Abstract
The leakage of hydrocarbon fluids through cracks in the annular cement and CO2 storage is a major concern to the Petroleum Industry. A significant risk is posed when repairing leakage in a micro annuli channel with smaller apertures. A low-viscosity sealant that can generate a long-lasting resilient seal is desired. The solution to sealing these channels might lie in a novel application using nano-silica Gel. In this study, laboratory tests were carried out to examine the capabilities of nano-silica gels to seal the cracks. Analyzing its rheological property, the gel strengths of nano-silica gels were found to increase with an increase in nano-silica concentration. Additionally, it was discovered that as the concentration of nano-silica increases, the sealing and leakage pressures, defined as the pressures before and after water breakthrough, respectively, increase as well. With a typical 15% concentration of nano silica in gel, a sealing pressure gradient of 80.2 psi/in and a leakage pressure gradient of 30 psi/in at a leaking rate of 1 cc/min were noted. To validate the validity of the experimental results, a mathematical model was developed to predict the leakage rate of sealed fractures. The model suggests that the young’s modulus of sealant is a key property of nano-sealants and further investigations are needed to validate the mathematical model for quantitative use. This study suggests a novel strategy for enhancing cement zonal isolation and reducing cement failure in oil and gas sector.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most crucial activities in drilling operations is cementing engineering. There are many variables at play when a well fails. Because of changes in the downhole environment, cement is vulnerable to cracking during a well's life. It is never acceptable to compromise the integrity of the cement during drilling or production operations. Leaks may develop at any moment during an active life of the well if the cement is not properly completed and abandoned. Through channels within the cement or between the cement and its surroundings, fluids (water or hydrocarbons) can migrate. When the wellbore integrity is compromised, these channels form, enabling formation fluids to move from the formation to the surface. Water, gases, and hydrocarbon compounds that escape through cement pathways endanger people and the environment.
Carbon Capture and Storage (CCS) has become a key technology used for cutting CO2 emission to the atmosphere to mitigate climate change. However, the leakage of injected CO2 is one of the major environmental concerns. CO2 leakage through wells is one of the critical risks identified in CO2 sequestration operations (Duguid et al. 2013; Zhang and Guo 2014; Duguid et al. 2017; Guo 2017). The leak is usually attributed to the “flow behind pipe” which is referred to as fluid flow through channels/fractures/cracks in the annular space between well casing and formation rock. The channels are usually caused by insufficient placement of cement in the annulus. The fractures/cracks can be induced by the cyclic pressure and thermal loading in well operations (Goodwin and Cook 1992; Nygaard et al. 2014; Weideman and Nygaard 2014). Goodwin and Crook (1992) analyzed cement sheath stress failure in various conditions. CO2 attack to the cement sheath can accelerate the development of the cracks (Nguyen et al. 2021; Zhang et al. 2021).
The flow channels in the wellbore annulus can be plugged using the conventional cement squeezing technique because of the large cross-sectional areas open for cement slurry to flow (Benge 2009; Hossain and Amro 2010). Guo et al. (2017a, b) predicted radial cracks in cement sheath due to casing internal pressure. Duguid et al. (2017) found radial cracks of approximately 1/2-mm-wide and 35- mm-deep in the sidewall-cores from an actual cement sheath. These cracks are too narrow to be sealed by cement squeezing because of the high pressure required to inject cement slurry of high viscosity. Recently nano-particle solutions have been studied to seal the cracks. The leak-off assessment of nanoparticle-stabilized CO2 foams for fracture/crack applications was performed by Nguyen et al. (2022), Fu et al. (2022), and Olayiwola et al.(2022a). Olayiwola et al. (2022b) demonstrated promising properties of nanoparticle-based gels to seal fractures in lab conditions. However, gels are soft, and their sealing performance depends on their mechanical properties such as young’s modulus. It is highly desirable to know the effect of gel mechanical properties on its sealing performance under different crack conditions.
Investigation of CO2 leak rate through fractures/cracks sealed by nanoparticle falls in the study area of leak of seal with a gap. The fluid flow through the gap is fundamentally described by the Hagen–Poiseuille equation (Jousten 2008). Lorenz and Persson (2009) presented experimental result for the leak rate of a rubber seal with rectangular cross-section. They compared the result to a theoretical model developed based on the percolation theory and their contact mechanics theory. They found good agreement between theoretical model and experiment. Persson (2022) investigated fluid leakage around a rubber seal with cylindrical cross-section based on the percolation at the rough contact area. Uniform fluid pressure distribution along the gap was assumed in these investigations. This assumption is not valid for studying the fluid leak rate through fractures/cracks sealed by nanoparticle because the fluid pressure varies greatly along the fractures/cracks of hundreds of meters long in the axial orientation of wellbore.
