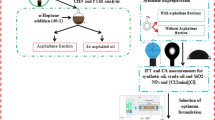
Abstract
The effects of the main components of crude oil, especially resin and asphaltene fractions, are essential concerns for efficient enhanced oil recovery (EOR) processes, especially during chemical injection processes. This importance comes from the nature of these two fractions which can act as surface active agents with undeniable effects on the used chemical for interfacial tension (IFT) reduction and wettability alteration. In this way, the effect of silicon oxide nanoparticles (SiO2-NPs) concomitant with two ionic liquids (ILs), namely 1-dodecyl-3-methyl imidazolium chloride ([C12mim][Cl]) and 1-octadecyl-3-methyl imidazolium chloride ([C18mim][Cl]), is investigated on the wettability alteration and IFT reduction using synthetic oils prepared by dissolving the extracted resin and asphaltene fractions with a concentration of 1–5 wt%. The measurements reveal that the effect of resin fraction is less than the asphaltene fraction for IFT reduction and wettability alteration. The sole presence of resin fraction reduces the IFT from 35.3 to 28.3 mN/m as the concentration is increased from 1 to 5 wt%, while a similar increase in the asphaltene fraction concentration reduces the IFT from 35.5 to 19.1 mN/m. Besides, the results reveal that the presence of [C12mim][Cl] in the range of 0–1000 ppm leads to a reduction in IFT from its maximum value of 35.3 to 0.81 mN/m, while in the case of [C18mim][Cl] with similar concentration variation, IFT is reduced from 35.3 to 0.7 which means the better effect of IL with longer chain length on the IFT reduction. Further analysis revealed that the effect of asphaltene fraction on the IFT is higher than resin fraction since the minimum IFT value was observed for [C18mim][Cl] with the value of 0.58 mN/m, while the contact angle (CA) values revealed revers effect for asphaltene fraction compared with the resin fraction. In general, regardless of the used IL, it seems that ILs leading to better wettability conditions which are crucial for EOR purposes and even better IFT values that can mobilize the trapped oil toward production points. Besides, further measurements revealed a positive effect of SiO2-NPs concomitant with the ILs to move the wettability toward the strongly water-wet condition with CA values of 29.2° and 28.3° for SiO2-NPs concentration of 1000 ppm and 1000 ppm of concentration for [C12mim][Cl] and [C18mim][Cl], respectively, for resinous synthetic oil (RSO) (5 wt%) while no meaningful effect regarding the SiO2-NPs presence at the different concentrations (100–2000 ppm) is found on the IFT reduction. A similar trend is observed for asphaltenic synthetic oil (5 wt%)/aqueous solution (SiO2-NPs with a concentration of 1000 ppm + ILs with a concentration of 1000 ppm) which reduces the CA to 26.3° and 37.8° for [C12mim][Cl] and [C18mim][Cl]), respectively.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
It is well accepted that only 30% of the original oil in place (OOIP) is recovered after primary and secondary oil recovery processes. On the other side, the increasing rate of energy consumption, population, and reservoir depletion (shortage of energy) forced the oil companies to propose new methods for a higher rate of oil recovery from the depleted reservoirs which is known as enhanced oil recovery (EOR) methods (Cheraghian 2015; Abhishek et al. 2015). In general, the EOR processes can be categorized into different methods mainly chemicals (Moradi et al. 2017; Barahoei et al. 2016; Hosseini et al. 2020; Najimi et al. 2019; Zabihi et al. 2020a, b; Zeinolabedini Hezave et al. 2014), thermal methods, low salinity water injection, smart water injection, carbon dioxide injection, etc. (Lashkarbolooki et al. 2019; Rajaei et al. 2013; Zabihi et al. 2020a, b). Among different EOR methods, chemical and carbon dioxide (CO2) injection (which are applicable chemicals for different industries such as pharmaceuticals and oil industries) are gaining increasing attention although chemical injection is widely examined, especially when the oil price increases (Najimi et al. 2020; Nowrouzi et al. 2022a, b; Nowrouzi et al. 2019a, 2019b, 2020a, b; Nowrouzi et al. 2020a, b, 2021a, b, c, 2022a, b).
Unfortunately, since using only one EOR method cannot provide the desired outcomes for extracting the trapped oil from the reservoirs, various hybrid and innovative techniques were proposed and examined to recover trapped oil from abandoned or depleted reservoirs (Cheraghian 2016). In this way, during the past decade, a new concept that combines the advantages of different EOR methods such as surfactant injection, nanoparticle (NPs) injection and smart water injection is introduced since using one EOR method is disabled to activate multiple mechanisms. Among the different methods which can be combined with each other, nanotechnology is one of the most widely interested EOR methods dealing with particles in the range of 1–100 nm (Khalil et al. 2017). In the light of its unique characteristics NPs were widely used in EOR industries for IFT reduction, (Zaid et al. 2013; Amirsadat et al. 2017), changing the wettability of rock surface toward water-wet condition (Al-Anssari et al. 2016; Saien and Gorji 2017), viscosity modification and even enhancing the swelling factor of the crude oil (Ehtesabi et al. 2015), producing nano-emulsion (Bobbo et al. 2012), selective pore plugging (Anganaei et al. 2014; Hashemi et al. 2013), and disjoining pressure (Aveyard et al. 2003; McElfresh et al. 2012a, b), or even increasing the thermal conductivity of the rocks using metal-based NPs. NPs are on the radar since they can change the wettability, reduce the IFT, and disjoining pressure which means better sweep efficiency (Rodriguez et al. 2009). Considering all of these unique features, NPs are going to be examined by different researchers to find if they can reduce the IFT, change the wettability of the surfaces or not (de Castro et al. 2017; Moradi et al. 2015; Suleimanov et al. 2011).
For example, Onyekonwu and his coworker (Onyekonwu and Ogolo 2010) investigated the possible effect of polysilicon NPs (PSNP), e.g., lipophobic and hydrophilic, and hydrophilic and lipophobic for changing the wettability of rock surfaces which is highly required through EOR processes. Also, several researchers (Cheraghian and Hendraningrat 2016; Hendraningrat et al. 2013a, b; Torsater et al. 2012) reported the significant effect of silica-based NPs on changing the rock surface wettability. Moreover, Mohammadi et al. (Mohammadi et al. 2014) used the aluminum oxide-NPs (Al2O3-NPs) for the sandstone reservoir to investigate their effects during the EOR processes by changing different effective parameters, namely rock surface wettability. After that, Tarek (Tarek 2015) investigated combinations of different types of NPs as an alternative to individual applications of NPs to provide possible synergy between NPs from different types. In detail, Tarek reported that using a mixed solution of different NPs such as Al2O3, iron oxide (Fe2O3-NPs) and silicon oxide (SiO2) was more effective than the solution that includes only one type of NPs.
According to these findings and the fast growth of using NPs, many researchers are going to examine the effect of these particles in different industries, especially EOR processes (Cheraghian and Hendraningrat 2016; Sheng 2010). More recently, several investigations have performed using copper oxide (CuO)/Titanium oxide (TiO2)-NPs/polyacrylamide, magnesium oxide (MgO), γ-Al2O3, and TiO2-NPs, to find the effect of different NPs on the IFT and contact angle (CA) of the binary systems (Nowrouzi et al. 2022a, b; Nowrouzi et al. 2019a).
For example, Nowruzi and his colleagues (Nowrouzi et al. 2022a, b) reported that the IFT experienced a further reduction as the NPs and ions existed in the system. They reported that an optimum IFT value of 4.68 mN/m was achieved for the solution including 2000 ppm of magnesium sulfate (MgSO4) + 500 ppm of MgO concomitant with the NPs. Furthermore, the measured CA values revealed that the modified water with salts is more effective for wettability alteration, especially if it is combined with NPs. They reached the minimum CA value of 18.33° using optimum chemical formulation which means highly hydrophilic conditions of the rock surface. Furthermore, they performed several core flooding experiments using an optimum chemical formulation containing nano-γ-Al2O3 leading to maximum oil recovery of 64.1–68.7% based on the original oil in place (OOIP).
