Abstract
Nanometer scaled pores are critical to studying gas shale reservoirs. In order to obtain the information of the evolution mechanism of nanoscale pore within lacustrine organic-rich shales, artificially matured shale samples from the Ordos Basin were treated using hydrous pyrolysis experiment. Low-temperature nitrogen adsorption, inductively coupled plasma atomic emission spectrometry (ICP-AES), and field emission scanning electron microscopy (FE-SEM) experiments were used to investigate the nanopore evolution with migration and precipitation of materials. The results show that the pore sizes were distributed from 1.1 to 500 nm, and the overall porosity tends to increase first and then decrease. The micropores and fine mesopores (< 10 nm) increased gradually from the 250 to the 350 °C, calcite appeared dissolution following a small peak of feldspar dissolution at this stage, and the CO2 reaches a partial pressure peak at 350 °C. The micropores, mesopores and macropores increased steeply from the 370 °C to the 450 °C. Organic pores were not developed until 350 °C, and well developed at 370 and 400 °C. Organic pores, intergranular pores of clay and intragranular pores of pyrite were well developed at 370 °C. The cumulative specific surface areas increased at 400 °C caused by the dehydration and transformation reaction of clay minerals. This study could provide a reference for the exploration of shale gas in lacustrine shales with different thermal maturities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unconventional hydrocarbon resources are 8.3 times as large as conventional oil and gas resources (USGS 2000, 2007; IEA 2008, 2009; Zou et al. 2012). Great efforts toward exploring unconventional tight oil and gas has been devoted and has been made significant breakthroughs in China, especially in the Sichuan Basin and Ordos Basin in recent years (Chen and Xiao 2014; Cui et al. 2013; Tang et al. 2015; Wu et al. 2015; Xue et al. 2015). The "three dessert zone" of oil and gas reservoirs are mostly distributed in the source reservoir interlayer symbiotic or close to the tight sandstone reservoir of the Triassic Yanchang formation with relatively good physical property (Yao et al. 2013).
Numerous laboratory experiments have been performed to investigate the static characterization of porosity in geological shale reservoirs (Alessa et al. 2021; Chalmers et al. 2012; Clarkson et al. 2013; Liang et al. 2014; Loucks et al. 2009; Sun et al. 2016; Yang et al. 2015a, b). The shale pore structure is the dominant factor influencing the capacity of an unconventional reservoir (Sakhaee-Pour and Li 2016; Gregg and Sing 1982), and the nanometer pores is the main channels of shale gas migration and storage within a shale (Yang et al. 2013; Xue et al. 2015). Nanoscale pores in shale are the carrier of free gas and adsorbed gas (Loucks et al. 2009; Ambrose et al. 2010; Sakhaee-Pour and Bryant 2012; Curtis et al. 2012), Besides, scholars have carried out related studies on how the pore structures changed with thermal evolution (Hu et al. 2015; Chen and Xiao 2014; Kuila et al. 2014; Tiwari et al. 2013; Curtis et al. 2012; Mastalerz et al. 2012; Clarkson et al. 2013). The methods of low-temperature nitrogen adsorption, argon ion polishing and scanning electron microscopy are widely used to directly observe the pore size, pore size and distribution of nanoscale under the microscope, and yielded substantial results regarding the evolutionary process of their pore type, size, shape, spatial distribution and connectivity (Alessa et al. 2021; Mastalerz et al. 2012; Sun et al. 2015; Xue et al. 2015; Yang et al. 2017).
However, the evolution law of nanopore is quite different between thermal simulation samples and actual geological samples with maturity differences in Ordos (Yin et al. 2018). The porosity of geological samples shows a trend of decreasing and then increasing, and the whole range of porosity is smaller. While the porosity of the thermal simulation sample presents a rule of increasing first and then decreasing, and the porosity is too large (Sun et al. 2015; Xue et al. 2015). Most of the thermal simulation experiments are columnar samples (Sun et al. 2015; Xue et al. 2015). Considering the influence of the lattice mechanical properties of columnar rock on the simulation experiment, we selected fragmented samples for thermal simulation experiments. Given the abovementioned facts, we chose the immature oil shales of the Yanchang Fm. in the Ordos Basin as an objective to investigate the evolution of pores. Artificially matured samples were obtained through hydrous pyrolysis experiments, which were conducted on the same samples with different lithostatic pressure, hydrodynamic pressure, heating rate, time and temperature. The evolution of the nanopore structure was analyzed using low-temperature N2 adsorption and field emission scanning electron microscopy (FE-SEM).
