Abstract
The study of interfacial tension (IFT) of fluid/liquid systems is vital to optimize the design and operation of liquid-fluid contactors, and in this case, the design and performance of carbon dioxide (CO2) dehydration by absorption using tri-ethylene glycol (TEG). Several studies on improving CO2 dehydration with an absorption column containing TEG focused on system simulation and modeling but so far neglect the impact of interfacial tension of TEG–CO2 on the overall system performance. This study analyzes the interfacial tension, fluid mixture densities and drop volumes of TEG and TEG + water in CO2 medium. The pendant drop and the oscillating u-tube methods with a high-pressure densitometer were used to measure the IFT and the mixture densities of TEG–CO2 and TEG + water–CO2, respectively, at temperatures ranging from 10 °C to 50 °C and pressures up to 250 bars. The saturated TEG–CO2 and TEG + water–CO2 densities were used to evaluate the IFT from recorded drop profiles. The static IFT of TEG–CO2 shows a strongly decreasing trend from above 40 mN/m to less than 5 mN/m as the pressure increases up to 250 bars at a constant temperature (30 °C) but strongly increases by a factor of nearly 6 as the temperature is raised from 10 °C to 50 °C at a fixed pressure of 70 bars. The same trend is observed for IFT of TEG–water–CO2, although a slight increase in IFT \((\sim\) 2 mN/m) is observed due a water content of 5 wt% which would have a negative effect on the mass transfer efficiency. Conversely, a lower interfacial tension at higher pressures and lower temperatures favors the formation of a larger interface which is advantageous for mass transfer. Dynamic IFT of TEG–CO2 attains a constant after around 5 min at 70 bars and 50 °C while the drop volume increases leveling off after 10 min corresponding to a volumetric expansion of nearly 14% which corresponds to a CO2 solubility in TEG of 14%. The drop volume of a pendant drop at higher pressures hardly depends on temperature due to the superposed counteracting effects of a slightly increasing buoyancy and slightly dropping IFT. For a process design, static values of IFT can be applied with a reasonable precision, e.g., for using We-correlations to estimate drop sizes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbon dioxide accounts for about 76% of the global greenhouse gas emissions (World Energy Outlook 2022) and approximately 34,000 million metric tons of carbon dioxide is released to the environment annually (Global Energy Review 2021). The vast majority of anthropogenic carbon dioxide emissions comes from combustion of fossil fuels, principally coal, petroleum (including oil and natural gas), with additional contributions from cement manufacturing, fertilizer production, deforestation and other changes in land use. As meeting the energy transformation in terms of decarbonization, carbon capture utilization and storage (CCUS) projects have been implemented and some CCUS are in planning stage around the world, mostly in European countries including Germany. CCUS projects include but are not limited to carbon dioxide transportation and storage, carbon dioxide enhanced oil recovery (CO2–EOR), carbon dioxide hydrogenation (carbon–neutral fuels), enhanced gas recovery (EGR) and CO2 conversion to ammonia in order to form urea for fertilizers. A recent study conducted by Rütters et al. (2022) aims at specifying a composition threshold for a safe transport, injection and storage. Captured carbon dioxide is practically always saturated with water which forms the major contaminant. Water must be removed from CO2 streams to prevent corrosion, gas hydrates and unwanted phase transition during CO2 transportation (Peletiri et al. 2018). The acceptable water content of CO2 ranges from 40 to 500 ppm depending on the specific use of CO2 (Buit et al. 2010). Also, Rütters et al. (2022) recommended a CO2 water content ≤ 50 ppm to prevent acid formation and limit pipeline corrosion. Most CO2 transportation pipelines are made of carbon steel which are susceptible to corrosion. In order to meet CO2 quality requirements and protect the pipeline and storage infrastructure, the moisture content must be continuously monitored. CO2 dehydration can principally take place by absorption, adsorption, cooling (compressor inter-stage cooling, adiabatic cooling via gas expansion), refrigeration, cryogenic (depending on the operating temperature) or membrane separation. Carbon dioxide dehydration using liquid desiccant in a countercurrent column contactor has been recommended as a viable CO2 dehydration option from several studies (Ahmari and Mufti 2022; Jumar 2021; Alhashboul et al. 2017; Hossain 2017; McKay and Maddocks 2012; Ho et al. 1996; Best 1981; West 1974). This method is widely known and established in natural gas dehydration, using countercurrently operated high-pressure columns. Countercurrent column contactors are also commonly being applied in the chemical, pharmaceutical and petrochemical industry. The applied contactors may contain sieve or cap trays or random as well as structured packings in order to intensify the contact between the entering gas and the liquid absorbent for facilitating the mass transfer of the undesired compounds. Usually, these processes are conducted at pressures above atmospheric for which pressure resistant equipment is required. When applying the absorption method, a liquid desiccant, e.g., a glycol is used to remove water vapor from wet carbon dioxide. Commonly applied tri-ethylene glycol (TEG) is contacted with the wet carbon dioxide in the absorber in a countercurrent flow pattern. Lean glycol enters the top of the absorption column while the wet carbon dioxide is injected at the bottom. The TEG flowing downward absorbs water vapor from the feed CO2 moving upward, the CO2 becomes lean and the TEG becomes rich in water. The absorbed water is removed from the rich TEG at the regenerator by distillation and the lean TEG is recirculated in a closed cycle. An efficient CO2 dehydration process has become increasingly important in order to maintain the required CO2 specifications and also prevent the devastating effects of unwanted fluids/solids which could cause formation of gas hydrates, corrosion, fouling and damaging of pipes/vessels leading to costly maintenance and repair operations. The efficiency of the separation process depends on the extension of the mass transfer interface that on its turn depends on the hydrodynamic and interfacial forces. The hydrodynamic forces tend to breakup droplets thereby creating new interface. On the other hand, the system always tends to minimize its energetic state, i.e., to reduce the interface, with the interfacial tension as the governing parameter: a low value of the interfacial tension facilitates creation of large interfaces while at the same time care needs to be taken to avoid formation of stable dispersions. For the simulation of the process, the interfacial tension as well as the mixture density needs to be known at the operating conditions that especially in the case of CO2 dehydration using TEG are above atmospheric pressure. Specifically, detailed knowledge of the interfacial tension in systems containing TEG, water and CO2 is required at pressures up to 150 bars at variable compositions in order to be able to describe and optimize the process.