An experimental set-up was used to look at the ability of Nano-silica gel to stop fluid leakage through channels in the cement. There hasn't been much research done on crosslinked micro-use gels in cement zonal isolation. Additionally, this study will provide petroleum engineers with a fundamental understanding of how to apply Nano-silica sealer for wellbore integrity applications. In this study, it will be examined how the concentration of nano-silica affects the gelation characteristics of the sealant, its ability to block routes, and its capacity to stop leakages. The laboratory investigations include rheological testing, gelation time predictions, blocking effectiveness, and mechanical properties. A prior assessment of CO2 leak rate through fractures sealed by nano-particle gels was carried out in this study based on experimental investigation and mathematical modeling. It was found that the CO2 leak rate is very sensitive to fracture/crack width and young’s modulus of gel, influenced by fracture/crack height and CO2 viscosity, and controlled by pressure differential.
Methodology
Materials
Nano-silica gel
Nouryon Pulp and Performance Chemicals Inc. supplied the nano silica used in this investigation. DI water and sodium chloride from Fisher Scientific were also used in this experiment. The nano silica utilized has grains that are 50 nm in size. According to the supply specification, the silica that is utilized is a colloidal, aqueous dispersion that is alkaline and has a solids content of around 50%. The surface-modified amorphous silica particles have a negative surface charge. The silica particles have a broad particle size variety, are discrete, and have a smooth, spherical form. For optimal performance as an emulsion stabilizer, the particles' surfaces have been treated with silanes. The dispersion physically resembles a white liquid that is a little bit more viscous than water. The material was used exactly as it was supplied from the company, with no chemical changes.
Brine
A brine solution was prepared using commercially available NaCl with a purity of 99.99 percent.
Cement core
All the cement core specimens used in this investigation were prepared with Class H cement and distilled water. Using a gas pycnometer, the specific gravity of the cement was determined to be 3.18. X-ray fluorescence spectroscopy was used to determine the chemical composition of Class-H cement (XRF).
Preparation of cement paste
The cement slurry was blended at room temperature using a bottom-drive blender with two speeds. A precise amount of water was poured to the blender, and dry cement was added in a consistent manner. The blender was then combined on low speed for around 15 s. The blender was then covered, and the mixing continued for an additional 35 s at high speed (API RP 10B-2). According to API specification 10A for API cement Class-H, the cement slurry's water/cement ratio (WCR) was 0.38. (API 2010).
Fracturing the cement cores
A two-inch diameter tube was filled with the cement slurry inside, closed on one end, and left open on the other for 72 h to solidify, resulting in the split cement cores. The cement core was taken out of the pipe after it had hardened. The dry core was then gently pressed against the tile-saw blade while it was running to split it into two halves. Using proppant or thin-metal plate, the two pieces of the core are then separated by the necessary fracture width, as depicted in Fig. 1.
Gel preparation
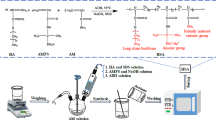
A simple experimental procedure was used to create silica gels at room temperature using Nano silica concentrations ranging from 15 to 40%, salt concentration, and water, as illustrated in Fig. 2. The required concentration of nano-silica particles was initially synthesized. The 3%-concentrated brines were then added to the nano-silica particle and given time to gel. The rheological and elastic characteristics of the resulting gel particles were then assessed using an Anton Par Rheometer. Figure 2a and b shows the appearance of the nano-silica gel before and after gelation. An OFITE 900 Rheometer was used to examine how the concentration of nano-silica affects gel's rheological and sealing properties.
Nano silica particles are dispersed in a stable inorganic aqueous solution as colloidal silica. It is distinct from other organic solutions due to the substance's ease of handling and environmental friendliness. The solution exists in ambient conditions as a low viscosity fluid, like water, which is advantageous for simple pumping. Colloidal silica is thought to gel because of particle collision, bonding, and aggregation into long-chain networks. pH, salts or cations, particle concentration level and temperature can cause Nano silica solution to gel. Hydroxyl ion (OH−) is drawn to the surface of silica in aqueous solution and forms the silanol (Si–OH) group.