Geological setting
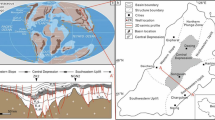
The study area is a part of the Weibei uplift region tectonically, which is located at Tongchuan city, south of the Ordos Basin (Fig. 1). According to the analysis of sedimentary records and lithofacies paleogeographic evolution history, the Ordos Basin experienced multiphase evolutionary stages from Paleozoic sedimentary marine facies, Mesozoic sedimentary continental facies, to Cenozoic basin peripheral fault-depression activities (Jiang et al. 2014). Mesozoic includes Upper Triassic Yanchang Formation, Lower Jurassic Fuxian Formation, and Yanan Formation (Ming 2006), which constitute 2 cycles and 3 oil and gas-bearing combinations vertically. According to lithology, electric and oil-bearing properties, Yanchang Formation was divided into 10 members (Chang1-Chang10 from top to bottom). The block samples were collected from Chang 7 Member, which was deposited at the stage of Lake Basin expansion with an area of 5 × 104 km2, and maximum water depth of 150 m (Hua 2010).
Materials and methods
Samples
The samples in this study were selected from the Triassic 7th Member of the Yanchang Formation in the Hejiafang area of the Ordos Basin. The bulk block samples were collected from an outcrop in an oil shale mine. Table 1 lists the TOC, RockeEval,and Vitrinite reflectance data of original shale. Before pyrolysis, the source rock was divided into seven aliquots to conduct parallel experiments, with one sample (HJF2-0) retained for comparison.
Pyrolysis
The pyrolysis experiments were conducted in a WYMN-3 HTHP simulation instrument at the Lanzhou Institute of Geology, Chinese Academy of Sciences (LIGCAS), as used by Sun et al. (2015), Wu et al. (2016) and Li et al. (2017). According to the buried history of the Upper Triassic Yanchang Formation, the fluid pressure set in the experiment is calculated. The experiments in this study investigated six different pressures: 16.9, 22.1, 32.5, 37.7, 42.9 and 52.2 MPa. Every experiment at a different pressure was conducted for 72 h. Table 2 lists the relevant conditions of the experiments.
Nitrogen adsorption
The low-pressure nitrogen adsorption measurements for quantified the changes of micropore were conducted with an ASAP 2020 HD88 surface area analyzer at the LIGCAS. The specific instrument parameters were described by Sun et al. (2015) and Yang et al. (2018) in detail.
Results and discussion
Nitrogen adsorption isotherms
Figure 2 shows the nitrogen adsorption–desorption isotherms of the shale samples at different simulation temperatures and pressures. The hysteresis loops for the original and thermal simulation samples are Type H3 indicating that intragranular pores with shapes of parallel and tubular holes with both ends open, which is irregular and open with good connectivity (Tran and Sakhaee-Pour 2018, 2019; Kruk and Jaroniec 2001; Xue et al. 2015; Yu et al. 2018). With the increase in simulation buried depth, the adsorbed nitrogen quantities increased compared to that of the unheated sample, with adsorbed quantities of 4.18, 3.85, 2.91, 2.50, 4.22, 22.18 and 6.57 cm3/g. Determine the pore volume size in the adsorption capacity of P/P0 ≈ 1 the size of the sample(Yang et al. 2014), indicating that more samples and large pore development, it can be seen that the sample HJF2-5 has the maximum pore volume, while the sample HJF2-3 has the smallest.
Response of pore structure parameters to temperature
Table 3 summarizes the detailed pore structure parameters at the different simulation condition. The cumulative specific surface area ranged from 0.587 to 6.2449 m2/g with an average of 2.0507 m2/g. Figure 3 plots the relationship between the specific surface area and the average diameter, and cumulative specific surface area. We can roughly divide the temperatures into two stages. From the unheated sample to 400 °C (Fig. 3a), the specific surface area increased rapidly with a peak of 1–2 nm and a peak of 2–50 nm (Fig. 3b). From 400 to 450 °C, the specific surface area reduced gradually.
Figure 4 shows the distributions of the pore volumes and cumulative pore volumes. The cumulative pore volume ranged from 0.004442 to 5.569 cm3/g with an average of 1.1216 cm3/g. In these samples, the pore size distributions ranged from 1.7 to 500 nm with an average of 29.27 nm.