Many studies have been carried out on interfacial tension of hydrocarbon liquid and aqueous solutions in carbon dioxide, among others to expand the knowledge on CO2 flooding as a method of enhanced oil recovery as well as for the purpose of CO2 storage (Cumicheo et al. 2014; Zolghadr et al. 2013; Jaeger and Eggers 2012; Shariat et al. 2012; Bikkina et al. 2011; Georgiadis et al. 2010; Bachu and Bennion 2009; Kvamme et al. 2007; Chiquet et al. 2007; Hebach et al. 2002; Yan et al. 2001; Shaver et al. 2001; Ren et al. 2000; McCaffery 1972). Sutjiadi-Sia et al. (2008) investigated interfacial phenomena of aqueous systems in dense carbon dioxide. They evaluated the interfacial tension of H2O + EtOH–CO2 + H2O + EtOH and also H2O–CO2 + EtOH at a temperature of 40 °C and pressures up to 270 bars. Both, static as well as dynamic interfacial tension values were measured. They reported that the interfacial tension of the investigated fluid systems does not only depend on the composition within the interface but also on the bulk phase. This work is relevant to extraction columns run with aqueous phases. Other studies on the interfacial tension of alcohols–carbon dioxide and alcohol–methane are tabulated in Table 1. So far, process simulation software and thermodynamic-based models (Jiang et al. 2019; Best 1981; Jumar 2021; Øi and Rai 2016; Kumar et al. 2010; Hossain 2017) have been applied to study the design and performance of CO2 dehydration by absorption method using tri-ethylene glycol. To the best of our knowledge, no study has been conducted on the interfacial tension of tri-ethylene glycol-water and CO2 up to date while there is still a lack of detailed knowledge with respect to mass transfer and hydrodynamic processes in glycol absorption columns operating at elevated pressures. The aim of this study is to provide new data required for the design and optimization of the glycol dehydration process and to help gain knowledge on the principal phenomena taking place.
Therefore, this work focuses on investigating the phase and interfacial behavior of systems containing TEG, TEG–water and carbon dioxide (CO2) at non-atmospheric conditions representing the conditions in absorption columns for glycol dehydration.
Theory
The interfacial tension represents the work which must be applied to increase the interface between two coexisting phases by a unit area:
Hence, a change in interfacial area is related to the energy input through the interfacial tension. Transferred to a column contactor, this means that energy has to be applied in order to provide sufficient mass transfer area for an efficient process to be performed. This energy originates from hydrodynamic forces. The dimensionless Weber-Number expresses the balance between hydrodynamic forces and surface forces as:
d being the droplet diameter (m), ρ the density of the surrounding phase (kg/m3), u the flow rate (m/s) and σ the interfacial tension (N/m). In case the hydrodynamic forces prevail, the surface tension is no longer able to maintain a stable drop and the drop will deform and eventually breakup (de Gennes et al. 2004). The critical We-number is associated to the maximum drop size that is still stable under the actual hydrodynamic conditions.
The droplets in column contactor cover a range of sizes and shapes. The equivalent diameter (\(d_{e}\)) of a spherical drop may be estimated using Lewis et al. (1951) equation,
\(d_{1}\) and \(d_{2}\) are major and minor axes of an ellipsoidal-drop image. For a spherical drop, \(d_{e}\) is the same as the drop diameter. The surface-mean diameter, \(d_{vs}\) (also known as Sauter mean diameter) is appropriate for mass transfer calculations because it gives the same surface area as the entire population of drops for the same mass of drops.
The drop size distribution data for N drops is given by,
\(d_{{{\text{vs}}}}\). is calculated from Eq. (4) as
The interfacial area per unit volume of a two-phase mixture is estimated as,
The dispersed-phase holdup \({\Phi }_{D}\), is another property that is crucial for the process design being defined as the volume fraction of the active section of an absorption column that is occupied by the dispersed phase. The interfacial area available for mass transfer in a countercurrent absorption column depends upon the volume fraction or holdup of the dispersed phase, as well as on the mean droplet size.
