp decrease in the SDBS concentration of 100 ppm if it dissolves in the NaCl solution, while the MgCl2 solution experiences moderate IFT reduction. The other point that must be explained is that the depicted results in Fig. 11 revealed that for the SDBS concentration of 200–1000 ppm, the IFT variation keeps its linear trend considering the fact that the IFT experiences an extreme reduction for the NaCl solution, especially if the asphaltenic synthetic oil with a concentration of 9 wt% is used. The IFT measurements revealed that for the NaCl aqueous solution and 9 wt% ASO, IFT reduces to the value of 0.08 mN/m compared with the asphaltenic synthetic oil of 3 wt. % with an IFT value of 0.65 mN/m. On the other hand, the IFT measurements demonstrated a reduction in IFT from 1.5 to 0.4 mN/m for the MgCl2 aqueous solution being contacted with ASO with concentrations of 3 and 9 wt%, respectively. It is completely obvious that the effect of SDBS on the IFT reduction of NaCl aqueous solution is much higher (eight times) than the effect of SDBS on the IFT of MgCl2 aqueous solution (three times).
In general, SDBS, which is an anionic surfactant with high molecular weight, can efficiently reduce the IFT to ultra-low values and create a more stable solution at both alkaline and acidic pH (Yang et al. 2010). Moreover, sulfonate-type surfactants such as SDBS not only are capable of tolerating harsh temperatures without any distinguished precipitation but also can be used for EOR purposes if the crude oils are highly viscous, with high-level wax and asphaltene contents. Similar results were reported by Hirasaki et al. (2011) who claimed that the main factors determining the IFT values are surfactant concentration in aqueous solutions, molecular structure, salinity, and temperature. They also reported that an increase in the surfactant concentration to a high value leads to achieving low and ultra-low IFT values if the concentration reaches the CMC at least.
Moreover, Kwok et al. (1993) reported that anionic surfactants SDBS is one of the surfactants that are sensitive to high-salinity aqueous solutions, as the surfactants begin to precipitate due to interaction between the salts and surfactant molecules.
Considering these facts, it seems that using low salinity conditions for SDBS surfactant is an ideal situation to provide the chance of IFT reduction to an acceptable level without precipitation risk which is one of the most crucial concerns during the chemical EOR processes. Respecting these findings, the SDBS concentration was considered as 1000 ppm for the rest of the experiments including wettability alteration analysis and core flooding experiments. Besides, in contrast to the current investigation, Trabelsi et al. (2011) reported transient IFT measurements of surfactant-enhanced alkaline/diluted heavy crude oil systems. They demonstrated that the dissolution of a slight amount of Triton X-405 and SDS led to a further IFT reduction, especially the SDBS which reduced the IFT of the binary solution to ultra-low values ~ 4 × 10–4 mN/m at a concentration of only 0.05% and a pH of 11. They mentioned according to their findings that this observed ultra-low IFT value provides a great chance for suitable oil recovery level as a function of salinity and pH.
Besides the aforementioned facts, it is possible to correlate the better effect of NaCl salt on the IFT reduction of SDBS compared with MgCl2 to hydrolysis of the SDBS head group. As the SDBS head group is hydrolyzed, the negatively charged head groups of the SDBS molecules tend to spontaneously surround the Na+ ions present in the liquid, and as a result, their surface charges reduce in a way that they can form aggregates. In this situation, it is easier for the SDBS aggregates to move toward the surface and interact with the asphaltene molecules of the synthetic oil compared with the aggregates formed by the Mg2+. In other words, due to the bigger hydrate radius of Mg2+, the formed aggregates of SDBS with Na + are smaller, leading to easier packing of these aggregates in the interface and consequently increasing the number of SBDS ion the interface. So, the interfacial concentration of SDBS is higher in the case of Na+, leading to better IFT reduction (Wang et al. 2022).
Effect of optimum chemical formulation on the wettability alteration of the rock surface
In this phase of this investigation, the possible influence of the optimum chemical formulation was investigated on the CA variation of the rock surface provided by the carbonate rock. The measurements revealed that the presence of chemicals and salts has a profound effect on the wettability alteration of the rock surfaces to move them toward strongly water-wet conditions (see Fig. 12). The reason behind this finding can be correlated with the influence of used alkalis, salts, and surfactants.