According to the IUPAC classifications (Zapata and Sakhaee-Pour 2016; Alipour et al. 2022; Gregg and Sing 1982; Sing 2009), pores are subdivided into three parts: macropores (pore diameter > 50 nm), mesopores (50 nm > pore diameter > 2 nm) and micropores (pore diameter < 2 nm). Generally, the pore volumes of the micropores, mesopores and macropores increased with an increase in the simulated temperature and pressure (Sun et al. 2015).
However, before 350 °C, the pore volume of simulated samples are lower than that of original sample (Fig. 4b). The main reason for this may be that the pressure plays a leading role in the diagenesis of this stage. After 350 °C, the volume of the sample pore is larger than that of the original sample, which is caused by hydrocarbon generation and dissolution of organic matte. At 400 °C, the volumes of micropores, mesopores and macropores increased steeply, especially for the macropores, the cumulative volume of which was much higher than those of the previous samples. When the temperature and pressure exceeded 374 °C, 22.1 MPa, respectively, the water will be vaporized. The gasification water has great influence on the pore volume. The gasification exists only in theory instead of geological condition. After 400 °C, even with further increases of the simulated temperature and pressure, the cumulative pore volume reduced gradually.
Figure 5 shows the linear relationships of the cumulative pore volume and specific surface area with the average diameter at different final simulation temperatures. There is a significant correlation-ship between BET surface area and micropore, mesoporous, macropore, and total pore volume, with correlation coefficients (R2) of 0.89 (Fig. 5a), 0.88 (Fig. 5b), 0.84 (Fig. 5c) and 0.81(Fig. 5d), respectively. It is shown that in the process of buried depth, the pore volumes are changed with diagenesis. The changes of specific surface area are the result of compaction effect and organic matter generating hydrocarbon, the main contributors to the specific surface area are micropores and fine mesopores
Morphological and distribution features of pore characteristics
The seven samples (original sample and simulated samples) were observed by scanning electron microscope (SEM). The morphological changes of the pores are varied during the process of thermal evolution (Fig. 6). The overall porosity tends to increase first and then decrease. Scanning electron microscopy (SEM) showed that the amount of pores developed in the shale were mainly inorganic pores, relatively few organic pores, and a few microfractures. Inorganic pores mainly include intergranular pores, pyrite microspheres, intergranular pores and intragranular pores. The micropore types of original samples are mainly primary residual pores, secondary dissolution pores (Fig. 6a), clay mineral intergranular pores (Fig. 6b), and pyrite intergranular pores. Meanwhile, some shrinkage pores formed by epigenetic action are developed. The organic pores were poorly developed at 250 °C and 300 °C (Fig. 6a–c). As the increase in simulated burial depth, the hydrocarbon generation of organic matter was strengthened and the organic pore increased. Organic pores began to develop at 350 °C (Fig. 6d) and were well developed at 370 and 400 °C (Fig. 6e, h). The main reason for the rapid increase in the porosity was the intense hydrocarbon generation causing organic pore development at 350 °C, after which the porosity decreased gradually and the transformed organic matter began to stabilize, resulting in a reduction in the rate of pore development at 370 °C.
Microscopic pore structure characteristics of samples with different simulated conditions: a unheated sample, secondary dissolution pores were developed, b at 250 °C, intergranular pore of flaky clay minerals were developed. c At 300 °C, organic pores were not developed. d At 350 °C, localized organic pores were developed, and clay mineral dissolution pores were developed. e At 370 °C, organic pores, intragranular pores of pyrite and dissolution pores of feldspar were greatly developed. f At 370 °C, feldspar dissolution pores were developed. g At 370 °C, the interlayer pores of clay minerals were developed, and accompanied by filamentous illite formation. h At 370 °C, organic pores and intragranular pores of pyrite were greatly developed. i At 370 °C, interlayer seams of clay minerals were developed. j At 400 °C, micro-crack were greatly developed
The intragranular pores of pyrite were not developed in the unheated sample (Fig. 6a) and were well developed at 400 °C (Fig. 6g, h). The smectite mixed layer would disappear, and that the amount of illite would increase after 370 °C (Fig. 6g). Thus, organic pores are not as good as inorganic pores, especially in the sample of HJF2-5 at 400 °C, the interlayer pores of clay minerals are obviously increased, and the pores are much larger than the volume of the organic pores (Fig. 6e–h). Many studies claimed that the TOC content is the main factor that controls the development of nanoscale pores (Zeng et al. 2014; Loucks et al. 2009). Jarvie et al. (2007) held that the porosity would increase 4.9% by per consumption of 35% of the TOC for shale with a TOC content of 7.0%. Before 300 °C, the thermal evolution was low maturity stage, which was a small amount of organic reduction, the pores developed are mainly inorganic pores. After 350 °C, localized organic pores were began developed and then increased gradually with the changed of TOC from 20.3% to 12.1%.