The salt solution injected during the gelation process functions as an activator or accelerator. The generated SiO group begins connecting with Si–OH to produce the siloxane bond when an activator is present. Finally, it is possible to imagine that long chain siloxane linkages arise while silica gelation takes place. Gelation happens when silica particle aggregation leads to the formation of a consistent 3D structural network. When fracture is induced in the well cement, it may generate either macro or micro sized fracture depending on the degree of fracturing effect. Regardless of the fracture size, nano-silica dispersion can penetrate through the size of fracture because of its initial low viscosity.
Injectivity test
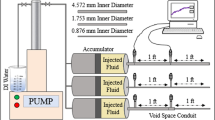
The gel's passage during the cement setting is described in Fig. 3. The apparatus consists of a 2-inch Core holder, two syringe pumps, and two accumulators with pistons. A 0.05-inch proppant was inserted between the two pieces of the broken core to gauge the fracture's width. Then, to create a complete core, the two sections are taped together. Then, confining pressure is applied to the core holder's rubber sleeve to stop fluid leaking around the fractured core and to simulate the confining pressure circumstances. Brine and nano-silica gel were injected into the core-holder through the accumulator. Pressure transducers were installed at the cell's intake, middle, and outflow to track the gel's performance along the fracture.
The Nano silica solution and the brine were co-injected in the laboratory core flooding test until the gel was formed inside the fractured core. An injection test was carried out after the cement core's cracks had been sealed with nano silica gel. The gel strength created by Nano silica imbedded in cement cracks during injection prevented fluid migration through the cracks until the gel lost its sealing strength, at which point water started to leak through. The pressure that Nano silica gel develops to close the fractures is referred to as the sealing pressure, whereas the pressure that develops after breakthrough is referred to as the leakage pressure.
The duration of the fluid blockage by Nano silica gel is a function of the gel strength developed during gelation process. This strongly support the fact that colloidal silica gel has enough strength to resist holdback pressure of reservoir fluid to escape through the fracture. The fundamental benefit of nano silica in maintaining well integrity is that the gelation mechanism accelerates in downhole conditions. The production of gel may be sped up when the solution enters the fracture aperture and meets the cement surface due to the possibility of bivalent Ca2+ leaching out of the cement. To simulate the initial condition where the cement concrete is already filled with formation fluid (normally water) before the silica injection, brine was pumped into the core-holder using an accumulator. After the brine was in place, a steady flow rate of 1 cc/min of nano-silica particle gel was injected into the core-holder.
The gel injection was continued till the formed gel was seen at the exit and the injection pressure differential across the fracture was steady. The injection pressure was measured by pressure sensors, and the angle of gel propagation across the fracture was monitored with a high-resolution camera. To test the gel's water sealing performance, brine was introduced into the gel packed fracture after the gel was set in place. The brine was injected at a continuous flow rate of 1 cc/min through an accumulator. The brine injection was continued till the pressure of the brine injection was stabilized. Pressure transducers were used to record pressure data in the testing period as shown in Fig. 3.
Equation (1) is applied to calculate the fracture conductivity during the injection test.
where Q (flowrate) in cc/min, µ (viscosity) in cp, \(L\left( {core length} \right)\) in inch, D (core diameter) in inch and \(C_{f}\) (fracture conductivity) in md-ft and, DP (pressure differential) in atmospheric pressure units (psi).
Equation (2) is used to determine the hydraulic aperture of the fracture, B.
In this equation, Hydraulic Aperture, B is expressed in inches, Flowrate, Q in cubic centimeters per minute, Viscosity, µ is expressed in centipoise (cp), Length of the core, L is expressed in inches, fracture width, wf is expressed in inches, and the pressure drop, DP is expressed in atmospheric pressure units (psi).
Leakage rate model
Figure 4 depicts a process of fluid-channeling in a gel-filled fracture. Due to the deficiency of gel placement in the fracture, fluid can enter the fracture under fluid pressure. Fluid squeezed into the gel-sealed fracture compresses the gel and induces stress and strain in the gel. The shrinkage of gel in the direction perpendicular to the fracture is equal to the width of fluid channel:
where wc(x) is the channel width at point x, e(x) is the strain in the gel at point x, and wf is fracture width which is assumed to be constant. The strain is expressed as
where s(x) is the stress at point x, p(x) is the fluid pressure at point x, and E is Young’s modulus of gel with nanoparticles. Substituting Eq. (4) into Eq. (3) gives:
Hagen–Poiseuille equation (Jousten 2008) takes the following differential form in consistent units:
where the fluid velocity can be expressed as
where h is the height (or depth) of fracture which is assumed to be constant. Substituting Eq. (7) into Eq. (6) yields:
Substituting Eq. (5) into Eq. (8) gives:
which is integrated as
where p1 is the pressure at flow inlet point (x = 0), p2 is the pressure at flow outlet point (x = L), and L is fracture length. The result of the integration is
This equation takes the following form in U.S. oilfield units.