In general, the previously performed laboratory studies showed that 5000 ppm salinity is the point that the highest level of wettability alteration was achieved (Bartels et al. 2019); due to the activation of several mechanisms, the most important one is the multi-ion exchange and double-layer effect. The findings revealed that among the different ions and cations, Ca2+, Mg2+, and SO42− ions are the active ions that participate in wettability alteration by separating the carboxylate groups from the rock surface (Berg et al. 2010). Moreover, Amirian et al. (2017) results revealed the wettability alteration toward water-wet conditions as the low-salinity water (LSW) was injected into the system.
Furthermore, Strand et al. (2006) reported that the underlying mechanism which is responsible for changing the rock wettability can be explained based on the multi-component ion exchange (MIE) concept involving Mg2+, Ca2+, and SO42− ions, resulting in the desorption of carboxylic material (Strand et al. 2006; Zhang and Austad 2006; Zhang et al. 2007). They mentioned that the MIE mechanism is a two-step process that firstly deals with SO42− ions adsorption onto the rock surface and consequent reduction in the positive leading to a reduction in electrostatic repulsion existing between the rock surface and divalent cations. After that, Ca2+ and/or Mg2+ ions concomitantly adsorb on the surface of the rock which reacts with the carboxylic group with a negative charge to fabricate complex ions and desorb the carboxylic material from the rock surface (Strand et al. 2006; Zhang and Austad 2006). Besides the effect of salts on the wettability alteration, NaOH and SDBS surfactants may introduce a similar effect on the wettability alteration since the NaOH can react with the acidic contents to produce in situ surfactant, which means the similar role of NaOH and SDBS. Carbonate wettability change using surfactant is described based on two main mechanisms. The first mechanism is called the coating mechanism, and it is related to anionic surfactants. In the first mechanism of action, anionic surfactant molecules form a monolayer on the surface of the carbonate rock where the adsorbed oil and hydrophobic tails interact with the surfactant molecules. At this point, the oil-wet rock surface moves toward water-wet conditions due to the adsorption of surfactant hydrophilic head groups into the rock surface. The next mechanism known as the cleaning mechanism is directly related to the cationic surfactants. This phenomenon can be related to the ion–pair formation between acidic portions of crude oil which is adsorbed on the rock surface and the surfactant cationic heads. The point is that based on the obtained results, the presence of anionic surfactants introduces a lower influence to change the wettability than the cases used the cationic surfactants due to the higher potential of the ion–pair interactions for these type of surfactants (Hammond and Unsal 2011; Salehi et al. 2008).
Effect of optimum formulation on the tertiary oil recovery using core flooding experiments
In the last stage of this investigation, the obtained optimum chemical formulations for NaCl and MgCl2 were used to find their effects on the tertiary oil recovery, while the concentration of asphaltenic fraction in toluene was changed between 3 and 9 wt%. The injection rate for all the stages was held constant at 0.3 cc/min well close to the velocity of the fluids in the reservoir which is about 1 ft/day to satisfy the laminar flow in the porous media. The performed core flooding experiments revealed the used optimum chemical formulations regardless of using NaCl or MgCl2 lead to tertiary oil recovery of between 5.3 and 10.1% based on OOIP using different asphaltene concentrations (3–9 wt%) as the synthetic oil (Table 1).