\({V}_{\mathrm{d}}\) and \({V}_{t}\) are the volume of the dispersed phase and the total volume of the two phases based on the effective length of the absorption column, respectively. On the other hand, the volume of the dispersed phase depends on the mean (Sauter) diameter as small droplets will be retained to a higher extent, increasing the holdup. The mean drop size on its turn depends on the critical We-number, containing the interfacial tension as a decisive parameter (see above).
Experimental
Materials
TEG was supplied by Tokyo chemicals industry Ltd. Japan, with a grade ≥ 99.8%; and CO2 was purchased from Westfalen Austria GmbH at a grade of 99.99%, as aqueous phase distilled water was used. Acetone was used for cleaning purpose only.
Methods and Setup
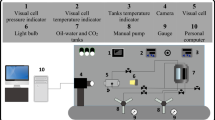
The interfacial tension of TEG and TEG–water in CO2 medium is measured using the pendant drop method in a high-pressure view cell in this experiment, (PD-E1700 MD-H, Eurotechnica GmbH, Germany). The equipment has a maximum pressure and temperature of 689 bars and 200 °C, respectively. The flow sheet is shown in Fig. 1. The apparatus has several components which include: the view cell, electric heating jacket, hand pump, mini-dosing, rupture disk support and disk, pressure gauge, thermocouple, safety switch, liquid supply tank and valves. The electric heating jacket is used to heat up the fluids in the view cell at a temperature stability of 1 K. The thermocouple and pressure probes are connected to the view cell to take digital readings of the view cell temperature (± 0.1 °C) and pressure (± 0.1 bar), respectively. The liquid supply tank with the high-pressure hand pump was used to pump water into the view cell for cleaning purposes only while setting up the experiment but could also be used to pressurize the view cell with solvents. The mini-dosing device is used to create a liquid drop via the capillary reaching into the view cell from the top connection. Valves are used to control the flow of fluid in and out of the equipment.
Flow sheet of the PD-E1700 MD-H (Eurotechnica 2019)
Experimental procedures
Two different solutions were prepared as the drop phase. One liquid is only composed of pure tri-ethylene glycol and the other contains 95wt% of tri-ethylene glycol blended with 5 wt% of distilled water. TEG water content of 5 wt% is used in this study to represent the worst-case scenario of TEG concentration obtainable in a dehydration column. The lines of the high-pressure pendant drop apparatus were flushed with water to remove any water-soluble contaminant. Afterward, acetone was applied and at the end pressurized air was used to dry the equipment. The IFT of water was determined and compared to the known value at room conditions to assure the equipment is clean. The cell was dried from water and the apparatus set up for the experiment with tri-ethylene glycol (TEG). The view cell temperature was set to the required temperature using the electrical heating jacket and also was pressurized to the desired pressure by opening the gas valve (G) to allow passage of CO2 from the gas cylinder to the view cell. An automated syringe pump (100DM Teledyne ISCO, USA) was used to pressurize the system to the designated conditions. A liquid drop was created in the view cell at the tip of the capillary using the mini-dosing device (PG1). Images of the drop at the capillary tip (Fig. 2) were captured by a camera attached to the drop shape analyzer (DSA100E). The DSA100E (Krüss GmbH, Germany) records the drop images that are analyzed by the ADVANCE software afterward (Krüss GmbH, Germany) in terms of the interfacial tension. The experiments were conducted at pressures up to 250 bars and temperatures ranging between 10 °C and 50 °C.
As the pendant drop technique requires accurate knowledge of the density difference of the coexisting fluids for IFT calculations, the density of saturated TEG–CO2 and TEG + water–CO2 mixtures were measured by use of the oscillating u-tube method with a high-pressure densitometer (DMA-HPM, Anton Paar-Austria) with an accuracy of ± 0.0001 g/cm3. The mixture density values were used to compute the IFT of TEG–CO2 and TEG + water–CO2, the results being shown in the result section.
Results and discussion
Pure phase and fluid mixture densities
The results of the density of pure TEG, TEG–CO2, TEG + H2O and TEG + H2O–CO2 mixtures are shown in Figs. 3 and 4. The densities of pure TEG, TEG–CO2, TEG + H2O and TEG + H2O–CO2 mixtures increase as pressure increases at constant temperature and decrease with increase in temperature at fixed pressure. The density of saturated TEG–CO2 is higher than density of pure TEG at 30 °C and 50 °C. This is as a result of solubility and high interaction of CO2 in TEG. Similar trends were reported in several technical papers, associating this effect likewise with strong interactions between the quadrupole CO2 with organic liquids, e.g., CO2 with Montney light crude oil (Habibi et al. 2017); CO2 with Lloydminster heavy oil (Li et al. 2013); CO2 with West Texas oil (Lansangan and Smith 1993); CO2 with a model oil (Samara et al. 2022). Also, the density of saturated TEG + H2O–CO2 is slightly higher than the density of TEG + H2O at 30 °C because of the solubility of CO2 in TEG and also due to strong water–CO2 interaction. This trend was also reported in studies conducted by several researchers on comparable systems (Samara et al. 2022; Li et al. 2011, 2004; Hebach et al. 2004). At 50 °C, the impact of CO2 on the density of TEG + H2O is neglectable, the elevated temperature decreasing the solubility of CO2 in the liquid phase. An increase in the gas saturated TEG density favors higher gas loads of the column contactor used for dehydration without the column being flooded. Gas dehydration columns operate at pressures up to 200 bars (Wang and Economides 2010). The velocity of the gas flowing up the column packing is confined by the risk of liquid loading or flooding but must be high enough for sufficient throughput. The column packing efficiency depends on the extension of the mass transfer interface and the flow conditions in terms of convective mass transfer. Highest efficiency is obtained close to the flooding point for which even small increments in liquid phase densities are desirable.
