Abstract
The chief source of the oilfield scale is the mixing of incompatible waters. This study demonstrated that mixing the reservoir of Mishrif formation (Halfaya oilfield) with six types of injection water sources, including Tigris River water, producing water formation, Gulf seawater, Marshes water, Middle Kirkuk formation water, and Main Outfall Drain water (AL-Masab AL-Aam Channel), leads to the formation of salt crusts that cause the reduction of reservoir rock permeability. According to the Piper diagram, the Mishrif formation water of all extant water samples was of the sodium chloride type (NaCl), except for HF-81, which was between (NaCl) and mix (CaMgCl) type. A geochemical simulation model of water alignment (PHREEQC) was used to simulate this problem, and it revealed the mineral scaling from mixing processes. These minerals precipitate in rock pores and clog them, which then cause damage to the petrophysical properties of the reservoir and prevent the passage of liquids. Results showed that the best water types used for injection are Middle Kirkuk formation water, followed by the general downstream, then Gulf seawater, but treatment before injection is needed. The study of geochemical modeling method can help to better understand scaling issues by efficiently identifying the best injection water from various selected types with the lowest possible cost, which in turn improves oil production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Scale deposition is one of the most critical oilfield issues that water injection systems face, especially when two incompatible fluids are present. Scales form when two incompatible waters are mixed and supersaturation occurs (Merdhah and Yassin 2008). Supersaturation with scale-forming salts caused by changes in physical conditions in which water exists can cause scale deposition in one water type. Millions of oil barrels per day are produced using the water injection technique; however, one of the difficulties associated with this effective production method is scale buildup due to the various dissolved particles and high salinity of the injected water (Al-Samhan et al. 2020; Jumaah 2021). Down-hole pumps, tubing, casing flow lines, heater treaters, tanks, and other production equipment and facilities are also damaged by scales, which can develop near any point in the production system to generate supersaturation. Variations in pressure and temperature or mixing two incompatible fluids can cause supersaturation in water. Calcium carbonate, calcium sulfate, strontium sulfate, and barium sulfate are the most popular oilfield scales, which require useful modeling tools to better predict and understand the complicated scale evolution (Moghadasi et al. 2007).
Hydrogeochemical modeling is a practical tool to comprehend the groundwater evolution in a particular aquifer, and the integration of a management plan can be set for the total regime. Geochemical models are means of dealing with geochemical reactions. Over the past three decades, many geochemical models have been developed that quantify the thermodynamics, ionic facies, saturation indices (SI), and mixing processes among water bodies (Ghalib 2014; Ghalib and Sogut 2014; Al-Mallah et al. 2022).
Many case histories of oil well scaling by calcium carbonate, calcium sulfate, strontium sulfate, and barium sulfate have been reported (Mitchell et al. 1980; Lindlof and Stoffer 1983; Vetter et al. 1987; Shuler et al. 1991; Liu et al. 2009; Olajire 2015; Liang et al. 2019; Murtaza et al. 2022). Problems in connection to oil well scaling in Russia, where scale has seriously plugged wells and is similar to cases in North Sea fields, have also been raised (Mitchell et al. 1980). Oilfield scale problems have occurred due to waterflooding in Saudi oilfields, Algeria, Indonesia in South Sumatra oilfields, China, Malaysia, Mabruk oil field in Libya and Egypt in the El-Morgan oilfield where calcium and strontium sulfate scales have been found in surface and subsurface production equipment (El-Hattab 1982; Yan et al. 2021; Alnajar and Refai 2022). Iraq has a long history of using water injection on a relatively small scale, dating back to 1961. Water injection is the most appropriate recovery mechanism for most of the reservoirs in southern and central Iraq, would give the highest recovery factors and is technically relatively straightforward (Liang et al. 2019).
Regarding the water types suggested to be used for injection, Iraq has two major rivers; Euphrates and the Tigris that run across the country from north to south. But, increasing competition from other users for water resources, ongoing drought and reduced river flows from upstream dam building means that operators can no longer rely on river water for field injection purposes (Liang et al. 2019). Therefore, other types of water available in the study area were chosen for the injection purpose. They include Tigris River water (TRW), producing water formation (PWF), Gulf seawater (GSW), Marshes water (MW), Middle Kirkuk formation water (MKFW), and Main Outfall Drain water (MODW), as shown in Fig. 1b, which each of them has specific quality of the source water.
This number of different water type, which are locally available in Iraq, have been proposing to use for injection process and enhancement of oil recovery. However, testing different types of water in different reservoir conditions to model and predict the compatibility and resultant scales issues has not yet addressed and comprehensively understood.