The experiments revealed that using the optimum chemical formulation consisting of NaCl led to better oil recovery with a maximum value of 10.1% based on OOIP than the optimum solution consisting of MgCl2 with a maximum oil recovery of about 7.3 wt%. The reason behind this finding can be correlated with the better effect of NaCl solution (SDBS concentration = 1000 ppm, NaOH concentration = 2000 ppm, NaCl concentration = 5000 ppm) for IFT reduction with IFT values of 0.65 mN/m, 0.25 mN/m and 0.08 mN/m for asphaltene concentration of 3, 6, and 9 wt%. However, the IFT values for the optimum MgCl2 solution (SDBS concentration = 1000 ppm, NaOH concentration = 2000 ppm, MgCl2 concentration = 5000 ppm) were 1.5, 0.88, and 0.4 mN/m, which are larger than the values obtained for NaCl solution, especially for the asphaltene concentration of 9 wt% which is eight times larger. In this way, the better efficiency of the NaCl solutions for oil recovery is directly correlated with the better potential of this solution for IFT reduction by considering the fact that the effect of these two optimum solutions on the wettability alteration is rather the same for the asphaltene concentration of 9 wt%. Besides, although the wettability alteration measurements revealed the strongly water-wet conditions for solutions being contacted with synthetic asphaltenic oil (asphaltene concentration of 3 wt%) with values of 29.30 and 24.30 for NaCl and MgCl2, respectively, lower oil recovery values were obtained for these binary solutions. So, one can conclude that the dominant mechanism through the performed core flooding experiments is IFT reduction due to its rapid nature instead of wettability alteration which is a time-consuming phenomenon that required more time to introduce its ultimate effect on the rock surface and oil recovery results. On the other hand, the alkaline agent of NaOH in the current investigation aids crude oil displacement by raising the pH of the injected water and generating in situ surfactants as a result of alkali reactions with acidic components existing in crude oil. After in situ surfactant formation, this mixture mobilizes the crude oil and removes it from the pore spaces in the reservoir. The possible mechanism for the effect of alkaline on the oil recovery can be correlated with Fig. 13.
The possible mechanism of chemicals on the extraction of oil drops (Druetta et al. 2019)
Conclusions
The synergy between the salts under low salinity conditions up to 5000 ppm for NaCl and MgCl2, NaOH in the range of 0–2000 as an effective alkali, 0–1000 ppm sodium dodecyl benzene sulfonate (SDBS) as an effective anionic surfactant and asphaltenic synthetic oil activated by resin fraction in different concentrations of 0–9 wt% and 0–5 wt%, respectively, was examined. This investigation was performed to find the possible effect of these chemicals on the interfacial tension (IFT) and wettability alteration which was then followed by core flooding experiments. The findings revealed that:
-
Increasing the concentration of asphaltene fraction from 0 to 9 wt% led to IFT reduction from 34.8 to 23.3 mN/m, which was due to the interfacial activity of the asphaltene molecules that can act as a surfactant.
-
The findings also revealed that the presence of salts regardless of whether being divalent or monovalent has a deep effect on the IFT reduction especially if the concentration of 5000 ppm (low salinity condition) is dissolved in the aqueous solution.
-
The findings revealed IFT reduction from its maximum value of 34.8 to 17.3 mN/m for MgCl2 concentrations of 5000 ppm.
-
Further investigations demonstrated that the presence of NaOH as an alkali is significantly effective to reduce the IFT of the binary system to the minimum value of 7.8 mN/m and 5.7 mN/m for NaCl and MgCl2, respectively, which means a higher effect of NaOH on the MgCl2 solution.
-
Besides, the addition of resin in the range of 1–5 wt% revealed the undeniable effect of resin fraction on the IFT of the system.
-
The synergy between the SDBS as an effective surfactant was investigated using the optimum aqueous formulation of MgCl2 and NaCl concentrations of 5000 ppm, NaOH concentrations of 2000 ppm, and synthetic oil with asphaltene concentration between 3 and 9 wt% and resin concentration of 5 wt%. The findings revealed that in contrast to the previous sections, the addition of SDBS has the highest effect on the solution prepared by NaCl instead of MgCl2, leading to IFT reduction to values of 0.08 and 0.4 mN/m, respectively.
-
The optimum formulation of an aqueous solution including 1000 ppm of SDBS, 5000 ppm of NaCl and MgCl2, and NaOH concentrations of 2000 ppm was contacted with the synthetic oil prepared using a dissolution of asphaltene fraction (3–9 wt%) and resin concentrations of 5 wt%. The findings revealed a significant effect of the obtained chemical formulation regardless of the used salts including NaCl and MgCl2 on the wettability alteration to change the wettability of the rock surface toward strongly water-wet conditions of 29.3° and 24.3° for asphaltene concentrations of 3 wt%.
-
The core flooding experiments using optimum formulations revealed the maximum oil recovery of 10.1% based on original oil in place (OOIP).