Static interfacial tension
Figure 5 shows the interfacial tension of TEG in CO2 at pressures ranging from atmospheric up to 250 bars and temperature of 30 °C and 50 °C. The interfacial tension readings were taken after the fluid phases have reached equilibrium (i.e., the change in interfacial tension < 0.1 mN/m over 5 min). The left y-axis in Fig. 5 represents the interfacial tension as a function of pressure at 30 °C and 50 °C. On the right-hand side, the TEG–CO2 density difference is plotted against pressure at the respective temperature conditions. The slope of IFT of TEG–CO2 at pressures < 70 bars at 30 °C and < 100 bars for 50 °C is generally steeper than at higher pressures, where IFT is only slightly decreased as a function of pressure. The decrease in IFT with increasing pressure is attributed to the adsorption of CO2 at the TEG drop surface. At increased pressure, CO2 adsorption at the interface tends to decrease due to a decreasing compressibility. Thus, further increase in pressure (> 70 bars at 30 °C and > 100 bars at 50 °C) results only in a slight decrease in IFT. A similar trend was reported by Harrison et al. (1996) in his studies on the interfacial tensions of polyethylene glycol in dense carbon dioxide at different surfactant concentrations. The density difference between the drop and the surrounding phases decreases gradually with increase in pressure at both temperature conditions (30 °C and 50 °C). The stronger decline in density difference at low pressures, P ≤ 100 bars at 30 °C and 50 °C is mainly due to the compressibility of CO2: from 851.6 kg/m3 to 416.9 kg/m3 at 70 bars to 80 bars and T = 30 °C and from 717.1 kg/m3 to 403.75 kg/m3 at 100 bars to 150 bars and T = 50 °C. These sharp declines mark the proximity of the critical point (31 °C and 74 bars) of CO2 with a sudden change from vapor-like CO2 to liquid-like CO2.
Figure 6 shows the IFT values of TEG–CO2 at the same conditions as in Fig. 5, but here the drop volume is represented at the right-hand side of the graph. It should be noted that the drops are formed to a size that is just as large still to be stable before detaching. The drop volume at 50 °C decreases with increase in pressure up to 100 bars where it becomes fairly constant at further increase in pressure. Also, there is a decrease in drop volume at 30 °C with increase in pressure up to 70 bars where it starts to slightly increase and remain fairly constant at higher pressures. The decrease in drop volume results from the counteracting effects of decreasing IFT (more pronounced for lower temperatures) and decreasing density difference (increasing buoyancy). While the IFT levels off at around 70 bars, the density difference keeps decreasing associated to an increased buoyancy that results in an increased drop volume until both, the IFT and the density difference start to align leading to a fairly constant value of the drop volume above 150 bars. The lower interfacial tension at higher pressures (Figs. 5 and 6) favors the formation of a larger interface (smaller drops) which is advantageous for mass transfer during process performance. TEG having a larger interface at higher pressure will be able to absorb water vapor from the wet carbon dioxide (mass transfer of water vapor into the absorbent-TEG) in less volume and time. Carbon dioxide dehydration is more efficient at lower temperature (T = 30 °C) as low IFT is recorded when compared to IFT at higher temperature (T = 50 °C).
In Fig. 7, IFT of TEG–CO2 is depicted as a function of temperature at 70 bars. IFT increases gradually as the temperature increases from 10 °C to 20 °C. There is a sudden increase in IFT as the temperature increases to 30 °C. A further increase in temperature results only in a slight increase in IFT. CO2 dehydration is more efficient at low temperatures (T = 10 °C and 20 °C) as lower IFTs are recorded at 70 bars (Fig. 7) and higher water solubility in TEG. It shall be noted though that compressed gases such as CO2 and light hydrocarbons (C1 to C3) tend to form gas hydrates at low temperatures (T < 10 °C) (Sloan et al. 2007). Thus, all temperature conditions for CO2 dehydration operations shall preferably be maintained above 10 °C. On the other side, higher temperatures are not beneficial in CO2 dehydration as the phase equilibrium results in higher water contents in CO2 (Campbell 2001).