The present study aims to simulate the compatibility of Mishrif formation water (MFW) with the six water types (TRW, PWF, GSW, MW, MKFW, and MODW). The amount of probable scaling, which can happen from mixing different ratios by determining mineral SI using a hydrogeochemical PHREEQC V.3 2019 program, is predicted. This estimation is based on Pitzer electrolyte theory, which is widely regarded as the most accurate method for calculating the influences of high temperature, pressure, and total dissolved solids on the composition of activity factors using Pitzer equations (Pitzer and Press 1991). Accomplishing these goals help to a better understanding of serious scaling issues in water injection processes. This study is a step forward to enhancing oil production by identifying appropriate water types for injection processes and reducing corrosion.
Theoretical background
The aim of the fluid injection into porous rock is to raise hydrocarbon recovery during the exploration and production process. Changes in reservoir rock characteristics are generally expected due to incompatibility between injected and native fluids. On the other hand, the reservoirs' heterogeneity will have an inevitable impact. Pore-fluid sensitivity in reservoir sandstones, for example, is highly affected by reservoir heterogeneity and sandstone microstructure, and it is, therefore, essential to include these geologic factors in the rock-physics analysis (Avseth et al. 2010). The major problem in the shale reservoirs is heterogeneity, and overcoming this obstacle needs an amount of 10 million gallons of water (Muther et al. 2021). In comparison, fracture evaluation is an essential step in the evaluation of heterogeneous carbonate reservoirs, especially where the permeability trend is not describable by core data because of secondary porosity (Aghli et al. 2020).
Geochemical modeling of the water–rock-petroleum interactions generally includes three activities: chemical speciation, mineral mass transfer that relies on mass balance calculations along specific reaction paths, and geochemical mixing process among water bodies, while hydrogeochemical interactions are either single-phase reactions called homogenous phases or more than one phase called heterogeneous phase interactions, which happen between two statuses, such as water–rock, gas–rock, and gas–water (Ghalib 2017; Ghalib and Almallah 2017). These interactions occur and are modified based on surrounding environmental alterations. To conduct the hydrogeochemical modeling technique, many agents, such as SI, ionic strength (ionic activity %), and CO2 partial pressure, should be recognized (Allen and Suchy 2001; Frenier 2018). Mathematical surface complexation models (SCMs) are essential in reactive transport modeling.
The surface complexation models (SCMs) are a general concept considering the interfacial equilibrium caused by specific reactions of bulk species with active surface groups. The SCMs have taken a prominent stage in capturing the electrostatic effect of brine salinity and ionic adsorption on the calcite surface. Also, it provides molecular and thermodynamic descriptions of the electrostatic and geochemical interactions on a colloidal surface. Different types of SCMs have been proposed in the literature to describe the adsorption of ions on the colloidal surface (Tetteh et al. 2022). The research on enhanced oil recovery within the last decade has led to an increased number of publications reporting both zeta potential measurements and SCMs for calcite. The SCMs are usually used to interpret experimental results by breaking down and quantifying the contribution of different surface interactions to the overall measured zeta potential. These mathematical models initially developed to describe the reactivity of oxide–water interfaces assume that the energy of adsorption is a contribution of a chemical and an electrostatic term (Bonto et al. 2022). Despite their generalized use, some aspects of these SCMs are still questioned; the unclear thermodynamic significance of the relationship between the apparent and intrinsic equilibrium constants or the lack of validation of the equilibrium constants over diverse experimental conditions are among the primary concerns (Bonto et al. 2022).
The hydrogeochemical models can be used for several purposes, including estimating prevailing geochemical reactions and determining the extent to which these reactions happen, predicting mineralogical contaminants and estimating groundwater flow rates and directions (Plummer 1992). Waterflood projects stay under the unit mobility ratio limit; some may unintentionally do so, producing erroneous conclusions from the injection data. A heated oil reservoir is typically filled with cold water from the surface, causing a temperature gradient and, as a result, a viscosity gradient near the injection well. A saturation gradient also exists, particularly toward the flood front (Rahman and Obathani 2019; Varfolomeev et al. 2022). Geochemical modeling of the water–rock-petroleum interactions also helps identify the controlling factors of H2S generation and predict the concentration and distribution of subsurface H2S before drilling and mapping (Lu et al. 2022). PHREEQC V.3 2019 is one of the crucial Hydrogeochemical modeling applications utilized in the current study to identify chemical model factors that can determine ionic strength, SI, and aqueous species distribution (Parkhurst and Appelo 1999).