Abbreviations
- Al2O3 :
-
Aluminum oxide
- ASO:
-
Asphaltenic synthetic oil
- CA:
-
Contact angle
- CMC:
-
Critical micelle concentration
- CuO:
-
Copper oxide
- EOR:
-
Enhanced oil recovery
- Fe2O3 :
-
Iron oxide
- FTIR:
-
Fourier transform infrared spectroscopy
- GC:
-
Gas chromatography
- IFT:
-
Interfacial tension
- ILs:
-
Ionic liquids
- MgO:
-
Magnesium oxide
- MgSO4 :
-
Magnesium sulfate
- NISOC:
-
National Iranian South Oil Company
- NPs:
-
Nanoparticle
- OOIP:
-
Original oil in place
- ppm:
-
Parts per million
- PSNP:
-
Polysilicon NPs
- RSO:
-
Resinous synthetic oil
- SDBS:
-
Sodium dodecyl benzene sulfonate
- SiO2 :
-
Silicon oxide
- SiO2-NPs:
-
Silicon oxide nanoparticles
- TAN:
-
Total acid number
- TiO2 :
-
Titanium oxide
- wt%:
-
Percentage by weight
- [C18mim][Cl]:
-
1-Octadecyl-3-methyl imidazolium chloride
- [C12mim][Cl]:
-
1-Dodecyl-3-methyl imidazolium chloride
- D :
-
Equatorial diameter, m
- d :
-
Diameter at the distance D from the top of the drop, m
- g :
-
Acceleration of gravity, m s−2
- H :
-
Shape-dependent parameter, m
- L :
-
Drop length, m
- R and R m :
-
Radius of the drop at equator, m
- R o :
-
Radius of the drop at edge, m
- γ :
-
Interfacial tension, mN/m
- Δ:
-
Difference between two parameters
- ρ :
-
Density, kg m−3
- ω :
-
Rotational speed, rad/s
References
Abbood NK, Mayahi N, Obeidavi A, Hosseini S (2022) Effect of SiO2 nanoparticles + 1-dodecyl-3-methyl imidazolium chloride on the IFT and wettability alteration at the presence of asphaltenic-synthetic oil. J Pet Explor Prod Technol 12(11):3137–3148
Abhishek R, Kumar GS, Sapru R (2015) Wettability alteration in carbonate reservoirs using nanofluids. Pet Sci Technol 33(7):794–801
Adamson AW, Gast AP (1967) Physical chemistry of surfaces, vol 150. Interscience Publishers, New York
Agi A, Junin R, Gbonhinbor J, Onyekonwu M (2018) Natural polymer flow behaviour in porous media for enhanced oil recovery applications: a review. J Pet Explor Prod Technol 8:1349–1362
Alves CA, Yanes JFR, Feitosa FX, de Sant’Ana HB (2022) Influence of asphaltenes and resins on water/model oil interfacial tension and emulsion behavior: comparison of extracted fractions from crude oils with different asphaltene stability. J Pet Sci Eng 208:109268
Amin JS, Nikooee E, Ghatee M, Ayatollahi S, Alamdari A, Sedghamiz T (2011) Investigating the effect of different asphaltene structures on surface topography and wettability alteration. Appl Surf Sci 257(20):8341–8349
Amirian T, Haghighi M, Mostaghimi P (2017) Pore scale visualization of low salinity water flooding as an enhanced oil recovery method. Energy Fuels 31(12):13133–13143
Amirsadat SA, Moradi B, Hezave AZ, Najimi S, Farsangi MH (2017) Investigating the effect of nano-silica on efficiency of the foam in enhanced oil recovery. Korean J Chem Eng 34(12):3119–3124
Andreas J, Hauser E, Tucker W (2002) Boundary tension by pendant drops1. J Phys Chem 42(8):1001–1019
Aske N, Kallevik H, Sjöblom J (2001) Determination of saturate, aromatic, resin, and asphaltenic (SARA) components in crude oils by means of infrared and near-infrared spectroscopy. Energy Fuels 15(5):1304–1312
Azam MR, Tan IM, Ismail L, Mushtaq M, Nadeem M, Sagir M (2013) Static adsorption of anionic surfactant onto crushed Berea sandstone. J Pet Explor Prod Technol 3:195–201
Barahoei M, Hezave ZA, Sabbaghi S, Ayatollahi S (2016) Copper oxide nano-fluid stabilized by ionic liquid for enhancing thermal conductivity of reservoir formation: Applicable for thermal Enhanced Oil Recovery processes. Chem Ind Chem Eng Q 22(2):211–225