Unfortunately, using NPs for EOR purposes has an intrinsic drawback that must be eliminated before using these particles in the field scale. This drawback is the precipitation of NPs in the aqueous solution before being effectively injected into the reservoir which can bring a catastrophic impact on the reservoir which is pore plugging consequently leading to the entrapment of residual oil in the pore networks maybe forever in severe cases. In other words, the compatibility of the injectable aqueous solutions into the reservoir has a great importance since any precipitation of particles may introduce catastrophic results in the reservoir by trapping the oil drops forever or long period. In this way, it is highly required to use a solution that can prevent the precipitation of NPs or at least retard it for a long period. One of the most common approaches is the addition of surfactants into the aqueous solutions leads to prevention of the fast precipitation of NPs. Among the different possible surfactants, using IL-based surfactants from imidazolium or pyridinium families gains a great interest during the past decade due to their unique features, especially their stability at the harsh salinity and thermal conditions.
ILs are recently proposed as new solvents which can provide unique characteristics such as lower melting and glass transition temperature (Dharaskar Swapnil 2012; Domańska 2005) concomitant with their economic advantages (Chen et al. 2014), or being non-flammable with a wide range of solubility and miscibility (Martins et al. 2014), etc. The reason behind their unique functionalities is their unique structure which comprises two different sections (cationic and anionic groups). In detail, it is possible to tailor any type of IL with a specific characteristic that makes them a good candidate for different industries. In detail, ILs are molecules that can be tuned for any specific application such as EOR by changing the combination of its anions and cations compartments (Khupse and Kumar 2010).
For example, Smit et al. (Smit et al. 1991) and Rajaei et al. (Rajaei et al. 2013) reported similar results for the ILs surface activity in the presence of high salinity. In detail, they reported that the dissolution of cationic ILs in saline water leads to an interaction between the positive charges of cationic ILs and negative charges of ions leading to the accumulation of ILs molecules at the oil–water interface and occurrence of low IFT values may be with the minimum value of 10−1 mN/m (in special cases 10–2 mNm−1) which is not low enough to consider the IFT reduction as the main effective mechanisms for these types of ILs (Hezave et al. 2013a). So it seems that individual application of ILs is not satisfactory for oil recovery purposes (Rodríguez-Palmeiro et al. 2015).
As a final point, crude oil is a combination of a large number of chemicals may be more than thousands of different compounds mainly divided into resin, asphaltene, saturates, and aromatics (Overton et al. 2016). So, it is impossible to correctly characterize crude oil using only one specific molecular type (Demirbas and Taylan 2015). As an alternative to eliminate such a limitation, hydrocarbon group type analysis is commonly employed with the knowledge of the distribution of major structural classes of hydrocarbons (Demirbas 2016; Muhammad et al. 2013). Among these fractions, resin and asphaltene are the most active fractions which act as a natural surfactant and react with the other chemicals presented in the solution. Among these two fractions, both of them are vital for crude oil stability but the resin fraction is responsible to stabilize the asphaltene fraction either (Fakher et al. 2020). In other words, asphaltene and resin fractions can act as natural surfactants in which they can manipulate the IFT values and stability of crude oil and water emulsions. Resin fractions of crude oil include polar molecules with heteroatoms such as nitrogen, oxygen, or sulfur which are among the heteroatoms ( Demirbas et al. 2015). The resin fraction is soluble in light alkanes such as pentane and heptane, but insoluble in liquid propane. In the literature, few works have reported the properties of the resins (Aske et al. 2001). The point is that it is possible to categorize the crude oil fractions into resin or asphaltene fractions using the fact the H/C ratio of resins is between 1.2 and 1.7 while this value for asphaltene fractions is between 0.9 and 1.2 although resins are structurally similar to asphaltenes (Ficken et al. 2002).
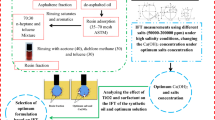
Respecting to the important role of the resin and asphaltene fractions which are limitedly investigated in the previously published literature, and since hybrid methods are applicable methods for higher oil production, a new hybrid chemical method is proposed in the current investigation. In this way, for the first time, hybrid solutions including ILs from the imidazolium family, namely 1-dodecyl-3-methyl imidazolium chloride ([C12mim][Cl]) and 1-octadecyl-3-methyl imidazolium chloride ([C18mim][Cl]), were selected as the chemical surfactants and probable stabilizers of the SiO2-NPs while the sample oils were synthetic types provided by dissolving a known portion of asphaltene and resin fractions. In detail, since crude oil is a complex mixture and performing investigations using crude oil puts the conclusions in a dark, it is highly required to investigate the influence of different parameters using specific fractions of the crude oil; resin and asphaltene fractions are the most important ones. Besides, since a limited number of investigations using asphaltene and resin are performed in the literature, especially in the absence of different chemicals such as NPs and ILs as the stabilizers, it seems that proposing hybrid solutions including NPs, ILs from the imidazolium family, and asphaltene and resin fractions dissolved in the toluene as the synthetic oil can provide a good insight into the interactions may exist between these chemicals for possible EOR applications.
Since based on the best knowledge of the authors there are no reports regarding these complex and hybrid solutions regarding the IFT reduction and wettability alteration in the presence of resin and asphaltene factions, the current work is focused on the effect of different combinations of these chemicals including 1-dodecyl-3-methyl imidazolium chloride ([C12mim][Cl]) and 1-octadecyl-3-methyl imidazolium chloride ([C18mim][Cl]) (100–1000 ppm), SiO2-NPs (100–2000 ppm) and concentrations of resin and asphaltene fractions (1–5 wt%) on the IFT reduction and wettability alteration for the first time.
Materials and methods
Materials and solutions
Crude oil with a density of 0.796 g cm−3 @ 15 °C was used as the sample oil for the IFT and CA measurements. The crude oil which was kindly provided by the National Iranian South Oil Company (NISOC) from one of the southern Iranian oilfields, comprised N. paraffin of 10.31%, I. paraffin of 10.884%, olefinic of 0.04%, naphthenes of 9.888%, aromatics of 6.373%, saturates (C15 < C20) of 6.489%, aromatics (C15 < C < C20) of 3.466%, unknowns (C < 20) of 14.537% and C20+ of 37.643% according to the gas chromatography (GC) analysis. Besides, the formation brine with Na+ & K+ (73,498 ppm), Ca2+ (10,206 ppm), Mg2+ (1880 ppm), Fe2+ (56 ppm), Cl− (134,881 ppm), HCO3− (1716 ppm) and SO42− (1078 ppm) was used for all of experiments as it was received.
The worth mentioning point is that the required IL, namely [C12mim] [Cl] (286.9 g/gmol) and [C18mim][Cl] (371.0 g/gmol), was synthesized using 1-methylimidazole, 1-chlorododecyl (Merck/Fluka, purity > 99.5%). A brief description of the used method for synthesizing is previously reported elsewhere (Hezave et al. 2013b, c). After synthesizing, the Fourier-transform infrared spectroscopy (FTIR) was used to characterize the synthesized [C12mim] [Cl] and [C18mim][Cl] (Abbood et al. 2022). In addition, the FTIRs of the synthesized [C12mim] [Cl] are compared with those synthesized by Bennet et al. (Bennett and Larter 1997) to check if the mentioned synthesized method was accurately performed or not. Moreover, the required SiO2-NPs with a particle size range of 20–30 nm were prepared from Finenano, Iran with a minimum purity of 99%.
Total acid number (TAN)
The total acid number (TAN) of the used crude oil was measured based on the ASTM D 664 method which revealed that the TAN of the crude oil was obtained at about 0.11 mg KOH/g oil. According to this definition given by Xin-heng (Xin-heng and Tian 2011), acidic crude oil has a TAN higher than 0.5 mg KOH/g, and the used crude oil for asphaltene and resin fractions extraction is not an acidic crude oil.
Extraction of asphaltene and resin
The required asphaltene and resin for preparing the resinous synthetic oil (RSO) and asphaltenic synthetic oil (ASO) were extracted from crude oil using a standard method known as IP 143/90 (Petroleum 1985). The reason behind this extraction and synthetic oils usage is that crude oil is comprised of thousands of compartments which make it difficult to extract reliable and generalized conclusions regarding the interactions between different chemicals and crude oil. In other words, although resin and asphaltene fractions are active with an essential roles, their effects on different fluid/fluid or fluid/solid interfaces and interactions are not well understood (Lashkarbolooki et al. 2014).