Study area and geological setting
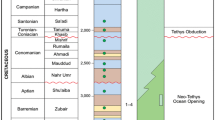
Halfaya oilfield is placed in Maysan governorate, 35 km southeast Amara city (Fig. 1). The structure, which is composed of two domes, runs along a northwest–southeast coordinate east (E 726,000–739,000) and north (N 3,500,000–351,400) and has a gentle elongated anticline of about 38 km long and 12 km wide. Halfaya oilfield is located in the Maysan region, southeast of Iraq, around 400 km from its capital, Baghdad. Tectonically, it is located at the unstable continental shelf in the northern Persian/Arabian Gulf basin at the northern brink of Gondwanaland and at the east side of the unstable continental shelf zone, Mesopotamian main belt, and the south section of the Tigris sub-belt (Zhong et al. 2018; Almalikee and Sen 2021). The Mesopotamian basin has a great deep buried area with thick sediments, somewhat stable tertiary tectonic units, and a well-presented platform environment (Fig. 1) (Ameen 1992; Azzam and Taher 1993). The study region is a gentle northwest–southeast anticline, influenced by the Alpine movement. The Mishrif formation was formed during the middle Cretaceous period, with a sedimentary thickness of 350–400 m that gradually thins to 150 m as it moves southwest. In the Iraq–Iran borders and Basra area, it is striped in a southeast–northwest direction (Alkersan 1975; Aqrawi 1998; Buday and Jassim 1987; Dunnington 1958; Ghalib 2014; Owen and Nasr 1958). It is part of the Qamchuqa group, and its reservoir rocks are carbonate with rudist deposited in a shallow marine platform. The most common are intergranular, intragranular (dissolved), and frame pores (Gao et al. 2013). The Late Cenomanian to Early Turonian was a period of generally favorable conditions worldwide for high organic productivity and the eustasy was the major element controlling the growth, development and location of built (Van Buchem et al. 2002). Mishrif formation, the most prominent oil-producing formation, may be as thick as 400 m and separated into 15 distinct strata. The late Cretaceous Laramide Orogeny results in a regional unconformity surface on top of the Mishrif formation (Aqrawi et al. 2010). The Mishrif is composed of two major sedimentary cycles abruptly terminated by the unconformity, which separates the Mishrif from the overlying Khasib formation (Aqrawi et al 2010). The top zone of Mishrif, MA1, is the regional first-order sequence boundary and consists of brecciated lime mudstone and packstone. The oil-bearing MA2 comprises dolomitized packstone, deposited and diagenetically formed in a shoal environment, with biomoldic/dissolution pores. The average reservoir thickness of MA2 is 8.9 m (17 m, maximum) (Fig. 2) (Zhong et al. 2018). The lower boundary of Mishrif formation represents the change from basinal Rumaila formation to shallow open marine facies. It is a conformable surface. The upper boundary with the Khassib formation is truncated by an unconformity surface separating the Middle from Late Cretaceous. The top Mishrif runcation forms the AP9/AP8 megasequence boundary at ~ 92 million years (Aqrawi et al. 2010).
Halfaya Oilfield's Cretaceous stratigraphic column and cored wells with interval distribution of drilling wells. adapted from (Zhong et al. 2018)
Materials and methods
Figure 3 outlines the main steps of current research. This research mainly focuses on 12 cored wells having thin sections, namely wells HF-1, HF-3, HF-4, HF-5, HF-7, HF-26, HF-161, HF-81, HF-115, HF-137, HF-195, and HF-316. These 12 wells cover all parts of the study area (Fig. 1). The six water sources selected for their injection into the Mishrif formation wells are the treated PWF, TRW, GSW, MW, MKFW, and MODW. Mishrif formation is the sampling target to collect oilfield water.
The multiparameter probe (HQ11d Portable pH Meter) was employed to record temperature, pH, electrical conductivity (EC). Based on standards guidelines of hydrogeological surveys, each well was properly purged prior groundwater sampling. Also, samples collection bottles were treated (HDPE), cleaned and labeled before sampling. In May 2020, water samples were subjected to physicochemical analyses in the quality central laboratories of Maysan Oil Company in Iraq following Standard Methods for Examination of Water and Wastewater (APHA 2017). The chemical analysis of water samples (oilfield water samples with injection waters) includes the analysis of major cations (Na+, Mg2+, K+, and Ca2+) and major anions (Cl−, \({\text{SO}}_{4}^{2 - }\), HCO3−, and \({\text{CO}}_{3}^{2 - }\)) (Tables 1, 2). Generally speaking, the salinity of water Tigris and Euphrates increases downstream, but at different rate. Salinity increases gradually at the northern part compare with another river in the world. Inverse geochemical modeling (PHREEQC program) is used to calculate SI, ionic strength, and aqueous species distribution, for testing the compatibility of MFW with six distinct water sources and subsequently for deciding whether it is practical and can be used as water injection in oil production.