Bartels W-B, Mahani H, Berg S, Hassanizadeh S (2019) Literature review of low salinity waterflooding from a length and time scale perspective. Fuel 236:338–353
Berg S, Cense A, Jansen E, Bakker K (2010) Direct experimental evidence of wettability modification by low salinity. Petrophys SPWLA J Form Evaluation Reserv Descr 51(05)
Burnham P, Dollahon N, Li C, Viescas A, Papaefthymiou G (2013) Magnetization and specific absorption rate studies of ball-milled iron oxide nanoparticles for biomedicine. J Nanoparticles 2013:1
Cagna A, Esposito G, Quinquis A-S, Langevin D (2018) On the reversibility of asphaltene adsorption at oil-water interfaces. Colloids Surf A 548:46–53
Cai B-Y, Yang J-T, Guo T-M (1996) Interfacial tension of hydrocarbon+ water/brine systems under high pressure. J Chem Eng Data 41(3):493–496
Chan K, Shah D (1980) The molecular mechanism for achieving ultra low interfacial tension minimum in a petroleum sulfonate/oil/brine system. J Dispers Sci Technol 1(1):55–95
Chehrazi A, Rahimpour-Bonab H, Rezaee M (2013) Seismic data conditioning and neural network-based attribute selection for enhanced fault detection. Pet Geosci 19(2):169–183
Cheraghian G (2015) Effects of nanoparticles on wettability: a review on applications of nanotechnology in the enhanced Oil recovery
Demirbas A (2016) Deposition and flocculation of asphaltenes from crude oils. Pet Sci Technol 34(1):6–11
Demirbas A, Taylan O (2015) Recovery of gasoline-range hydrocarbons from petroleum basic plastic wastes. Pet Sci Technol 33(23–24):1883–1889
Demirbas A, Alidrisi H, Balubaid M (2015) API gravity, sulfur content, and desulfurization of crude oil. Pet Sci Technol 33(1):93–101
Druetta P, Raffa P, Picchioni F (2019) Chemical enhanced oil recovery and the role of chemical product design. Appl Energy 252:113480
Fakher S, Abdelaal H, Elgahawy Y, Imqam A (2019) A characterization of different alkali chemical agents for alkaline flooding enhanced oil recovery operations: an experimental investigation. Sn Appl Sci 1:1–11
Fakher S, Ahdaya M, Elturki M, Imqam A (2020) Critical review of asphaltene properties and factors impacting its stability in crude oil. J Pet Explor Prod Technol 10(3):1183–1200
Fang Y, Yang E, Guo S, Cui C, Zhou C (2022) Study on micro remaining oil distribution of polymer flooding in Class-II B oil layer of Daqing oilfield. Energy 254(Part C):124479
Ficken KJ, Wooller MJ, Swain D, Street-Perrott FA, Eglinton G (2002) Reconstruction of a subalpine grass-dominated ecosystem, Lake Rutundu, Mount Kenya: a novel multi-proxy approach. Palaeogeogr Palaeoclimatol Palaeoecol 177(1–2):137–149
Gbadamosi AO, Junin R, Manan MA, Agi A, Yusuff AS (2019) An overview of chemical enhanced oil recovery: recent advances and prospects. Int Nano Lett 9:171–202
Hammond PS, Unsal E (2011) Spontaneous imbibition of surfactant solution into an oil-wet capillary: wettability restoration by surfactant− contaminant complexation. Langmuir 27(8):4412–4429
Hirasaki GJ, Miller CA, Puerto M (2011) Recent advances in surfactant EOR. SPE J 16(04):889–907. https://doi.org/10.2118/115386-PA
Hosseini S, Sabet M, Zeinolabedini Hezave AA, Ayoub M, Elraies KA (2020) Effect of combination of cationic surfactant and salts on wettability alteration of carbonate rock. Energy Sources Part A Recovery Util Environ Effe 1–17
Jian C, Poopari MR, Liu Q, Zerpa N, Zeng H, Tang T (2016) Reduction of water/oil interfacial tension by model asphaltenes: the governing role of surface concentration. J Phys Chem B 120(25):5646–5654
Kume G, Gallotti M, Nunes G (2008) Review on anionic/cationic surfactant mixtures. J Surfactants Deterg 11(1):1–11