According to the results obtained based on the spectroscopic analysis, resins and asphaltene fractions consisted of hydroxyl groups, ester, acid, carbonyl functions, and long paraffinic chains with naphthenic rings and polar functions which provide them a surfactant-like structure and nature with high complexities (Lashkarbolooki et al. 2016; Wu et al. 1998). Besides, the analyses revealed that although resin and asphaltene fractions usually appear with each other, they are different in size, aromaticity, polarity, and physical appearance.
For isolation purposes, n-heptane with a ratio of 40:1 was used to isolate the asphaltene fraction in the first stages. After that, the remaining molten and de-asphalted oil was used to extract the resin fraction using the column chromatography method described elsewhere (Amin et al. 2011; Soorghali et al. 2014).
The maltene (de-asphalted oil + n-heptane) was adsorbed to a silica gel column (Merck, 35–70 mesh ASTM), followed by rinsing of saturates and aromatics by a solution of 70:30 n-heptane and toluene. Finally, an acetone/dichloromethane/toluene mixture with a ratio of 40:30:30) was used to isolate and remove the resins from the column (Miller 1982; Yarranton et al. 2000).
Moreover, the extracted fractions were analyzed by a CHNSO analyzer (Thermo Flash EA 1112 series) to identify the mass ratios of C, H, N, S, and O atoms present in the resin and asphaltene extracts. According to the findings based on the elemental analysis, it seems that the resin fraction, which has the H/C ratio of 1.44, has lower aromaticity than the asphaltene with the H/C ratio of 1.12 which means that the resin molecules are more branched compared with asphaltene molecules. So, due to the more branched molecular structure of resin than asphaltene molecules and the presence of heteroatoms of sulfur and oxygen with negative charges, resin molecules act like surfactant which can reduce the IFT value. In this way, it is highly crucial to investigate the effect of different chemicals in contact with the RSO and ASO prepared by dissolving a specific amount of resin or asphaltene fraction into the toluene for IFT reduction and wettability alteration purposes.
Interfacial tension (IFT) and contact angle (CA) measurements
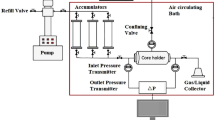
The pendant drop and sessile drop methods are the most accurate methods for IFT and CA measurements (Yang et al. 2014), respectively. Respecting this fact, pendant drop equipment (Fanavari Atiyeh Pouyandegan Exir Co., Arak. Iran) (see Fig. 1) was used to measure the IFT and CA of different solutions in the presence of RSO and ASO.
In general, the used pendant drop included different sections such as an automatic drop dispenser system, image capturing system, and image processing software. In brief, the aquarium was filled with the bulk phase and then the required drop was injected on the tip of the nozzle. At this point, the drop must be injected to the volume in which the gravity and buoyancy forces are equal and the drop is close to being detached from the nozzle. The point is that not only the volume of the drop must be sufficient, but also it is highly required to use a proper magnification in which the maximum space of the image is occupied by the drop to ensure accurate IFT measurement using the drop shape analysis approach (see Eq. 1):
where Δρ, g, and H are the difference between the densities of bulk and drop phases, acceleration of gravity, and the shape-dependent parameter. The H value is depending on the shape factor value, i.e., S = d/D, where D is the equatorial diameter and d is the diameter at the distance D from the top of the drop. Also, the software is developed in a way that it is possible to calculate the CA of the drop under the captive or non-captive condition by injecting the oil drop on the top of the surface or beneath the thin sections. Using the aforementioned Equation (Eq. 1), it is possible to analyze the captured images to calculate the IFT using the drop shape analysis concept, while the sensible drop concept can be used to calculate the CA (Adamson and Gast 1967; Andreas et al. 2002; Stauffer 1965).
The last noteworthy point is that the maximum uncertainty of the measured IFT values which is the average of at least three independent measurements for each data point was about ± 0.2 mN m−1 which was impossible to indicate these uncertainties for the measured IFT values in the plotted IFT values due to a broad range of IFT values measured in the current investigation.
Results and discussion
Evaluation of performance of ionic liquids (ILs) on the resinous synthetic oil
The measured IFT and CA values using different concentrations of resin fractions in the toluene, and different concentrations of [C12mim][Cl] and [C18mim][Cl] dissolved in the aqueous solution revealed considerable effects of resin and IL on the IFT and wettability. In detail, the measurements revealed that the dissolution of resin in the range of 1–5 wt% while no chemicals were dissolved in the aqueous solution led to a reduction in IFT from 35.3 to 28.3 mN/m (Table 1). This reduction can be related to the nature of resin fraction for IFT reduction since it has a surface activity that makes the resin molecules similar to the surfactant molecules.
Besides, the measurements revealed the capability of resin fraction for changing the wettability of the rock surface toward oil-wet conditions which increased the CA of the rock surface to a maximum value of 137.1° (see Table 1).
On the other side, the investigations of the results given in Table 1 revealed that the presence of [C12mim][Cl] in different concentrations of 100–1000 ppm led to a reduction in IFT for all the examined concentrations of RSO (1–5 wt%) (see Fig. 2). A closer look into the results depicted in Fig. 2b revealed that for a specific resin fraction, an increase in the [C12mim][Cl] concentration changed the wettability toward more water-wet condition while an increase in the resin fraction concentration from 1 to 5 wt% changed the rock surface toward more oil-wet condition. The reason behind this increase in the CA value as a function of resin fraction can be correlated to the repulsive forces that appear as the resin concentration increases. In other words, as the concentration of resin fraction increases, the number of resin molecules at the rock interface increases which can increase the number of ILs can be trapped in their structure. As a consequence of this increase in the resin molecules and trapped molecules of ILs in the resin structure an enhancement in repulsive forces is inevitable which moves the ILs molecules to a far distance to each other to reduce the repulsive forces. So, it is harder for the ILs molecules to penetrate the resin film formed on the rock surface in the light of this far distance consequently leading to lower capability of ILs for wettability alteration or at least longer time would be required to reach the ultimate effect of ILs for wettability alteration toward water-wet condition.
In the next stage of this investigation, the effect of longer chain length IL, namely [C18mim] [Cl] (0–1000 ppm), on the IFT and CA was investigated at the different concentrations of resin fraction. The measurements (see Fig. 3a, and b) revealed that similar to the effect of [C12mim] [Cl] on the IFT, an increase in the IL concentration reduced the IFT value for all of the examined resin concentrations and even introduced a higher impact on the IFT compared with the effect of [C12mim] [Cl] (see Fig. 4).
In contrast to the results obtained for the effect of [C12mim] [Cl] on the CA, the measurements revealed that there is a shifting point at the resin concentration of 3 wt% where the effect of [C18mim] [Cl] is different on the CA (see Fig. 3b). The other point is that, for a constant concentration of [C18mim][Cl], increasing the resin fraction from 1 to 3 wt% leads to an increase in the CA (more oil-wet conditions) while further increase in the resin concentration from 3 to 5 wt% reduced the CA which means more water-wet conditions.
The reason behind this observed shifting point can be correlated to the longer chain length of [C18mim] [Cl] compared with [C12mim] [Cl] leading to a more stabilized packing of the IL molecules on the rock surface. In other words, since the [C18mim] [Cl] has longer chain length molecules, they can neutralize the repulsive forces that may appear if they reach the surface leading to better packing of the IL molecules at the interface and easier penetration into the resin film formed on the rock surface render the rock surface toward more water-wet condition.
Evaluation of performance of ionic liquids (ILs) on the asphaltenic synthetic oil
In general, the IFT measurements revealed the higher impact of asphaltene fraction on the IFT reduction compared with the resin fraction for similar concentrations ranging between 1 and 5 wt% (see Figs. 5a and b and 6).
Similar to the results obtained for RSO, it seems that the overall trend for the effect of IL concentration on the wettability alteration is the same for all the examined asphaltene concentrations and it is reducing effect which means more water-wet surfaces can be obtained using higher concentrations of IL. But, a closer look into the results depicted in Fig. 5b revealed that for the asphaltene concentration of 3 wt% and IL concentration of 500 ppm which was selected as the critical micelle concentration (CMC) based on the IFT measurements, the situation is more complicated.