The generation and migration of pyrolysis inorganic minerals
Dissolved Na, Mg, K, Ca, Al, Mn and Si of minerals in mass exchange were determined (Fig. 4). The contents of Na+, K+, Ca2+, Si4+ and Mn2+ increased at 300 and 370 °C, respectively, reaching the maximum at 370 °C and then rapidly declining. The contents of Al3+, Mg2+ and P4+ are relatively low and remaining stable. The curves K+ Na+ and Ca2+ are similar, but the content of Na+ is obviously higher than others. Mg2+ has a small sudden increase at 370 °C, and then becomes stabile.
The acidic solution products experienced dissolution of feldspar minerals and partially transformed into other minerals. Some of them existed in the form of ions. The dissolutions of calcium feldspar and albite played a leading role at 250 °C (Fig. 6a). More feldspar minerals will release the corresponding ions in the dissolution process [Eq. (1)]:
Following the peak of feldspar dissolution, a small peak of calcite dissolution appeared. The dissolution of calcite belongs to the dissolve congruently [Eq. (2)]. Through the XRF element analysis (Fig. 7), the content of HJF2-2 presented a low Ca2+ concentration, but a high total ions concentration value, which indicates that the water–rock equilibrium transfer was easy to happen.
The reason for the observed behavior is the influence of calcite dissolution from other minerals in the reaction system [Eq. (3)]. For HJF2-3 samples, the corrosion degree of feldspar minerals reduced to a minimum value because of carbonate cementation. At 350 °C, as decarboxylation of acetic acid can result in CO2 points pressure, theoretically this diagenetic environment favors the dissolution of carbonate rocks. But the balance of pH (such as feldspar and kaolinite petrochemical) had a buffer. Therefore, CO2 could not produce organic acid dehydroxy reducing the pH, it caused the precipitation of carbonate, and leaded to the densification of the reservoir. For sample HJF2-3, the result of XRF analysis shows that the content of calcium oxide had a small peak, and indicated that the temperature point is influenced by the CO2 partial pressure.
The clay minerals transformation is affected by dissolution of feldspar minerals. After feldspar minerals are corroded, a large amount of K+, Al3+ and Si4+ will be released. The released dissolution product of feldspar precipitate into kaolinite, smectite and illite which will promote the transformation process of clay minerals, which is a reduction in volume of the reaction (Eqs. 3.4–6). The pore observation of illite indicated that it was the highest at 400 °C (Fig. 6g).
At 400 °C, the sample shows the highest values of K, Ca, Mg, Al, Si, Fe, S, Cl and P (Fig. 7). The dehydration reaction of clay minerals is consistent with the graphs of interlayer seams of clay by the electron microscope and the N2 adsorption. The dehydration of clay was adsorption water to constitution water in turn.
Conclusions
Lacustrine artificially matured shale samples were treated using hydrous pyrolysis. Low-temperature nitrogen adsorption, X-ray fluorescence, and field emission scanning electron microscopy (FE-SEM) experiments were used to investigate the nanopore evolution of artificially matured shale samples. The following conclusions were obtained:
-
(1)
The hysteresis loops corresponding to temperature ranges from unheated to 450 °C were Type H3, indicating that the pores developed were irregular and open with good connectivity between intragranular pores with shapes of parallel, and slit-like and open-ended tubes.
-
(2)
The pore sizes were distributed from 1.1 to 500 nm. From the 250 °C to the 325 °C, the micropores and fine mesopores (< 10 nm) increased gradually, but less than the unheated sample; at 370, 400 and 450 °C, the micropores, mesopores and macropores increased significantly.
-
(3)
The cumulative specific surface area and cumulative pore volume presented the same trend on the whole. The overall porosity tends to increase first and then decrease. The main contributors to the specific surface area are micropores and fine mesopores.
-
(4)
Organic pores were not developed until 350 °C, and well developed at 370 and 400 °C. Organic pores, intergranular pores of clay and intragranular pores of pyrite were well developed at 370 °C.