Kwok W, Nasr-El-Din HA, Hayes R (1993) Propagation of an anionic surfactant in radial sandstone cores. J Can Pet Technol 32(06)
Lashkarbolooki M, Ayatollahi S (2016) Effect of asphaltene and resin on interfacial tension of acidic crude oil/sulfate aqueous solution: experimental study. Fluid Phase Equilib 414:149–155
Lashkarbolooki M, Ayatollahi S (2018) Effects of asphaltene, resin and crude oil type on the interfacial tension of crude oil/brine solution. Fuel 223:261–267
Lashkarbolooki M, Ayatollahi S, Riazi M (2014) Effect of salinity, resin, and asphaltene on the surface properties of acidic crude oil/smart water/rock system. Energy Fuels 28(11):6820–6829
Lashkarbolooki M, Riazi M, Ayatollahi S, Hezave AZ (2016) Synergy effects of ions, resin, and asphaltene on interfacial tension of acidic crude oil and low–high salinity brines. Fuel 165:75–85
Lashkarbolooki M, Hezave AZ, Ayatollahi S (2019) The role of CO2 and ion type in the dynamic interfacial tension of acidic crude oil/carbonated brine. Pet Sci 16(4):850–858
Miller R (1982) Hydrocarbon class fractionation with bonded-phase liquid chromatography. Anal Chem 54(11):1742–1746
Najimi S, Nowrouzi I, Khaksar Manshad A, Mohammadi AH (2020) Experimental study of the performances of commercial surfactants in reducing interfacial tension and wettability alteration in the process of chemical water injection into carbonate reservoirs. J Pet Explor Prod Technol 10(4):1551–1563
Najimi S, Nowrouzi I, Manshad AK, Farsangi MH, Hezave AZ, Ali JA, Keshavarz A, Mohammadi AH (2019) Investigating the effect of [C8Py][Cl] and [C18Py][Cl] ionic liquids on the water/oil interfacial tension by considering Taguchi method. J Pet Explor Prod Technol 9(4):2933–2941
Nasr-El-Din HA, Taylor KC (1992) Dynamic interfacial tension of crude oil/alkali/surfactant systems. Colloids Surf 66(1):23–37
Nowrouzi I, Manshad AK, Mohammadi AH (2019a) Effects of concentration and size of TiO2 nano-particles on the performance of smart water in wettability alteration and oil production under spontaneous imbibition. J Pet Sci Eng 183:106357
Nowrouzi I, Manshad AK, Mohammadi AH (2019b) Effects of TiO2, MgO, and γ-Al2O3 nano-particles in carbonated water on water-oil interfacial tension (IFT) reduction in chemical enhanced oil recovery (CEOR) process. J Mol Liq 292:111348
Nowrouzi I, Manshad AK, Mohammadi AH (2020a) Effects of TiO2, MgO and γ-Al2O3 nano-particles on wettability alteration and oil production under carbonated nano-fluid imbibition in carbonate oil reservoirs. Fuel 259:116110
Nowrouzi I, Mohammadi AH, Manshad AK (2020b) Water-oil interfacial tension (IFT) reduction and wettability alteration in surfactant flooding process using extracted saponin from Anabasis Setifera plant. J Pet Sci Eng 189:106901
Nowrouzi I, Mohammadi AH, Manshad AK (2021a) Chemical enhanced oil recovery by different scenarios of slug injection into carbonate/sandstone composite oil reservoirs using an anionic surfactant derived from rapeseed oil. Energy Fuels 35(2):1248–1258
Nowrouzi I, Mohammadi AH, Manshad AK (2021b) Double-Chain Single-Head modification of extracted saponin from Anabasis Setifera plant and its effects on chemical enhanced oil recovery process by surfactant-alkali slug injection into carbonate oil reservoirs. J Pet Sci Eng 201:108438
Nowrouzi I, Mohammadi AH, Manshad AK (2021c) Synergic effects of dissolved carbon dioxide and an anionic surfactant synthesized from Rapeseed oil on interfacial tension (IFT) reduction, wettability alteration, and oil swelling in the process of chemical water injection into carbonate oil reservoirs. Fuel 290:120011