Surfactants which are normally dissolved in water and organic solvents are organic compounds comprised of a hydrophobic tail and a hydrophilic head. Surfactant molecules are leveled at the liquid level due to their hydrophilic–hydrophobic structure so that the hydrophilic head is dissolved in an aqueous solution and the hydrophobic head avoids it. In general, as the surfactant concertation increases, it is possible to occupy a higher amount of interface space using surfactant molecules, and even experience aggregations of surfactants due to the absorption of the same parts of each surfactant molecule commonly known as the micelle. The formation of the micelle reduce the strength of surfactant molecules by limiting their release (Schramm 2000; Torrealba and Johns 2017).
Surfactants are designed and manufactured to reduce the IFT of water/oil system. When the micelles begin to form, they form a nucleus like a drop of oil and their ionic head creates an outer shell that improves contact with water. In general, it is possible to determine the efficiency of a surfactant by measuring the CMC value of each desired surfactant. Besides, measuring the CMC is important since it is an indication of surfactants efficiency. In detail, formation of the micelles regardless of the CMC point gives the ability to form an oil-soluble emulsion. In this way, for the surfactant with lower CMC value, lower amount of surfactant is required to reach the minimum IFT value (Joos et al. 1992; Ward and Tordai 1946).
It is well accepted that there is a direct relationship between the hydrophobic length and the surfactant capacity for IFT reduction. In other words, longer tails of the hydrophobic tail mean better efficiency of surfactant at lower concentrations (Painter et al. 2010). As mentioned earlier, the IFT reduction due to salinity enhancement can be explained by the mechanism of the synergic effects of ions and surfactants. The negatively charged ions neutralize the positive surface charges of the cationic surfactants which consequently make it easier the accumulation of surfactant molecules at the oil–aqueous solution interface leading to higher IFT reduction.
In detail, it seems that for 3 wt% asphaltene and IL concentrations below the CMC value, the wettability reduces compared with the higher concentration of asphaltene (5 wt%) while further increase in the IL concentration to 500 and 1000 ppm (higher than the CMC value) moves the wettability of the rock surfaces toward more water-wet condition. The reason behind this observed trend can be correlated to the fact that for the IL concentrations lower than 500 ppm, the competition between the IL molecules to reach the rock surface (penetrate the asphaltenic oil film) and asphaltene molecules to stick into the rock surface leading to accumulation of IL molecules at the interface and undesired repulsive forces leading to more oil-wet conditions. But, as the IL concentration reaches the CMC value in which the IL molecules tend to form micelles, the system gets more stabilized gradually leading to the easier formation of the IL bridge on the rock surface and wettability alteration toward water-wet condition. Moreover, the other possible hypothesis behind this observed trend can be correlated to the fact that as the ILs molecules start to form micelles, they would be more stable considering the surface charge, and at this point, they can get closer to the rock surface and pack on the rock surface more easily where they can defeat the bonds existed between the asphaltene molecules and rock surface and substitute those molecules and change the wettability toward more water-wet condition.
But in the case of using [C18mim] [Cl] for IFT reduction and wettability alteration, although the IFT variation and reduction were similar to those patterns that were previously observed for RSO and ASO, the wettability alteration was slightly different from that observed for [C12mim] [Cl] and ASO (see Fig. 7b). In detail, it seems that similar to the IFT values obtained for [C12mim] [Cl], an increase in the IL concentration leading to a considerable reduction in the IFT, especially around 500 ppm which can be selected as the CMC value of the current system.
Moreover, the investigations regarding the effect of asphaltene and [C18mim][Cl] concentrations on the wettability alteration revealed that there is a shifting point where the reducing pattern in the CA values changes to an increasing pattern. This observed trend can be correlated to the higher concentration of asphaltene molecules at the interface and their interactions with the IL molecules leading to repulsive forces that put the IL molecules away from the rock interface leading to the less water-wet condition compared with the synthetic oils comprised of a lower amount of asphaltene fraction.
The effect of ILs on the wettability alteration of the rock surface can be contributed to the two different mechanisms. In detail, two mechanisms are generally accepted for the alteration of wettability by surfactant solutions: the creation of a stable thin film by surfactant on the surface (Salathiel 1973) and the surfactant molecules packing at the surface (Spinler et al. 2000). When a thin film of water is stable, oil is spread in the middle of large pores, and water between oil and rock is generally in bubble form. But, it is possible to destabilize this thin film by introducing surfactant molecules in the solution which can be adsorbed in the interface. But the instability of a thin film of water in the site creates a continuous oil pathway that mobilizes the oil drops leading to mixed wettability conditions (Spinler et al. 2000). In other words, the continuous emission and absorption of surfactant molecules into the environment change the physical–chemical properties of the rock, resulting in a continuous pathway for the production of oil (Ayirala et al. 2006; Jadhunandan and Morrow 1995; Najimi et al. 2020; Salathiel 1973).
Effect of SiO2 −NPs on the IFT and wettability alteration
In the last stage of this investigation, the solutions including 1000 ppm of [C12mim] [Cl] and [C18mim] [Cl] were selected as the optimum aqueous solutions for the rest of the IFT and wettability alteration measurements while the RSO and ASO with a concentration of 5 wt% were selected as the sample oils (see Figs. 8 and 9). In this way, the SiO2-NPs concentration was changed between 100 and 2000 ppm to find the possible synergy that existed between the synthetic oils and ILs solutions.
The worth mentioning point is that there are two steps to prepare the nanofluids: the first step is preparing the nanofluid in the meantime that nanoparticles are produced which means a combination of the fluid and nanoparticles at the same time that NPs are produced and the second step that prepared NPs are added to the base fluid and dispersing the NPs in the base fluid using chemical or physical methods. Dispersion and stabilizing methods of NPs in the fluid would include surfactant addition (Yu and Xie 2012), pH control of the nanofluid (Ghadimi et al. 2011), and ultrasonic vibration (Chang et al. 2007). Considering the dispersion method of NPs in the base fluid for this work, using chemicals based on the IL was employed to keep the NPs dispersed in the aqueous solution.
The measured IFT values for both ILs and both synthetic oils including resinous and asphaltenic types revealed that increasing the SiO2-NPs from 0 to 2000 ppm leads to a complex behavior on the IFT reduction. In detail, considering the RSO revealed that increasing the SiO2-NPs for the solution including [C12mim][Cl] from 0 to 200 ppm leads to an increase in the IFT while further increase in the SiO2-NPs concentration lowers the IFT values close to the initial condition (solution includes no SiO2-NPs). But in the case of the aqueous solution which includes [C18mim][Cl], an increase in the SiO2-NPs concentration from 0 to 100 ppm reduced the IFT from 0.75 to 0.66 mN/m which means a 12% reduction in IFT values. A similar but stronger trend was also observed for the synthetic oil prepared using asphaltene fraction, especially for [C18mim][Cl] since the effect of NPs on the aqueous solutions including [C12mim][Cl] was insignificant which can be ignored. In detail, the measurements revealed that increasing the NPs concentration from 0 to 2000 ppm for the solution including [C12mim][Cl] led to a slight change in the IFT with no regular pattern while increasing the SiO2-NPs concentration from 0 to 100 ppm for the aqueous solution including [C18mim][Cl] ca reduce the IFT value from 0.58 to 0.46 which is about 20% reduction in IFT. But a further increase in the SiO2-NPs from 100 to 2000 ppm faded away the reducing effect of these NPs on the IFT by modifying the IFT value to a value of 0.55 mN/m which is rather the same as the solution that includes no SiO2-NPs. The reducing effect of SiO2-NPs on the IFT can be correlated to at least two mechanisms that are related to dispersed NPs and available ions in the aqueous solution. In detail, Li et al. (Lee and Lee 2017) and Dahle (Dahle 2013) reported that it is possible to generate a monolayer structure at the interface of the saline nanofluid and crude oil using NPs which can immerge a weak interfacial tension between immiscible phases which is almost similar to the function of the surfactants. The point is that the resulting concentration is directly proportional to NPs concentration in nanofluids meaning that the increase of the concentration intensifies this mechanism and decreases the IFT (Hendraningrat et al. 2013a, b). But, the point is that as the NPs concentration increases to a threshold, the effect of repulsive forces appears which move the SiO2-NPs to the bulk phase instead of the interface which reduces the density of the formed monolayer consequently leading to higher IFT values. In this way, it seems that there is an optimum concentration for SiO2-NPs leading to the minimum IFT value.