-
(5)
Calcite appeared dissolution following a small peak of feldspar dissolution, and influenced by the CO2 partial pressure at 350 °C. The clay minerals transformation have affected by dissolution of feldspar minerals. The smectite mixed layer would disappear, and that the amount of illite would increase at 400 °C.
References
Alessa S, Sakhaee-Pour A, Sadooni FN, Al-Kuwari HA (2021) Comprehensive pore size characterization of Midra shale. J Pet Sci Eng 203:108576
Alipour KM, Kasha A, Sakhaee-Pour A, Sadooni FN, Al-Kuwari HAS (2022) Empirical relation for capillary pressure in shale. Petrophys—SPWLA J Form Eval Reserv Descr 63(05):591–603
Ambrose RJ, Hartman RC,Campos MD, Akkutlu IY, Sondergeld C (2010) New pore-scale considerations for shale gas in place calculations. SPE 131772, presented at the uncowentional gas conference
Brunauer S, Deming LS, Deming WE et al (1940) On a theory of the van der waals adsorption of gases. J Am Chem Soc 62:1723–1732
Ceng WT, Zhang JC, Ding WL et al (2014) Characteristics and influence factors of nanopores in Yanchang Shale reservoir: a case study of Liuping-171 well in Erdos Basin. J China Coal Soc 39(6):1118–1126
Chalmers GR, Bustin RM, Power IM (2012) Characterization of gas shale pore systems by porosimetry, pycnometry, surface area, and field emission scanning electron microscopy/transmission electron microscopy image analyses: examples from the Barnett, Woodford, Haynesville, Marcellus, and Doig unit. AAPG Bull 96:1099–1119
Chen J, Xiao XM (2014) Evolution of nanoporosity in organic-rich shales during thermal maturation. Fuel 129:173–181
Chen SB, Zhu YM, Wang GY et al (2012) Structure characteristics and accumulation significance of nanopores in Longmaxi shale gas reservoir in the southern Sichuan Basin. J China Coal Soc 37(3):438-444(437)
Clarkson CR, Solano N, Bustin RM et al (2013) Pore structure characterization of North American shale gas reservoirs using USANS/SANS, gas adsorption, and mercury intrusion. Fuel 103:606–616
Cui JW, Zhu RK, Cui JG (2013) Relationship of porous evolution and residual hydrocarbon: evidence from modeling experiment with geological constrains. Acta Geol Sin 87(5):730–736
Curtis ME, Cardott BJ, Sondergeld CH et al (2012) Development of organic porosity in the Woodford Shale with increasing thermal maturity. Int J Coal Geol 103:26–31
Gregg SJ, Sing KSW (1982) Adsorption, surface area, and porosity. Academic Press, New York
Han H, Zhong NN, Chen C et al (2014) The gas potential of Jurassic continental shale in the middle-small basins, West China. Chin Sci Bull 59(9):809
Hu H, Zhang T, Wiggins-Camacho JD et al (2015) Experimental investigation of changes in methane adsorption of bitumen-free Woodford Shale with thermal maturation induced by hydrous pyrolysis. Mar Pet Geol 59:114–128
Jarvie DM, Hill RJ, Ruble TE et al (2007) Unconventional shale-gas systems: the Mississippian Barnett Shale of north-central Texas as one model for thermogenic shale-gas assessment. AAPG Bull 91:475–499
Jiang ZX, Zhang WZ, Liang C et al (2014) Characteristics and evaluation elements of shale oil reservoir. Acta Pet Sin 35(1):184–196
Kruk M, Jaroniec M (2001) Gas adsorption characterization of ordered organic−inorganic nanocomposite materials. Chem Mater 13:3169–3183
Kuila U, Mccarty DK, Derkowski A et al (2014) Nano-scale texture and porosity of organic matter and clay minerals in organic-rich mudrocks. Fuel 135:359–373
Liang X, Zhang TS, Yang Y et al (2014) Microscopic pore structure and its controlling factors of overmature shale in the Lower Cambrian Qiongzhusi Fm, northern Yunnan and Guizhou provinces of China. Nat Gas Ind 34(2):18–26
Loucks RG, Reed RM, Ruppel SC, Jarvie DM (2009) Morphology, genesis, and distribution of nanometer-scale pores in siliceous mudstones of the mississippian barnett shale. J Sediment Res 79:848–861
Mastalerz M, He L, Melnichenko YB et al (2012) Porosity of coal and shale: insights from gas adsorption and SANS/USANS techniques. Energy Fuel 26:5109–5120