where pressures p1 and p2 are in psi, dynamic viscosity m is in cp, young’s modulus E is in psi, flow rate Q is in ft3/s, fracture length L is in ft, fracture height (or depth) h is in inch, and fracture width wf is in inch. The flow rate is solved from Eq. (12) to obtain:
Results and discussions
This section summarizes experimental and model results of tests for gel strengths, sealing pressures, and leakage pressures of gels with different concentrations of nano-silica. The gel's capacity to plug cement fractures and reduce fluid migration through fractured cement was investigated using the Gel-Cement Plugging Efficiency setup. Using Eqs. (1) and (2), the fracture conductivity and the hydraulic aperture could be estimated.
The gel injection pressure is shown in Table 1 for various flowrates. The cement fracture that was employed in this study has a diameter of 2 inches, a length of 10 inches, and a width of 0.05 inches. The pressure of the gel injection increases with flowrate. The greatest flowrate of 4 cc/min was used to reach the peak pressure of 265 psi. When the flowrate was reduced to 3, 2, and 1 cc/min, the injection steady pressure fell to 173, 113, and 75 psi, respectively. The Darcy law of flow, which states that pressure drop increases as flowrate increases, is supported by these data. Average hydraulic aperture size and fracture conductivity for the cement core were found to be 0.64 md-ft and 0.08 inches, respectively, according to the results provided in Table 1.
Gel strength
Figure 5 demonstrates that the growth of gel strength is accelerated by increasing the concentration of nano-silica. The observed gel solution produced a gel strength of 504 100 lb/ft2 for a 15% nano-silica concentration, and when the nano-silica content was increased to 40%, the gel strength increased to 8,492.6 lb./100ft2. A saturation effect may be seen in the leveling-off above the nano-silica concentration of 35%. Due to the increased molecular weight brought on by the nano-silica particles' presence in the fluid, the gel's texture thickens as their concentration rises. The hydroxyl groups included in nano-silica particles' structure make it easier to create a filler network in polymer chains and increase gel strength. (Zareie et al. 2019). This is owing to the Nano-silica particle's aggressive filler network, whose rheological property increases as its concentration in the solution increases (Havet et al. 2003). This increase in rheological feature raises the corresponding gel strength by increasing the ionic strength of the mixture solution (Gallagher and Mitchell 2002).
Sealing and leakage pressures
Figure 6 displays the pressure curve for a fracture that was sealed using a 15% concentration nano-silica solution during the post-gel brine injection test. The sealing pressure, or greatest point of injection pressure, was 810 psi prior to brine breaching the sealant. The strength of the sealant is determined by this sealing pressure, which is equivalent to 802/10 = 80.2 psi/inch. Following the brine breakout, the injection pressure dropped to a steady leakage pressure of 300 psi. The flow must be kept at a leakage pressure gradient of 300/10 = 30 psi/inch. Therefore, the sealed fracture leaks at a rate of 1 cc/min when there is a 300-psi pressure difference.
The sealing pressures of gels with various amounts of nano-silica are shown in Fig. 7. It demonstrates how the sealing pressure rises as the concentration of nano-silica does. Under the 25% nano-silica concentration, the leveling-off indicating the saturation effect is not visible. Figure 8 depicts the leakage pressures of gels containing different concentrations of nano silica. It shows that the leakage pressure increases as nano silica concentration does. There is no leveling-off at a nano silica concentration of 25%, indicating the saturation effect.
Predicting the leakage rate with Eq. (13) requires the data for young’s modulus of sealant, the nano silica gel in this case. Unfortunately, this piece of information is not found from literature. Figure 9 presents a sensitivity test of fracture leakage rate against the uncertain value of Young’s modulus of sealant. It indicates that the leakage rate declines sharply with Young’s modulus according to Eq. (13). If the mathematical model is correct, the Young’s modulus of the nano-silica gel should be about 3050 psi for sealant with 15% nano-silica gel presented in Fig. 6.