On the other hand, investigating the effect of SiO2-NPs on the CA values of different aqueous solutions and synthetic oils using carbonate rock thin sections revealed a significant effect of NPs to change the wettability toward more water-wet conditions. Similar results were reported by Nowrouzi et al. (Nowrouzi et al. 2019a) that the ability of the used nanofluids in reducing the CA is more effective than their effect on the IFT reduction. They reported that increasing the SiO2-NPs concentration to 1000 ppm with a particle size of 10 nm and 20 times seawater dilution reduces the CA to the minimum value of 21.36° which is a strongly water-wet condition.
The measured CAs revealed that the CA variation was less dependent on the IL type if the synthetic oil be the resinous type. On the other hand, the measurements revealed that for the ASO, increasing the chain length of the IL ([C18mim] [Cl]) leads to lower effect of SiO2-NPs on the wettability alteration toward water-wet condition. The probable reason behind this observed trend can be correlated to the possible repulsive forces that appeared between the aqueous solution comprised of complex [C18mim][Cl] + SiO2-NPs and asphaltenic molecules leading to less adsorption of complex molecules into the interface rendering the surface less water-wet. In total, the NPs can change the wettability of the rock surface using the wedge film mechanism while it is possible to reduce this effect as the ILs existed in the solution. In detail, ILs are chemicals with a bulky structure in which the bulk volume of their molecules increases as the alkyl chain length increases. So, the bulky structure of [C18mim][Cl] is larger than the [C12mim][Cl] molecules. Respecting this fact, it seems that the bulky structure of ILs can be a positive parameter to adsorb the NPs into their structure and easily transfer them to the rock surface where the IL molecules can orient due to the surfactant-like structure. On the other hand, as the bulky structure of ILs gets bigger, the probability of repulsive forces which can exist between the IL molecules and the active components existing on the rock surface increases which can act like a barrier and reduces the chance of NPs to be transferred into the rock surface for wettability alteration. According to this possible phenomenon, it seems that the repulsive forces that existed between the asphaltenic molecules and the ILs with C18 chain length are dominant which can reduce the chance of reaching NPs adsorbed on the [C18mim][Cl] to the surface moves the system toward less water-wet condition.
The other possible theory which can describe the CA and wettability change from oleophilic to hydrophilic is disjoining pressure since it directly increases the crude oil movement (Sun et al. 2017) which consequently affects the wettability alteration and IFT. In detail, Chengara et al. (2004) claimed that the nanofluids form a thin film on the solid surface by placing themselves in sorted layers, and in this regard entropy of the suspension increases through the higher freedom for NPs leading to an emerge in high pressure compared to the volume since the pressure profile figure is dependent on the size and shape of the NPs. This diffused film on the rock surface is capable of separating oil, paraffin, water, and gas from the surface (McElfresh et al. 2012a, b).
The formed thin film of NPs is a result of a pressure named structural joining pressure, the Brownian motion and electrostatic repulsion forces (Aveyard et al. 2003). In brief, Aveyard and his coworkers claimed that NPs size and concentration, salinity and fluid temperature, and rock surface properties are influential to develop the formed layer. For example, when the concentration of NPs in the nanofluid was increased, the film will start to expand on a solid surface leading to more reduction in CA at higher concentrations.
Conclusions
In the current investigation, a hybrid chemical solution based on the simultaneous dissolution of ionic liquids (ILs), namely 1-dodecyl-3-methyl imidazolium chloride ([C12mim][Cl]) and 1–octadecyl-3-methyl imidazolium chloride ([C18mim][Cl]) and silicon oxide nanoparticles (SiO2-NPs), was proposed for IFT reduction and wettability alteration using the pendant drop approach. Besides the proposed new chemical formulation, the effects of these solutions were investigated on synthetic oils including resin or asphaltene fraction for the first time on the interfacial tension (IFT) and contact angle (CA) values. In this way, the concentrations of ILs ranged between 100 and 1000 ppm, SiO2-NPs ranged between 100 and 2000 ppm, and the concentrations of resin and asphaltene fractions dissolved in the toluene ranged between 1 and 5 wt%. According to the performed measurements, the following results can be highlighted:
-
The presence of resin or asphaltene fractions in the toluene can reduce the IFT values which is an indication of the surfactant nature of these fractions.
-
The presence of ILs, especially [C18mim][C] in the range of 100–1000 ppm, reduced the IFT to values of 1.3, 0.7, and 0.75 mN/m for resin concentrations of 1, 3, and 5 wt%, respectively.
-
Measured contact angles revealed the effect of ILs for moving the rock surface toward water-wet conditions although increasing the resin concentration from 1 to 5 wt% can reduce the effect of [C12mim][Cl] for wettability alteration.
-
Concerning the measured CA values for the resin/[C18mim][Cl], it seems that there is a shifting concentration for resin (3 wt%) where the effect of [C18mim][Cl] on the wettability alteration changes to being more effective for wettability alteration toward water-wet condition.
-
The results showed that although the asphaltene fraction is more effective for IFT reduction than the resin fraction, the dissolution of ILs can reduce the IFT to the values which are close to the IFT of systems including the resin fraction. In other words, regardless of the fractions used to prepare the synthetic oils, the presence of ILs, especially [C18mim][Cl], which has a longer chain length significantly changes the IFT values to a very low value.
-
The contact angle measurements revealed that for 3 wt% asphaltene and IL concentrations below critical micelle concentration (CMC) value, the wettability reduced compared with the higher concentration of asphaltene (5 wt%) while further increase in the [C12mim][Cl] concentration to 500 ppm and 1000 ppm which is the CMC value and higher than that value, the wettability alteration pattern turn to moving toward more water-wet condition instead of being more oil-wet.
-
The results also revealed that in the case of [C18mim][Cl], there is a shifting point where the reducing pattern in the CA values changes to an increasing pattern. This observed trend can be correlated to the higher concentration of asphaltene molecules at the interface and their interactions with the IL molecules leading to repulsive forces that put the IL molecules away from the rock interface leading to the less water-wet condition compared with the synthetic oils comprised of a lower amount of asphaltene fraction.
-
The measurements regarding the effect of SiO2-NPs on the IFT revealed no meaningful effect of SiO2-NPs on the IFT reduction although it has a significant effect on the wettability alteration of rock surfaces toward strongly water-wet conditions of 29.2° and 28.3° using 1000 ppm of [C12mim][Cl] and [C18mim][Cl] and 2000 ppm of SiO2-NPs for resinous synthetic oil (5 wt%).
-
Finally, the measurements revealed the higher impact of SiO2-NPs for wettability alteration toward strongly water-wet conditions using [C12mim][Cl] compared with the same concentrations of [C18mim][Cl] which was correlated to the longer chain length of [C18mim][Cl] which provides repulsive forces put the SiO2-NPs away from the rock surface to provide more stabilize condition.