Effect of tri-ethylene glycol purity
IFT values of TEG–CO2 and TEG + H2O–CO2 are shown in Fig. 8 as a function of pressure at 30 °C. Also, the drop volumes are represented on the right-hand side of the graph. The same decreasing trend of IFT with increase in pressure is observed but there is a slight increase in interfacial tension when 5wt% distilled water is added to TEG. This increase is due to the presence of water which has a higher IFT than TEG as also shown in Fig. 8 for IFT of H2O–CO2 (Bikkina et al. 2011). Less water vapor is likely to be absorbed by TEG containing 5wt% water due to the phase equilibrium and additionally the higher IFT counteracts formation of interface when compared to pure TEG in CO2 environment. This shows that a higher concentration of TEG is in any case beneficial for an efficient CO2 dehydration to take place. Also, the drop volume decreases with increase in pressure up to 70 bars in parallel with the IFT. Beyond 70 bars there is a considerable increase in drop volume due to the drastically decreased density difference between the drop and the surrounding phase. As this density difference remains fairly stable at higher pressures, the drop volume does the same. The drops containing water take larger dimensions in accordance with the higher IFT.
Static interfacial tension of TEG and TEG–H2O in CO2 as a function of pressure at 30 °C and 50 °C. The figure also shows the drop volume of TEG–CO2 and TEG–H2O–CO2. Also, IFTs of H2O–CO2 at 25 °C and 40 °C (Bikkina et al. 2011) are shown in the figure
Dynamic interfacial tension
The dynamic interfacial tension (time dependent) and the drop volume of TEG–CO2 are shown on the left- and right-hand sides of Fig. 9, respectively. IFT of TEG–CO2 remains fairly constant with time but the drop volume increases with time leveling off and only slightly increases on the long term. The increase in drop volume is due to the solubility of CO2 in TEG. The volumetric expansion of the TEG drop in the presence of CO2 and the associated solubility of CO2 in TEG are evaluated from Fig. 9 using Eqs. (8 and 9).
Equation (9) is derived from volume relationship with mass and density.
\(V_{{\text{E}}}\), volumetric expansion (%), \(\Delta V\) = change in volume (mm3), \(V_{i}\) = initial volume (mm3), \(\frac{{m_{{{\text{CO}}_{{2}} }} }}{{m_{{{\text{TEG}}}} }}\) = CO2 solubility in TEG (g CO2/g TEG), \(\rho_{{\left( {{\text{TEG + CO}}_{{2}} } \right)}}\) = density of CO2 saturated TEG, \(\rho_{{{\text{TEG}}}}\) = density of pure TEG.
\(\upsilon\) is related to \(V_{{\text{E}}}\) as expressed in Eq. (10)
A similar approach was applied by Samara et al. (2022) to calculate CO2 solubility in oil. The calculated results show a volumetric expansion of 13.7% and associated to a CO2 solubility in TEG of 14 wt%. The mass transfer of CO2 into the TEG drop is clearly reflected by a volumetric expansion while the interfacial tension takes a constant value more quickly. While the volumetric expansion reflects the solubility of the CO2 inside the bulk phase, the interfacial tension is a measure of the composition at the interface.
Conclusions
The interfacial tension and the mixture densities of TEG–CO2 and TEG + water–CO2 have been measured as a function of pressure and temperature in the range up to 250 bars between 10 °C and 50 °C. The static interfacial tension of TEG–CO2 and TEG + water–CO2 decreases with an increase in pressure at constant temperature but increases with a rising temperature at fixed pressure. After a short decrease in IFT over time, the dynamic IFT remains nearly constant while the mass transfer into the bulk takes considerably longer time, reflected by the volumetric expansion.
The following conclusions may be drawn from the current work, especially in view of the CO2 dehydration processes:
-
1.
The lower interfacial tension at higher pressures favors the formation of a larger interface through smaller droplets which is advantageous for mass transfer and as a consequence for process efficiency.
-
2.
At elevated gas pressures, the behavior with respect to temperature is inverted: an increasing temperature leads to an increasing IFT which is associated to a decreasing gas solubility in the liquid phase. As a consequence, CO2 dehydration efficiency is favored at lower temperature (T < 20 °C) but limited by the risk of formation of gas hydrates.
-
3.
The drop volume at higher pressures hardly depends on temperature because of superposed counteracting effects by the buoyancy and the IFT.
-
4.
As the water concentration increases in the TEG phase, the IFT increases which counteracts mass transfer efficiency.
-
5.
Since only at the very beginning of drop formation the IFT is decreasing, the static values of IFT may be applied for process design through correlations using the critical We-Number with a reasonable precision.
-
6.
The dynamic IFT takes constant values before the equilibrium concentration in the drop is attained indicating that the interfacial composition stabilizes before.
-
7.
The volumetric expansion of TEG exposed to CO2 was used to estimate the gas solubility when knowing the mixture density at the designated conditions. This method is easy to be applied from an experimental point of view and gives good insight into the phase behavior which is also required for design purposes.
In general, data are provided on properties at process conditions that are required for process simulation and optimization on the one hand and on the other give insight into governing mechanisms, especially at elevated pressures and temperatures under dynamic conditions.