Conclusions
A series of tests were carried out in this study to investigate the sealing properties of nano-silica gels for sealing underground fractures/cracks near oil/gas production and CO2 sequestration wellbores. The following conclusions are drawn.
-
1.
A resilient Silica seal can be created at room temperature by mixing Nano Silica solutions, Salt, and Water.
-
2.
Up to a concentration of 35% nano silica, the gel strength of nano silica gels gets stronger with increasing nano silica concentration. Above the 35% concentration, the line flattens out, illustrating the saturation effect.
-
3.
The concentration of nano-silica causes an increase in the sealing pressure, which is the maximum pressure before water breakthrough. Under the 25% nano-silica concentration, the leveling-off reflecting the saturation effect was not seen. The sealing pressure gradient is 80.2 psi/in when nano-silica is present in gel at a typical concentration of 15%.
-
4.
As the concentration of nano-silica increases, the leakage pressure—defined as the pressure that stabilizes after water breakthrough—increases. Under a 25% nano-silica concentration, the trend remains unchanged. To produce a leakage rate of 1 cc/min with a standard 15% concentration of nano-silica in gel, a leakage pressure gradient of 30 psi/in is needed.
-
5.
To anticipate the leakage rate of sealed fractures, a mathematical model was created. According to the model, the leakage rate of a sealed fracture is directly proportional to fracture height, fracture width raised to the third power, pressure differential raised to the fourth power, and inversely proportional to fluid viscosity, Young's modulus of sealant raised to the third power, and fracture length.
-
6.
The mathematical model's sensitivity analysis shows that as the sealant's Young's modulus is raised, the leakage rate of sealed fractures rapidly decreases. To efficiently seal cracks, strong sealants with high Young's modulus values need be created.
-
7.
Further investigations are needed to measure the Young’s modulus of nano-particle gels and validate the mathematical model for quantitative use. Without further validation, the model may only be used qualitatively for analyzing factors affecting the sealing performance of fracture sealants.
Abbreviations
- B:
-
Hydraulic Aperture, in
- Cf :
-
Fracture Conductivity, mD-ft
- D:
-
Core Diameter, in
- E:
-
Young’s Modulus, psi
- h:
-
Fracture height, in
- L:
-
Fracture length, ft
- Dp:
-
Pressure Drop, psi
- dp/dx:
-
Pressure gradient, psi/ft
- p(x):
-
Fluid pressure at point x, psi
- p1 :
-
Flow inlet pressure, psi
- p2 :
-
Flow outlet pressure, psi
- Q:
-
Fluid Flowrate, ft3/s
- v(x):
-
Fluid Velocity, ft/s
- wc :
-
Channel width, in
- wf :
-
Fracture width, in
- µ:
-
Dynamic viscosity, cp
- e:
-
Strain, psi
- s:
-
Stress, psi
- API:
-
American petroleum institute
- CCS:
-
Carbon capture and storage
- WCR:
-
Water cement ratio
- XRF:
-
X-ray fluorescence
- API:
-
American petroleum institute
- CCS:
-
Carbon capture and storage
- WCR:
-
Water cement ratio
- XRF:
-
X-ray fluorescence
References
Benge G (2009) Improving wellbore seal integrity in CO2 injection wells. SPE/IADC Drill Conf Exhib. https://doi.org/10.2118/119267-MS
Duguid A, Butsch R, Carey JW, Celia M, Chugunov N, Gasda S, Ramakrishnan TS, Stamp V, Wang J (2013) Pre-injection baseline data collection to establish existing wellbore leakage properties. Energy Proc 37:5661–5672. https://doi.org/10.1016/j.egypro.2013.06.488
Duguid A, Guo B, Nygaard R (2017) Well integrity assessment of monitoring wells at an active CO2-EOR flood. Energy Proc 114:5118–5138. https://doi.org/10.1016/j.egypro.2017.03.1667
Fu C, Guo B, Liu N (2022) Leak off assessment of nanoparticle-stabilized CO2 foams for fracturing applications. J Nat Gas Sci Eng 100:104493. https://doi.org/10.1016/j.jngse.2022.104493
Gallagher PM, Mitchell JK (2002) Influence of colloidal silica grout on liquefaction potential and cyclic undrained behavior of loose sand. Soil Dyn Earthq Eng 22:1017–1026. https://doi.org/10.1016/S0267-7261(02)00126-4