Abbreviations
- D :
-
Equatorial diameter, m
- d :
-
Diameter at the distance D from the top of the drop, m
- g :
-
Acceleration of gravity, m s−2
- H :
-
Shape-dependent parameter
- γ :
-
Interfacial tension
- Δ :
-
Difference between two parameters
- ρ :
-
Density, kg m−3
- Al2O3 :
-
Aluminum oxide
- ASO:
-
Asphaltenic synthetic oil
- CA:
-
Contact angle
- CMC:
-
Critical micelle concentration
- CuO:
-
Copper oxide
- EOR:
-
Enhanced oil recovery
- Fe2O3 :
-
Iron oxide
- FTIR:
-
Fourier-transform infrared spectroscopy
- GC:
-
Gas chromatography
- IFT:
-
Interfacial tension
- ILs:
-
Ionic liquids
- MgO:
-
Magnesium oxide
- MgSO4 :
-
Magnesium sulfate
- NISOC:
-
National Iranian South Oil Company
- NPs:
-
Nanoparticle
- OOIP:
-
Original oil in place
- ppm:
-
Part per million
- PSNP:
-
Polysilicon NPs
- RSO:
-
Resinous synthetic oil
- SiO2 :
-
Silicon oxide
- SiO2-NPs:
-
Silicon oxide nanoparticles
- TAN:
-
Total acid number
- TiO2 :
-
Titanium oxide
- wt%:
-
Percentage by weight
- [C18mim][Cl]:
-
1-Octadecyl-3-methyl imidazolium chloride
- [C12mim][Cl]:
-
1-Dodecyl-3-methyl imidazolium chloride
References
Abbood NK, Mayahi N, Hosseini S (2022) Effect of SiO2 nanoparticles+ 1-dodecyl-3-methyl imidazolium chloride on the IFT and wettability alteration at the presence of asphaltenic-synthetic oil. J Pet Explor Prod Technol. https://doi.org/10.1007/s13202-022-01509-x
Abhishek R, Kumar GS, Sapru R (2015) Wettability alteration in carbonate reservoirs using nanofluids. Pet Sci Technol 33(7):794–801. https://doi.org/10.1080/10916466.2015.1014967
Adamson AW, Gast AP (1967) Physical chemistry of surfaces. Interscience publishers, New York
Al-Anssari S, Barifcani A, Wang S, Maxim L, Iglauer S (2016) Wettability alteration of oil-wet carbonate by silica nanofluid. J Colloid Interface Sci 461:435–442. https://doi.org/10.1016/j.jcis.2015.09.051
Amin JS, Nikooee E, Ghatee M, Ayatollahi S, Alamdari A, Sedghamiz T (2011) Investigating the effect of different asphaltene structures on surface topography and wettability alteration. Appl Surf Sci 257(20):8341–8349. https://doi.org/10.1016/j.apsusc.2011.03.123
Amirsadat SA, Moradi B, Hezave AZ, Najimi S, Farsangi MH (2017) Investigating the effect of nano-silica on efficiency of the foam in enhanced oil recovery. Korean J Chem Eng 34(12):3119–3123. https://doi.org/10.1007/s11814-017-0242-7
Andreas J, Hauser E, Tucker W (2002) Boundary tension by pendant drops1. J Phys Chem 42(8):1001–1019. https://doi.org/10.1021/j100903a002
Anganaei H, Pourabdollah K, Rostami A (2014) Experimental improvement of nano-enhanced oil recovery using nano-emulsions. Arab J Sci Eng 39(8):6453–6461. https://doi.org/10.1007/s13369-014-1258-5
Aske N, Kallevik H, Sjöblom J (2001) Determination of saturate, aromatic, resin, and asphaltenic (SARA) components in crude oils by means of infrared and near-infrared spectroscopy. Energy Fuels 15(5):1304–1312. https://doi.org/10.1021/ef010088h
Aveyard R, Binks BP, Clint JH (2003) Emulsions stabilised solely by colloidal particles. Adv Colloid Interface Sci 100:503–546. https://doi.org/10.1016/S0001-8686(02)00069-6
Ayirala SC, Vijapurapu CS, Rao DN (2006) Beneficial effects of wettability altering surfactants in oil-wet fractured reservoirs. J Pet Sci Eng 52(1–4):261–274. https://doi.org/10.1016/j.petrol.2006.03.019
Barahoei M, Hezave ZA, Sabbaghi S, Ayatollahi S (2016) Copper oxide nano-fluid stabilized by ionic liquid for enhancing thermal conductivity of reservoir formation: applicable for thermal enhanced oil recovery processes. Chem Ind Chem Eng Q 22(2):211–225. https://doi.org/10.2298/CICEQ150407035B
Bennett B, Larter S (1997) Partition behaviour of alkylphenols in crude oil/brine systems under subsurface conditions. Geochim Cosmochim Acta 61(20):4393–4402. https://doi.org/10.1016/S0016-7037(97)88537-7
Bobbo S, Fedele L, Benetti A, Colla L, Fabrizio M, Pagura C, Barison S (2012) Viscosity of water based SWCNH and TiO2 nanofluids. Exp Therm Fluid Sci 36:65–71. https://doi.org/10.1016/j.expthermflusci.2011.08.004
Chang H, Jwo C, Fan P, Pai S (2007) Process optimization and material properties for nanofluid manufacturing. J Adv Manuf Technol 34(3):300–306. https://doi.org/10.1007/s00170-006-0597-0
Chen L, Sharifzadeh M, Mac Dowell N, Welton T, Shah N, Hallett JP (2014) Inexpensive ionic liquids:[HSO4]−-based solvent production at bulk scale. Green Chem 16(6):3098–3106. https://doi.org/10.1039/C4GC00016A
Chengara A, Nikolov AD, Wasan DT, Trokhymchuk A, Henderson D (2004) Spreading of nanofluids driven by the structural disjoining pressure gradient. J Colloid Interface Sci 280(1):192–201. https://doi.org/10.1016/j.jcis.2004.07.005
Cheraghian G (2015) Effects of nanoparticles on wettability: a review on applications of nanotechnology in the enhanced oil recovery. Int Nano Lett. https://doi.org/10.1007/s40089-015-0173-4
Cheraghian G (2016) Effects of titanium dioxide nanoparticles on the efficiency of surfactant flooding of heavy oil in a glass micromodel. Pet Sci Technol 34(3):260–267. https://doi.org/10.1080/10916466.2015.1132233
Cheraghian G, Hendraningrat L (2016) A review on applications of nanotechnology in the enhanced oil recovery part B: effects of nanoparticles on flooding. Int Nano Lett 6(1):1–10. https://doi.org/10.1007/s40089-015-0170-7
Dahle G (2013) The effect of nanoparticles on oil/water interfacial tension. Project thesis, NTNU
de Castro Dantas TN, de Souza TTC, Neto AAD, de Alencar Moura MCP, de Barros Neto EL (2017) Experimental study of nanofluids applied in EOR processes. J Surfactants Deterg 20(5):1095–1104. https://doi.org/10.1007/s11743-017-1992-2
Demirbas A (2016) Deposition and flocculation of asphaltenes from crude oils. Pet Sci Technol 34(1):6–11. https://doi.org/10.1080/10916466.2015.1115875
Demirbas A, Taylan O (2015) Recovery of gasoline-range hydrocarbons from petroleum basic plastic wastes. Pet Sci Technol 33(23–24):1883–1889. https://doi.org/10.1080/10916466.2015.1110594
Demirbas A, Alidrisi H, Balubaid M (2015) API gravity, sulfur content, and desulfurization of crude oil. Pet Sci Technol 33(1):93–101. https://doi.org/10.1080/10916466.2014.950383
Dharaskar Swapnil A (2012) Ionic liquids (a review): the green solvents for petroleum and hydrocarbon industries. Res J Chem Sci ISSN 2231:606X
Domańska U (2005) Solubilities and thermophysical properties of ionic liquids. Pure Appl Chem 77(3):543–557. https://doi.org/10.1351/pac200577030543
Ehtesabi H, Ahadian MM, Taghikhani V (2015) Enhanced heavy oil recovery using TiO2 nanoparticles: investigation of deposition during transport in core plug. Energy Fuels 29(1):1–8. https://doi.org/10.1021/ef5015605
Fakher S, Ahdaya M, Elturki M, Imqam A (2020) Critical review of asphaltene properties and factors impacting its stability in crude oil. Pet Explor Prod Technol 10(3):1183–1200. https://doi.org/10.1007/s13202-019-00811-5
Ficken KJ, Wooller MJ, Swain D, Street-Perrott FA, Eglinton G (2002) Reconstruction of a subalpine grass-dominated ecosystem, Lake Rutundu, Mount Kenya: a novel multi-proxy approach. Palaeogeogr Palaeoclimatol Palaeoecol 177(1–2):137–149. https://doi.org/10.1016/s0031-0182(01)00356-x
Ghadimi A, Saidur R, Metselaar H (2011) A review of nanofluid stability properties and characterization in stationary conditions. Int J Heat Mass Transf 54(17–18):4051–4068. https://doi.org/10.1016/j.ijheatmasstransfer.2011.04.014