Nowrouzi I, Khaksar Manshad A, Mohammadi AH (2022a) Effects of MgO, γ-Al2O3, and TiO2 nanoparticles at low concentrations on interfacial tension (IFT), rock wettability, and oil recovery by spontaneous imbibition in the process of smart nanofluid injection into carbonate reservoirs. ACS Omega 7(26):22161–22172
Nowrouzi I, Mohammadi AH, Manshad AK (2022b) Preliminary evaluation of a natural surfactant extracted from Myrtus communis plant for enhancing oil recovery from carbonate oil reservoirs. J Pet Explor Prod Technol 12(3):783–792
Olayiwola SO, Dejam M (2019) A comprehensive review on interaction of nanoparticles with low salinity water and surfactant for enhanced oil recovery in sandstone and carbonate reservoirs. Fuel 241:1045–1057
Overton EB, Wade TL, Radović JR, Meyer BM, Miles MS, Larter SR (2016) Chemical composition of Macondo and other crude oils and compositional alterations during oil spills. Oceanography 29(3):50–63
Petroleum I (1985) IP standards for petroleum and Its products: methods for analysis and testing
Rajaei H, Hezave AZ, Lashkarbolooki M, Esmaeilzadeh F (2013) Representing experimental solubility of phenylephrine hydrochloride in supercritical carbon dioxide and modeling solute solubility using semi-empirical correlations. J Supercrit Fluids 75:181–186
Rane JP, Harbottle D, Pauchard V, Couzis A, Banerjee S (2012) Adsorption kinetics of asphaltenes at the oil–water interface and nanoaggregation in the bulk. Langmuir 28(26):9986–9995
Rezaeian MS, Mousavi SM, Saljoughi E, Amiri HAA (2020) Evaluation of thin film composite membrane in production of ionically modified water applied for enhanced oil recovery. Desalination 474:114194
Rudin J, Bernard C, Wasan DT (1994) Effect of added surfactant on interfacial tension and spontaneous emulsification in alkali/acidic oil systems. Ind Eng Chem Res 33(5):1150–1158
Saha R, Uppaluri RV, Tiwari P (2018) Influence of emulsification, interfacial tension, wettability alteration and saponification on residual oil recovery by alkali flooding. J Ind Eng Chem 59:286–296
Salehi M, Johnson SJ, Liang J-T (2008) Mechanistic study of wettability alteration using surfactants with applications in naturally fractured reservoirs. Langmuir 24(24):14099–14107
Setudehnia A (1978) The mesozoic sequence in south-west Iran and adjacent areas. J Pet Geol 1(1):3–42
Sheng JJ (2015) Status of surfactant EOR technology. Petroleum 1(2):97–105
Soleimani Zohr Shiri M, Henderson W, Mucalo MR (2019) A review of the lesser-studied microemulsion-based synthesis methodologies used for preparing nanoparticle systems of the noble metals, Os, Ren Ir and Rh. Materials 12(12):1896
Soorghali F, Zolghadr A, Ayatollahi S (2014) Effect of resins on asphaltene deposition and the changes of surface properties at different pressures: a microstructure study. Energy Fuels 28(4):2415–2421
Stauffer CE (1965) The measurement of surface tension by the pendant drop technique. J Phys Chem 69(6):1933–1938
Strand S, Høgnesen EJ, Austad T (2006) Wettability alteration of carbonates—effects of potential determining ions (Ca2+ and SO42−) and temperature. Colloids Surf A 275(1–3):1–10
Sun Z, Kang X, Lu X, Li Q, Jiang W (2018) Effects of crude oil composition on the ASP flooding: a case from Saertu, Xingshugang and Lamadian Oilfield in Daqing. Colloids Surf A 555:586–594
Taylor KC, Hawkins BF, Islam MR (1990) Dynamic interfacial tension in surfactant enhanced alkaline flooding. J Can Pet Technol 29(01)
Trabelsi S, Argillier J-FO, Dalmazzone C, Hutin A, Bazin B, Langevin D (2011) Effect of added surfactants in an enhanced alkaline/heavy oil system. Energy Fuels 25(4):1681–1685