Goodwin KJ, Crook RJ (1992) Cement sheath stress failure. SPE Drill Eng 7:291–296. https://doi.org/10.2118/20453-PA
Guo B, Duguid A, Nygaard R (2017a) Statistical analysis of CO2 exposed wells to predict long term leakage through the development of an integrated neural-genetic algorithm. Univ of Louisiana at Lafayette. https://doi.org/10.2172/1373948
Guo B, Wei N, Song J, Lee J (2017b) Prediction of the maximum allowable bottom hole pressure in CO2 injection wells. J Petrol Sci Eng 156:575–581. https://doi.org/10.1016/j.petrol.2017.06.033
Havet G, Isayev AI (2003) A thermodynamic approach to the rheology of highly interactive filler-polymer mixtures. Part II. Comparison with polystyrene/nanosilica mixtures. Rheol Acta 42:47–55. https://doi.org/10.1007/s00397-002-0253-z
Hossain MM, Amro MM (2010) Drilling and completion challenges and remedies of CO2 injected wells with emphasis to mitigate well integrity issues. SPE Asia Pac Oil Gas Conf Exhib. https://doi.org/10.2118/133830-MS
Jousten K (2008) Handbook of vacuum technology. Wiley-VCH, Weinheim
Lorenz B, Persson BNJ (2009) Leak rate of seals: comparison of theory with experiment. Euro Phys Lett 86:44006. https://doi.org/10.1209/0295-5075/86/44006
Nguyen V, Olayiwola O, Guo B, Liu N (2021) Well cement degradation and wellbore integrity in geological CO2 storages: a literature review. Pet Petrochem Eng J. https://doi.org/10.23880/ppej-16000269
Nguyen V, Olayiwola O, Liu N, Guo B (2022) Investigation of nano-silica solution flow through cement cracks. Sustainability 15(1):577. https://doi.org/10.3390/su15010577
Nygaard R, Salehi S, Weideman B, Lavoie RG (2014) Effect of dynamic loading on wellbore leakage for the wabamun area CO2-sequestration project. J Can Pet Technol 53:69–82. https://doi.org/10.2118/146640-PA
Olayiwola O, Nguyen V, Yousuf N, Baudoin N, Liu N, Guo B (2022b) Experimental investigation of nano-silica gel properties for well integrity remediation. Energy Fuels 36:14094–14100. https://doi.org/10.1021/acs.energyfuels.2c02921
Olayiwola O, Nguyen V, Andres R, Liu N(2022a) The application of Nano-silica gel in sealing well micro-annuli and cement channeling. In: American associate of drilling engineering fluids technical conference and exhibition. https://www.aade.org/application/files/1516/5237/7567/AADE-22-FTCE-SPP-10_-_Olayiwola_-_Paper.pdf.
Persson BNJ (2022) Fluid Leakage in static rubber seals. Tribol Lett 70:31. https://doi.org/10.1007/s11249-022-01573-8
Weideman B, Nygaard R (2014) How cement operations affect your cement sheath short- and long-term integrity. In: American association of drilling engineers fluids technical conference and exhibition, Houston, Texas. AADE-14-FTCE-20
Zareie C, Bahramian AR, Sefti MV, Salehi MB (2019) Network-gel strength relationship and performance improvement of polyacrylamide hydrogel using nano-silica; with regards to application in oil wells conditions. J Mol Liq 278:512–520. https://doi.org/10.1016/j.molliq.2019.01.089
Zhang X, Guo B (2014) A review of CO2 behavior during geological storage and leakage assessment. Int J Recent Dev Eng Technol 3:14–23
Zhang P, Guo B, Liu N (2021) Numerical simulation of CO2 migration into cement sheath of oil/gas wells. J Nat Gas Sci Eng 94:104085. https://doi.org/10.1016/j.jngse.2021.104085
Acknowledgements
The authors are grateful to BIRD for supporting the project “Safe, sustainable, and resilient development of offshore reservoirs and natural gas upgrading through innovative science and technology: Gulf of Mexico – Mediterranean,” through Grant No. EC-19 Fossil Energy.
Funding
No external funding was used for this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Olayiwola, O., Nguyen, V., Liu, N. et al. Prior assessment of CO2 leak rate through cracks sealed by nanoparticle gels. J Petrol Explor Prod Technol 13, 1509–1517 (2023). https://doi.org/10.1007/s13202-023-01626-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-023-01626-1