Hashemi R, Nassar NN, Pereira Almao P (2013) Enhanced heavy oil recovery by in situ prepared ultradispersed multimetallic nanoparticles: a study of hot fluid flooding for Athabasca bitumen recovery. Energy Fuels 27(4):2194–2201. https://doi.org/10.1021/ef3020537
Hendraningrat L, Li S, Torsæter O (2013a) A coreflood investigation of nanofluid enhanced oil recovery. J Pet Sci Eng 111:128–138. https://doi.org/10.1016/j.petrol.2013.07.003
Hendraningrat L, Li S, Torsaeter O (2013b) Enhancing oil recovery of low-permeability Berea sandstone through optimized nanofluids concentration. Paper presented at the SPE enhanced oil recovery conference. https://doi.org/10.2118/165283-MS
Hezave AZ, Dorostkar S, Ayatollahi S, Nabipour M, Hemmateenejad B (2013a) Dynamic interfacial tension behavior between heavy crude oil and ionic liquid solution (1-dodecyl-3-methylimidazolium chloride ([C12mim][Cl]+ distilled or saline water/heavy crude oil)) as a new surfactant. J Mol Liq 187:83–89. https://doi.org/10.1016/j.molliq.2013.05.007
Hezave AZ, Dorostkar S, Ayatollahi S, Nabipour M, Hemmateenejad B (2013b) Effect of different families (imidazolium and pyridinium) of ionic liquids-based surfactants on interfacial tension of water/crude oil system. Fluid Phase Equilib 360:139–145. https://doi.org/10.1016/j.fluid.2013.09.025
Hezave AZ, Dorostkar S, Ayatollahi S, Nabipour M, Hemmateenejad B (2013c) Investigating the effect of ionic liquid (1-dodecyl-3-methylimidazolium chloride ([C12mim][Cl])) on the water/oil interfacial tension as a novel surfactant. Colloids Surf A Physicochem Eng Asp 421:63–71. https://doi.org/10.1016/j.colsurfa.2012.12.008
Hosseini S, Sabet M, Zeinolabedini Hezave A, Ayoub M, Elraies KA (2020) Effect of combination of cationic surfactant and salts on wettability alteration of carbonate rock. Energy Source Part A Recovery Util Environ Eff. https://doi.org/10.1080/15567036.2020.1778141
Jadhunandan P, Morrow NR (1995) Effect of wettability on waterflood recovery for crude-oil/brine/rock systems. SPE Reserv Eng 10(01):40–46. https://doi.org/10.2118/22597-PA
Joos P, Fang J, Serrien G (1992) Comments on some dynamic surface tension measurements by the dynamic bubble pressure method. J Colloid Interface Sci 151(1):144–149. https://doi.org/10.1016/0021-9797(92)90245-H
Khalil M, Jan BM, Tong CW, Berawi MA (2017) Advanced nanomaterials in oil and gas industry: design, application and challenges. Appl Energy 191:287–310. https://doi.org/10.1016/j.apenergy.2017.01.074
Khupse ND, Kumar A (2010) Ionic liquids: new materials with wide applications. Indian J Chem 49A:635–648
Lashkarbolooki M, Ayatollahi S, Riazi M (2014) Effect of salinity, resin, and asphaltene on the surface properties of acidic crude oil/smart water/rock system. Energy Fuels 28(11):6820–6829. https://doi.org/10.1021/ef5015692
Lashkarbolooki M, Riazi M, Ayatollahi S, Hezave AZ (2016) Synergy effects of ions, resin, and asphaltene on interfacial tension of acidic crude oil and low–high salinity brines. Fuel 165:75–85. https://doi.org/10.1016/j.fuel.2015.10.030
Lashkarbolooki M, Hezave AZ, Ayatollahi S (2019) The role of CO2 and ion type in the dynamic interfacial tension of acidic crude oil/carbonated brine. Pet Sci 16(4):850–858. https://doi.org/10.1007/s12182-019-0310-1
Lee JH, Lee KS (2017) Enhanced wettability modification and CO2 solubility effect by carbonated low salinity water injection in carbonate reservoirs. J Chem 5:1–10. https://doi.org/10.1155/2017/8142032
Martins MA, Neves CM, Kurnia KA, Luís A, Santos LM, Freire MG, Coutinho JA (2014) Impact of the cation symmetry on the mutual solubilities between water and imidazolium-based ionic liquids. Fluid Phase Equilib 375:161–167. https://doi.org/10.1016/j.fluid.2014.05.013
McElfresh P, Holcomb D, Ector D (2012a) Application of nanofluid technology to improve recovery in oil and gas wells. Paper presented at the SPE international oilfield nanotechnology conference and exhibition. https://doi.org/10.2118/154827-MS
McElfresh P, Olguin C, Ector D (2012b) The application of nanoparticle dispersions to remove paraffin and polymer filter cake damage. Paper presented at the SPE international symposium and exhibition on formation damage control. https://doi.org/10.2118/151848-MS
Miller R (1982) Hydrocarbon class fractionation with bonded-phase liquid chromatography. Anal Chem 54(11):1742–1746. https://doi.org/10.1021/ac00248a021
Moradi B, Pourafshary P, Jalali F, Mohammadi M, Emadi M (2015) Experimental study of water-based nanofluid alternating gas injection as a novel enhanced oil-recovery method in oil-wet carbonate reservoirs. J Nat Gas Sci Eng 27:64–73. https://doi.org/10.1016/j.jngse.2015.07.009
Muhammad I, Tijjani N, Dioha I, Musa A, Sale H, Lawal A (2013) SARA separation and determination of concentration levels of some heavy metals in organic fractions of Nigerian crude oil. Chem Mater Res 3(4):7–14
Najimi S, Nowrouzi I, Manshad AK, Farsangi MH, Hezave AZ, Ali JA, Mohammadi AH (2019) Investigating the effect of [C8Py][Cl] and [C18Py][Cl] ionic liquids on the water/oil interfacial tension by considering Taguchi method. J Pet Explor Prod Technol 9(4):2933–2941. https://doi.org/10.1007/s13202-019-0688-8
Najimi S, Nowrouzi I, Khaksar Manshad A, Mohammadi AH (2020) Experimental study of the performances of commercial surfactants in reducing interfacial tension and wettability alteration in the process of chemical water injection into carbonate reservoirs. J Pet Explor Prod Technol 10(4):1551–1563. https://doi.org/10.1007/s13202-019-00789-0
Nowrouzi I, Manshad AK, Mohammadi AH (2019a) Effects of concentration and size of TiO2 nano-particles on the performance of smart water in wettability alteration and oil production under spontaneous imbibition. J Pet Sci Eng 183:106357. https://doi.org/10.1016/j.petrol.2019.106357
Nowrouzi I, Manshad AK, Mohammadi AH (2019b) Effects of TiO2, MgO, and γ-Al2O3 nano-particles in carbonated water on water-oil interfacial tension (IFT) reduction in chemical enhanced oil recovery (CEOR) process. J Mol Liq 292:111348. https://doi.org/10.1016/J.MOLLIQ.2019.111348
Nowrouzi I, Manshad AK, Mohammadi AH (2020a) Effects of TiO2, MgO and γ-Al2O3 nano-particles on wettability alteration and oil production under carbonated nano-fluid imbibition in carbonate oil reservoirs. Fuel 259:116110. https://doi.org/10.1016/j.fuel.2019.116110
Nowrouzi I, Mohammadi AH, Manshad AK (2020b) Water-oil interfacial tension (IFT) reduction and wettability alteration in surfactant flooding process using extracted saponin from Anabasis Setifera plant. J Pet Sci Eng 189:106901. https://doi.org/10.1016/j.petrol.2019.106901
Nowrouzi I, Mohammadi AH, Manshad AK (2021a) Chemical enhanced oil recovery by different scenarios of slug injection into carbonate/sandstone composite oil reservoirs using an anionic surfactant derived from rapeseed oil. Energy Fuels 35(2):1248–1258. https://doi.org/10.1021/acs.energyfuels.0c03385
Nowrouzi I, Mohammadi AH, Manshad AK (2021b) Double-Chain Single-Head modification of extracted saponin from Anabasis Setifera plant and its effects on chemical enhanced oil recovery process by surfactant-alkali slug injection into carbonate oil reservoirs. J Pet Sci Eng 201:108438. https://doi.org/10.1016/j.petrol.2021.108438
Nowrouzi I, Mohammadi AH, Manshad AK (2021c) Synergic effects of dissolved carbon dioxide and an anionic surfactant synthesized from Rapeseed oil on interfacial tension (IFT) reduction, wettability alteration, and oil swelling in the process of chemical water injection into carbonate oil reservoirs. Fuel 290:120011. https://doi.org/10.1016/j.fuel.2020.120011