Romero-Sarmiento M-F, Rouzaud J-N, Bernard S et al (2014) Evolution of Barnett Shale organic carbon structure and nanostructure with increasing maturation. Org Geochem 71:7–16
Sakhaee-Pour A, Bryant SL (2012) Gas permeability of shale. SPE Reserv Eval Eng 15(04):401–409
Sakhaee-Pour A, Li W (2016) Fractal dimensions of shale. J Nat Gas Sci Eng 30:578–582
Sing KSW (2009) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 57:2201–2218
Sun LN, Tuo JC, Zhang MF et al (2015) Formation and development of the pore structure in Chang 7 member oil-shale from Ordos Basin during organic matter evolution induced by hydrous pyrolysis. Fuel 158:549–557
Sun MD, Yu BS, Hu QH et al (2016) Pore characteristics of Longmaxi shale gas reservoir in the West of Guizhou, China: investigations using small-angle neutron scattering (SANS), helium pycnometry, and gas sorption isotherm. Int J Coal Geol 171:61–68
Tang X, Zhang JC, Jin ZJ et al (2015) Experimental investigation of thermal maturation on shale reservoir properties from hydrous pyrolysis of Chang 7 shale, Ordos Basin. Mar Pet Geol 64:165–172
Tiwari P, Deo M, Lin CL et al (2013) Characterization of oil shale pore structure before and after pyrolysis by using X-ray micro CT. Fuel 107:547–554
Tran H, Sakhaee-Pour A (2018) Critical properties (Tc, Pc) of shale gas at the core scale. Int J Heat Mass Transf 127:579–588
Tran H, Sakhaee-Pour A (2019) The compressibility factor (Z) of shale gas at the core scale. Petrophys- SPWLA J Form Eval Reserv Descr 60(04):494–506
Wu ST, Zhu RK, Cui JW et al (2015) Characteristics of lacustrine shale porosity evolution, Triassic Chang 7 Member, Ordos Basin, NW China. Pet Explor Dev 42(2):185–195
Wu YD, Ji LM, He C et al (2016) The effects of pressure and hydrocarbon expulsion on hydrocarbon generation during hydrous pyrolysis of type-I kerogen in source rock. J Nat Gas Sci Eng 34:1215–1224
Xue LH, Yang W, Zhong JA et al (2015) Porous evolution of the organic-rich shale from simulated experiment with geological constrains, samples from Yanchang Formation in Ordos Basin. Acta Geol Sin 38(1):970–978
Yang F, Ning ZF, Hu CP et al (2013) Characterization of microscopic pore structures in shale reservoirs. Acta Pet Sin 34(2):301–311
Yang F, Ning ZF, Wang Q et al (2014) Characterization of shale nano pore typing. Nat Gas Geosci 25(4):618–623
Yang S, Chen GJ, Lv CF et al (2015a) Evolution of nanopore structure in lacustrine organic-rich shales during thermal maturation from hydrous pyrolysis, Minhe Basin, West China. Mar Pet Geol 88:127–140
Yang W, Chen GJ, Lv CF et al (2015b) Micropore Characteristics of the organic-rich shale in the 7~(th) member of the Yanchang Formation in the Southeast of Ordos Basin. Nat Gas Geosci 26(3):418–426
Yu C, Tran H, Sakhaee-Pour A (2018) Pore size of shale based on acyclic pore model. Transp Porous Media 124(2):345–368
Zapata Y, Sakhaee-Pour A (2016) Modeling adsorption-desorption hysteresis in shales: acyclic pore model. Fuel 181:557–565
Zou CN, Zhu RK, Wu ST et al (2012) Types, characteristics, genesis and prospects of conventional and unconventional hydrocarbon accumulations:taking tight oil and tight gas in China as an instance. Acta Pet Sin 33(2):173–187
Acknowledgments
This work was supported by the Youth Science and Technology Foundation of Hebei GEO University (Grants No. QN202233) and Science and Technology Project of Hebei Education Department (Grants No. ZD2022057).
Funding
The funding was provided by Youth Science and Technology Foundation of Hebei GEO University (Grants No. QN202233) and Science and Technology Project of Hebei Education Department (Grants No. ZD2022057).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, F., Wang, F., Du, J. et al. Fractal characteristics of artificially matured lacustrine shales from Ordos Basin, West China. J Petrol Explor Prod Technol 13, 1703–1713 (2023). https://doi.org/10.1007/s13202-023-01637-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-023-01637-y