Abbreviations
- \(a\) :
-
Interfacial area, m2
- \(d\) :
-
Diameter, m
- \(d_{1}\) :
-
Major axes of an ellipsoidal-drop image
- \(d_{2}\) :
-
Minor axes of an ellipsoidal-drop image
- \(d_{e}\) :
-
Droplet diameter, m
- \(d_{{{\text{vs}}}}\) :
-
Sauter mean diameter, m
- \(m_{{{\text{CO}}_{{2}} }}\) :
-
Mass of carbon dioxide, kg
- \(m_{{{\text{TEG}}}}\) :
-
Mass of tri-ethylene glycol, kg
- T :
-
Temperature, oC
- u :
-
Flow rate, m/s
- \(V_{{\text{d}}}\) :
-
Volume of the dispersed phase for the effective length of the column
- \(V_{{\text{E}}}\) :
-
Volumetric expansion, %
- \(V_{t}\) :
-
Total volume of the two phases for the effective length of the column
- \(V_{i}\) :
-
Initial volume, mm3
- \(W_{e}\) :
-
Weber-Number
- \(dW_{\sigma }\) :
-
Change in work done by interfacial tension
- \(\Delta V\) :
-
Change in volume, m3
- ρ :
-
Density, kg/m3
- \(\rho_{{\left( {{\text{TEG + CO}}_{{2}} } \right)}}\) :
-
Density of carbon dioxide saturated tri-ethylene glycol, kg/m3
- \(\rho_{{{\text{TEG}}}}\) :
-
Density of pure tri-ethylene glycol, kg/m3
- \(\sigma\) :
-
Interfacial tension, N/m
- \(\sigma dA\) :
-
Change in interfacial area, m2
- \(\Phi_{{\text{D}}}\) :
-
Dispersed-phase hold up
- CCUS:
-
Carbon capture utilization and storage
- EOR:
-
Enhanced oil recovery
- EGR:
-
Enhanced gas recovery
- TEG:
-
Tri-ethylene glycol
- EtOH:
-
Ethanol
- PD:
-
Pendant drop
- DSA:
-
Drop shape analyzer
References
Ahmari S, Mufti A (2022). CHG emission reduction at first Saudi Aramco CC&I project. IPTC-22267-EA. In: International petroleum technology conference held in Riyadh, Saudi Arabia, Accessed 21–23 February. https://doi.org/10.2523/IPTC-22267-EA
Alhashboul A, Almufti A, Kokal S (2017) Surface facilities design for the first CO2 EOR demonstration project in Saudi Arabia. In: SPE-185836-MS. SPE Europec featured at 79th EAGE Conference and Exhibition held in Paris, France, Accessed 12–15 June. https://doi.org/10.2118/185836-MS
Bachu S, Bennion DB (2009) Interfacial tension between CO2, freshwater and brine in the range of pressure from (20 to 270) bars, temperature from (20 to 125) °C, and water salinity from (0 to 334000) mg.L-1. J Chem Eng Data 54:765–775. https://doi.org/10.1021/je800529x
Becker S, Werth S, Horsch M, Langenbach K, Hasse H (2016) Interfacial tension and adsorption in the binary system ethanol and carbon dioxide: experiments, molecular simulation and density gradient theory. J Fluid Ph Equilib 427:476–487. https://doi.org/10.1016/j.fluid.2016.08.007
Best JW (1981) Rigorous computer simulation of CO2 dehydration facilities. SPE 10284. In: 56th annual fall technical conference and exhibition of the society of petroleum engineers of AIME, held in San Antonio, Texas, Accessed 5–7 October. DOI: https://doi.org/10.2118/10284-MS
Bikkina PK, Shoham O, Uppaluri R (2011) Equilibrated interfacial tension data of the CO2-water system at high pressures and moderate temperatures. J Chem Eng Data 56:3725–3733. https://doi.org/10.1021/je200302h
Buit L, Ahmad M, Mallon W, Hage F (2010) CO2 EuroPipe study of the occurrence of free water in dense phase CO2 transport. Energ Proced 4:3056–3062. https://doi.org/10.1016/j.egypro.2011.02.217
Campbell JN (2001) Gas conditioning and processing. In: Campbell JM, Robert A (eds) The equipment modules, 8th edn, vol 2. John Campbell & Company Publisher, Norman
Chiquet P, Daridon JL, Broseta D, Thibeau S (2007) CO2/water interfacial tensions under pressure and temperature conditions of CO2 geological storage. J Energy Convers Manag 48:736–744. https://doi.org/10.1016/j.enconman.2006.09.011
Chun B, Wilkinson GT (1995) Interfacial tension in high-pressure carbon-dioxide mixtures. Ind Eng Chem Res 34:4371–4377. https://doi.org/10.1021/ie00039a029
Cumicheo C, Cartes M, Segura H, Müller EA, Mejia A (2014) High-pressure densities and interfacial tensions of binary systems containing carbon dioxide + n-alkanes: (n-Dodecanes, n-tridecane, n-tetradecane). J Fluid Phas Equilib 380:82–92. https://doi.org/10.1016/j.fluid.2014.07.039
Dittmar D, Fredenhagen A, Oei SB, Eggers R (2003) Interfacial tensions of ethanol-carbon dioxide and ethanol-nitrogen. Dependence of the interfacial tension on the fluid density-prerequisites and physical reasoning. J Che Eng Sci 58:1223–1233. https://doi.org/10.1016/S0009-2509(02)00626-7