Nowrouzi I, Khaksar Manshad A, Mohammadi AH (2022a) Effects of MgO, γ-Al2O3, and TiO2 nanoparticles at low concentrations on interfacial tension (IFT), rock wettability, and oil recovery by spontaneous imbibition in the process of smart nanofluid injection into carbonate reservoirs. ACS Omega 7(26):22161–22172. https://doi.org/10.1021/acsomega.1c07134
Nowrouzi I, Mohammadi AH, Manshad AK (2022b) Preliminary evaluation of a natural surfactant extracted from Myrtus communis plant for enhancing oil recovery from carbonate oil reservoirs. J Pet Explor Prod Technol 12(3):783–792. https://doi.org/10.1007/s13202-021-01336-6
Onyekonwu MO, Ogolo NA (2010) Investigating the use of nanoparticles in enhancing oil recovery. Paper presented at the Nigeria Annual international conference and exhibition. https://doi.org/10.2118/140744-MS
Overton EB, Wade TL, Radović JR, Meyer BM, Miles MS, Larter SR (2016) Chemical composition of Macondo and other crude oils and compositional alterations during oil spills. Oceanography 29(3):50–63. https://doi.org/10.5670/oceanog.2016.62
Painter P, Williams P, Mannebach E (2010) Recovery of bitumen from oil or tar sands using ionic liquids. Energy Fuels 24(2):1094–1098. https://doi.org/10.1021/ef9009586
Petroleum I (1985) IP standards for petroleum and Its products: methods for analysis and testing
Rajaei H, Hezave AZ, Lashkarbolooki M, Esmaeilzadeh F (2013) Representing experimental solubility of phenylephrine hydrochloride in supercritical carbon dioxide and modeling solute solubility using semi-empirical correlations. J Supercrit Fluids 75:181–186. https://doi.org/10.1016/j.supflu.2012.11.014
Rodríguez-Palmeiro I, Rodríguez-Escontrela I, Rodríguez O, Arce A, Soto A (2015) Characterization and interfacial properties of the surfactant ionic liquid 1-dodecyl-3-methyl imidazolium acetate for enhanced oil recovery. RSC Adv 5(47):37392–37398. https://doi.org/10.1039/C5RA05247E
Rodriguez Pin E, Roberts M, Yu H, Huh C, Bryant SL (2009) Enhanced migration of surface-treated nanoparticles in sedimentary rocks. Paper presented at the SPE annual technical conference and exhibition. https://doi.org/10.2118/124418-MS
Saien J, Gorji AM (2017) Simultaneous adsorption of CTAB surfactant and magnetite nanoparticles on the interfacial tension of n-hexane–water. J Mol Liq 242:1027–1034. https://doi.org/10.1016/j.molliq.2017.07.115
Salathiel R (1973) Oil recovery by surface film drainage in mixed-wettability rocks. J Pet Technol 25(10):1216–1224. https://doi.org/10.2118/4104-PA
Schramm LL (2000) Surfactants: fundamentals and applications in the petroleum industry. Cambridge university press
Seid Mohammadi M, Moghadasi J, Naseri S (2014) An experimental investigation of wettability alteration in carbonate reservoir using γ-Al2O3 nanoparticles. IJOGST 3(2):18–26. https://doi.org/10.22050/ijogst.2014.6034
Sheng JJ (2010) Modern chemical enhanced oil recovery: theory and practice. Gulf Professional Publishing
Smit B, Hilbers P, Esselink K, Rupert L, Van Os N, Schlijper A (1991) Structure of a water/oil interface in the presence of micelles: a computer simulation study. J Phys Chem 95(16):6361–6368. https://doi.org/10.1021/j100169a052
Soorghali F, Zolghadr A, Ayatollahi S (2014) Effect of resins on asphaltene deposition and the changes of surface properties at different pressures: a microstructure study. Energy Fuels 28(4):2415–2421. https://doi.org/10.1021/ef500020n
Spinler E, Zornes D, Tobola D, Moradi-Araghi A (2000) Enhancement of oil recovery using a low concentration of surfactant to improve spontaneous and forced imbibition in chalk. Paper presented at the SPE/DOE Improved Oil Recovery Symposium
Stauffer CE (1965) The measurement of surface tension by the pendant drop technique. J Phys Chem 69(6):1933–1938. https://doi.org/10.1021/j100890a024
Suleimanov BA, Ismailov F, Veliyev E (2011) Nanofluid for enhanced oil recovery. J Pet Sci Eng 78(2):431–437. https://doi.org/10.1016/j.petrol.2011.06.014
Sun X, Zhang Y, Chen G, Gai Z (2017) Application of nanoparticles in enhanced oil recovery: a critical review of recent progress. Energies 10(3):345. https://doi.org/10.3390/en10030345
Tarek M (2015) Investigating nano-fluid mixture effects to enhance oil recovery. Paper presented at the SPE Annual Technical Conference and Exhibition.https://doi.org/10.2118/178739-STU
Torrealba V, Johns R (2017) Coupled interfacial tension and phase behavior model based on micellar curvatures. Langmuir 33(47):13604–13614. https://doi.org/10.1021/acs.langmuir.7b03372
Torsater O, Engeset B, Hendraningrat L, Suwarno S (2012) Improved oil recovery by nanofluids flooding: an experimental study. Paper presented at the SPE Kuwait international petroleum conference and exhibition. https://doi.org/10.2118/163335-MS
Ward A, Tordai L (1946) Time-dependence of boundary tensions of solutions I. The role of diffusion in time-effects. J Chem Phys 14(7):453–461. https://doi.org/10.1063/1.1724167
Wu J, Prausnitz J, Firoozabadi A (1998) Molecular-thermodynamic framework for asphaltene-oil equilibria. AIChE J 44(11):1188–1998. https://doi.org/10.1002/aic.690440516
Xin-heng C, Tian SB (2011) Review and comprehensive analysis of composition and origin of high acidity crude oils. China Pet Process Petrochem Technol 13(1):6
Yang Z, Li M, Peng B, Lin M, Dong Z, Ling Y (2014) Interfacial tension of CO2 and organic liquid under high pressure and temperature. Chin J Chem Eng 22(11–12):1302–1306. https://doi.org/10.1016/j.cjche.2014.09.042
Yarranton HW, Alboudwarej H, Jakher R (2000) Investigation of asphaltene association with vapor pressure osmometry and interfacial tension measurements. Ind Eng Chem Res 39(8):2916–2924. https://doi.org/10.1021/IE000073R
Yu W, Xie H (2012) A review on nanofluids: preparation, stability mechanisms, and applications. J Nanomater. https://doi.org/10.1155/2012/435873
Zabihi S, Faraji D, Rahnama Y, Zeinolabedini Hezave A, Ayatollahi S (2020a) Relative permeability measurement in carbonate rocks, the effects of conventional surfactants vs. Ionic liquid-based surfactants. J Dispers Sci Technol 41(12):1797–1811. https://doi.org/10.1080/01932691.2019.1637262
Zabihi S, Rahnama Y, Sharafi A, Borousan F, Zeinolabedini Hezave A, Shirazian S (2020b) Experimental solubility measurements of fenoprofen in supercritical carbon dioxide. J Chem Eng Data 65(4):1425–1434. https://doi.org/10.1021/acs.jced.9b00861
Zaid HM, Yahya N, Latiff NRA (2013) The effect of nanoparticles crystallite size on the recovery efficiency in dielectric nanofluid flooding. J Nano Res 21:103–108. https://doi.org/10.4028/www.scientific.net/JNanoR.21.103
Zeinolabedini Hezave A, Dorostkar S, Ayatollahi S, Nabipour M, Hemmateenejad B (2014) Mechanistic investigation on dynamic interfacial tension between crude oil and ionic liquid using mass transfer concept. J Dispers Sci Technol 35(10):1483–1491. https://doi.org/10.1080/01932691.2013.844075
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dahham, N.A., Abbood, N.K., Hosseini, S. et al. Investigation on the interactions of resinous and asphaltenic synthetic oils and silicon oxide nanoparticles stabilized by different ionic liquid-based surfactants: interfacial tension and wettability alteration studies. J Petrol Explor Prod Technol 13, 1963–1977 (2023). https://doi.org/10.1007/s13202-023-01650-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-023-01650-1