Vonnegut B (1942) Rotating bubble method for the determination of surface and interfacial tensions. Rev Sci Instrum 13(1):6–9
Wang Z, Xu Y, Khan N, Zhu C, Gao Y (2022) Effects of the surfactant, polymer, and crude oil properties on the formation and stabilization of oil-based foam liquid films: insights from the microscale. J Mol Liq 373:121194
Wu J, Prausnitz J, Firoozabadi A (1998) Molecular-thermodynamic framework for asphaltene-oil equilibria (vol 44, pg 1188, 1998). AIChE J 44(11):2568–2568
Yang Z, Li M, Peng B, Lin M, Dong Z, Ling Y (2014) Interfacial tension of CO2 and organic liquid under high pressure and temperature. Chin J Chem Eng 22(11–12):1302–1306
Yang H, Britton C, Liyanage PJ, Solairaj S, Kim DH, Nguyen Q, Pope GA et al (2010) Low-cost, high-performance chemicals for enhanced oil recovery. In: Paper presented at the SPE improved oil recovery symposium. https://doi.org/10.2118/129978-MS
Yarranton HW, Alboudwarej H, Jakher R (2000) Investigation of asphaltene association with vapor pressure osmometry and interfacial tension measurements. Ind Eng Chem Res 39(8):2916–2924
Zabihi S, Rahnama Y, Sharafi A, Borousan F, Zeinolabedini Hezave A, Shirazian S (2020b) Experimental solubility measurements of fenoprofen in supercritical carbon dioxide. J Chem Eng Data 65(4):1425–1434
Zabihi S, Faraji D, Rahnama Y, Zeinolabedini Hezave A, Ayatollahi S (2020a) Relative permeability measurement in carbonate rocks, the effects of conventional surfactants vs. Ionic liquid-based surfactants. J Dispers Sci Technol 41(12):1797–1811
Zeinolabedini Hezave A, Dorostkar S, Ayatollahi S, Nabipour M, Hemmateenejad B (2014) Mechanistic investigation on dynamic interfacial tension between crude oil and ionic liquid using mass transfer concept. J Dispers Sci Technol 35(10):1483–1491
Zhang P, Austad T (2006) Wettability and oil recovery from carbonates: effects of temperature and potential determining ions. Colloids Surf A 279(1–3):179–187
Zhang P, Tweheyo MT, Austad T (2007) Wettability alteration and improved oil recovery by spontaneous imbibition of seawater into chalk: Impact of the potential determining ions Ca2+, Mg2+, and SO42−. Colloids Surf A 301(1–3):199–208
Zhang J, Tian D, Lin M, Yang Z, Dong Z (2016) Effect of resins, waxes and asphaltenes on water-oil interfacial properties and emulsion stability. Colloids Surf A 507:1–6
Zhang S, Zhang L, Lu X, Shi C, Tang T, Wang X, Zeng H et al (2018) Adsorption kinetics of asphaltenes at oil/water interface: Effects of concentration and temperature. Fuel 212:387–394
Zhao C, Jiang Y, Li M, Cheng T, Yang W, Zhou G (2018) The effect of NaOH on lowering interfacial tension of oil/alkylbenzene sulfonates solution. RSC Adv 8(11):6169–6177
Zhong H, He Y, Yang E, Bi Y, Yang T (2021) Modeling of microflow during viscoelastic polymer flooding in heterogenous reservoirs of Daqing Oilfield. J Pet Sci Eng 210(1):110091
Zulkifli NN, Mahmood SM, Akbari S, Manap AAA, Kechut NI, Elrais KA (2020) Evaluation of new surfactants for enhanced oil recovery applications in high-temperature reservoirs. J Pet Explor Prod Technol 10:283–296
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abbood, N.K., Dahham, N.A., Dezfouli, M.A. et al. Effect of sodium hydroxide, sodium dodecyl benzenesulfonate, and resin on the interfacial tension of asphaltenic synthetic oil extracted from acidic crude oil under low salinity condition. J Petrol Explor Prod Technol 13, 2457–2474 (2023). https://doi.org/10.1007/s13202-023-01673-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-023-01673-8