Eurotechnica (2019) PD-E1700 MD-H operating manual
Fabien A, Lefebvre G, Calvignac B, Legout P, Badens E, Crampon C (2022) Interfacial tension of ethanol, water, and their mixtures in high pressure carbon dioxide: Measurements and modeling. J Coll Interface Sci 613(7):847–856. https://doi.org/10.1016/j.jcis.2022.01.058
Georgiadis A, Llovell F, Bismarck A, Blas FJ, Galindo A, Maitland GC, Trusler JPM, Jackson G (2010) Interfacial tension measurements and modelling of (carbon dioxide + n-alkanes) and (carbon dioxide + water) binary mixtures at elevated pressures and temperatures. J Supercrit Fluid 55:743–754. https://doi.org/10.1016/j.supflu.2010.09.028
Global Energy Review (2021) CO2 emissions from energy combustion and industrial processes, 1900–2021. Accessed 8 December 2022
Habibi A, Yassin MR, Dehghanpour H, Bryan D (2017) Experimental investigation of CO2-oil interactions in tight rocks: a Montney case study. Fuel 203:853–867. https://doi.org/10.1016/j.fuel.2017.04.077
Harrison KL, Johnston KP, Sanchez IC (1996) Effect of surfactants on the interfacial tension between supercritical carbon dioxide and polyethylene glycol. Langmuir 12:2637–2644. https://doi.org/10.1021/la9510137
Hebach A, Oberhof A, Dahmen N, Kögel A, Ederer H, Dinjus E (2002) Interfacial tension at elevated pressures-measurements and correlations in the water + carbon dioxide system. J Chem Eng Data 47:1540–1546. https://doi.org/10.1021/je025569p
Hebach A, Oberhof A, Dahmen N, Dinjus E (2003) Interfacial phenomena in the system water +CO2+Alcohol Ethoxylates. J Chem Eng Data 48:1557–1560. https://doi.org/10.1021/je034090+
Hebach A, Oberhof A, Dahmen N (2004) Density of water + carbon dioxide at elevated pressures: measurements and correlation. J Chem Eng Data 49(4):950–953. https://doi.org/10.1021/je034260i
Ho KT, McMullen J, Boyle P, Rojek O, Forgo M, Beatty T, Longworth HL (1996) Subsurface acid gas disposal scheme in Wayne-Rosedale, Alberta. SPE 35848.In: international conference on health, safety & environment held in New Orleans, Lousiana, Accessed 9–12 June. https://doi.org/10.2118/35848-MS
Hossain M, (2017) Design of injection facilities for CO2 recovery process: a case study. SPE-185474-MS. In: SPE Latin America and Caribbean petroleum engineering conference held in buenos aires, Argentina, Accessed 18–19 May. https://doi.org/10.2118/185474-MS
Jaeger P, Egger R (2012) Interfacial properties at elevated pressures in reservoir systems containing compressed or supercritical carbon dioxide. J Supercrit Fluids. 66:80–85. https://doi.org/10.1016/j.supflu.2011.12.024
Jiang Y, Taheri M, Yu G, Zhu J, Lei Z (2019) Experiments, modeling and simulation of CO2 dehydration by ionic liquid, triethylene glycol, and their binary mixtures. Ind Eng Chem Res 58:15588–15597. https://doi.org/10.1021/acs.iecr.9b02540
Jumar T (2021) CO2 compression and dehydration unit report. Drax BECCS Project. Department for business, energy and industrial strategy
Kumar S, Zarzour O, King G, Carbon M, (2010) Design of CO2 dehydration and compression facilities. SPE 137284. In: international petroleum exhibition and conference held in Abu Dhabi, Accessed 1–4 November. https://doi.org/10.2118/137284-MS
Kvamme B, Kuzunetsova T, Hebach A, Oberhof A, Lunde E (2007) Measurements and modelling of interfacial tension for water+carbon dioxide systems at elevated pressures. J Comp Mater Sci 38:506–513. https://doi.org/10.1016/j.commatsci.2006.01.020
Lansangan RM, Smith JL (1993) Viscosity, density and composition measurements of CO2/west texas oil systems. SPE Res Eng 8(03):175–182. https://doi.org/10.2118/21017-PA
Lewis JB, Jones I, Pratt HRC (1951) Droplet behaviour in packed columns. Trans Inst of Chem Eng 29:126–144
Li Z, Dong M, Li S, Dai L (2004) Densities and solubilities for binary systems of carbon dioxide + water and carbon dioxide + brine at 59°C and pressures to 29 MPa. J Chem Eng Data 49(4):1026–1031. https://doi.org/10.1021/je049945c
Li D, Graupner BJ, Bauer SA (2011) A method of calculating the liquid density for CO2-H2O-NaCl system under CO2 storage condition. Energy Proced 4:3817–3824. https://doi.org/10.1016/j.egypro.2011.02.317
Li H, Zheng S, Yang D (2013) Enhanced swelling effect and viscosity reduction of solvent(s)/CO2/heavy-oil systems. SPE J 18(04):696–707. https://doi.org/10.2118/150168-PA
McCaffery FG (1972) Measurement of interfacial tensions and contact angles at high temperature and pressure. J Can Pet Technol 11(03):26–32. https://doi.org/10.2118/72-03-03
McKay W, Maddocks JR, (2012) Acid gas dehydration-Is there a better way? OTC 23549. In: offshore technology conference held in Houston, Texas. USA, 30 April-3 May. https://doi.org/10.4043/23549-MS
Øi LE, Rai B, (2016) Simulation of glycol processes for CO2 dehydration. In: Proceedings of the 9th EUROSIM & the 57th SIMS held at Oulu, Finland, 12th–16th September. https://doi.org/10.3384/ecp17142168
Peletiri SP, Rahmanian N, Mujtaba IM (2018) CO2 pipeline design: a review. Energies 11(9):2184. https://doi.org/10.3390/en11092184
de Gennes P, Brochard-Wyart F, Quere D (2004) Capillary and wetting phenomena. Springer, pp 7–8. ISBN 978-0-387-00592-8. https://doi.org/10.1007/978-0-387-21656-0
Ren QY, Chen GJ, Yan W, Guo TM (2000) Interfacial tension of (CO2+CH4) plus water from 298 K to 373 K and pressures up to 30 MPa. J Chem Eng Data 45:610–612. https://doi.org/10.1021/je990301s
Rütters H, Fischer S, Hoa LQ, Bettge D, Bässler R, Massmann J, Ostertag-Henning C, Wolf JL, Pumpa M, Lubenau U, Knauer S, Jaeger P, Neumann A, Svensson K, Pöllmann H, Lempp C, Menezes FF, Hagemann B (2022) Towards defining reasonable minimum composition threshold-impacts of variable CO2 stream compositions on transport, injection and storage. Int J Greenhouse Gas Control 114:103589. https://doi.org/10.1016/j.ijggc.2022.103589
Samara H, Al-Eryani M, Jaeger P (2022) The role of supercritucal carbon dioxide in modifying the phase and interfacial properties of multiphase systems relevant to combined EOR-CCS. Fuel 323:124271. https://doi.org/10.1016/j.fuel.2022.124271
Shariat A, Moore RG, Mehta SA, Van Fraassen KC, Newsham KE, Rushing JA (2012) Laboratory measurements of CO2-H2O interfacial tension at HP/HT conditions: implications for CO2 sequestration in deep aquifers.In: carbon management technology conference (CMTC 150010). https://doi.org/10.7122/150010-MS
Shaver RD, Robinson RL, Gasem KAM (2001) An automated apparatus for equilibrium phase compositions, densities, and interfacial tensions: data for carbon dioxide + decane. J Fluid Phase Equilib 179:43–66. https://doi.org/10.1016/S0378-3812(00)00475-1
Sloan ED, Koh CA (2007) Clathrate hydrates of natural gases, 3rd edn. CRC Press. https://doi.org/10.1201/9781420008494
Sutjiadi-Sia Y, Jaeger P, Eggers R (2008) Interfacial tension of aqueous systems in dense carbon dioxide. J Supercrit Fluids 46:272–279. https://doi.org/10.1016/j.supflu.2008.06.001
Villablanca-Ahues R, Nagl R, Zeiner T, Jaeger P (2022) Phase Equilibria and interfacial properties at elevated pressure. ESAT 2022, Graz 17. Accessed 20 July 2022
Wang X, Economides M (2010) Advanced natural gas engineering. Gulf Pub Co. https://doi.org/10.1016/C2013-0-15532-8
West JM (1974) Design and operation of a supercritical CO2 pipeline-compression system. SPE 4804.In: permian basin oil recovery conference, Midland, Texas, Accessed 11–12 March 1974. https://doi.org/10.2118/4804-MS
World Energy Outlook (2022) Global carbon dioxide emissions. Accessed 8 December 2022
Yan W, Zhao GY, Chen GJ, Guo TM (2001) Interfacial tension of (methane + nitrogen) + water and (carbon dioxide + nitrogen) + water systems. J Chem Eng Data 46(6):1544–1548. https://doi.org/10.1021/je0101505
Zolghadr A, Escrochi M, Ayatollahi S (2013) Temperature and composition effect on CO2 miscibility by interfacial tension measurement. J Chem Eng Data 58(5):1168–1175. https://doi.org/10.1021/je301283e
Acknowledgements
We acknowledge the financial support by the Open Access Publishing Fund of Clausthal University of Technology. The principal author thanks PTDF/DAAD for a personal Grant No. 91759688. We are grateful to the reviewers for their insightful comments.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research is part of principal author PhD work funded by PTDF/DAAD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing financial and non-financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibeh, S., Jaeger, P. Interfacial tension of tri-ethylene glycol-water mixtures in carbon dioxide at elevated pressures. J Petrol Explor Prod Technol 13, 1379–1390 (2023). https://doi.org/10.1007/s13202-023-01615-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-023-01615-4