Chemical compatibility and saturation index (SI)
SI is an index that is used for chemical interaction model studies. It shows the mineral saturation status in the groundwater system and then determines the rock type contacting with groundwater. Pitzer equations have defined it. Therefore, the minerals in the aqueous phase are in equilibrium, supersaturation or undersaturation status (Gedamy et al. 2011).
Here, SI is saturation index, IAP is product of the ionic activity in dissolution reaction, and KSp is equilibrium constant of the mineral dissolution at a specific temperature.
SI are given in three cases as follows:
-
S.I (0): Mineral phase is in equilibrium status in the water
-
S.I (˃ 0): Precipitation of mineral phase in the water (super saturated status)
-
S.I (˂ 0): Dissolution or alteration of mineral phase in the water (under saturated status).
SI type controls the contents of inorganic constituents of groundwater. All minerals usually react with groundwater to some extent. However, minerals that react slowly have less influence on chemical compositions than minerals that react rapidly. Mineral stability is different because of the changing equilibrium conditions during mineral formation. Mineral abundance in any aquifer system also affects water chemistry. SI is essential for calculating chemical compatibility. Chemical compatibility is a holding group that simulates the compatibility through which kinds mix different injection sources with oilfield water at different rates (Wilkin and DiGiulio 2010). Subsequently, the properties of the new water are tested to determine whether it is compatible or incompatible as injection water of the produced oil. The six water types are selected to be mixed by different ratios (10–90%), (20–80%), (30–70%), (40%–60%), (50–50%), (60–40%), (70–30%), (80–20%), and (90–10%) with the oilfield water of Mishrif formation in Halfaya oilfield. Waters that can be mixed without precipitates are called compatible if they can be mixed without producing any chemical reaction among the dissolved solids in the waters and thus precipitating insoluble compounds (Henkel 1953). Water used for injection usually contains an amount of inorganic salts and sometimes organic salts in solution; testing the compatibility of the injection water and water in the formation before starting the injection operation is common practice (Collins 1977). In general, scale (salt crust), precipitated due to mixing, is one of the most critical problems that inflict damage on injection systems and the main oil reservoir. It can also plug production lines, equipment, and impaired fluid flow (Moghadasi et al. 2003; Merdhah and Yassin 2008). In the case of injection systems, the formed scale may cause a plug in formation pores, thus decreasing permeability and causing obstruction to the fluid flow with time (Collins and Wright 1985). One of the most critical and hazardous phenomena is mineral scale deposition due to the incompatibility between injected and formation waters and changes in temperature, pressure, gas dissolution, and ph. It is a scale deposition process from aqueous mineral solutions, referred to as brines, when they become supersaturated solutions as a result of changes in the state of their thermodynamic and chemical equilibrium (Taheri et al. 2011). The most important ions present in oilfield water and cause scale precipitation are Ca2+, Sr2+, Ba2+, Fe2+, HCO−, and CO2− (Oddo et al. 1982; Paulo et al. 2001). Scale deposition can occur in a particular water type due to supersaturation with scale-forming salts attributable to variations in physical conditions based on which the water exists (Yuan and Todd 1991). Scales form when two incompatible liquids are set together and supersaturation happens. The primary source of oilfield scale is waters that are named incompatible if they react chemically and precipitate minerals when mixed (Merdhah and Yassin 2007). The common scale types known in oil reservoirs are listed in Table 3. Sulfates such as calcium sulfate (anhydrite, gypsum), barium sulfate (barite), strontium sulfate (celestite), and calcium carbonate (calcite) are important scales in oilfield operations. The proposed ARN acid structure suggested by (Lutnaes et al. (2006) is presented in Fig. 4.
Proposed predominant structure of ARN acid (Modified after Olajire 2015)
Mathematical model of chemical compatibility
This model is designed to determine the scale type formed and calculate SI while mixing two water types with differences in components’ chemical and physical properties. First, SI indicates if a solution is in equilibrium, undersaturated or supersaturated condition concerning a solid phase (Warsinger et al. 2015). One value signifies a tenfold supersaturation, whereas a value of (− 2) refers to a 100-fold undersaturation about a particular mineral phase. Equilibrium can be assumed for a range of (− 0.5) to (+ 0.5). If the SI value is below (− 0.5), then the solution is undersaturated about the corresponding mineral; if SI exceeds (+ 0.5), then the water is supersaturated for this mineral (Ghalib 2017; Ghalib and Almallah 2017; Hussain et al. 2019; Al-Qurnawi et al. 2022). Therefore, PHREEQC V.3 is used in this study because it can simulate the interactions that occur during the mixing and precipitation of mineral types.
Results and discussion
Oilfield water and source water properties
Oilfield water is natural water presented in pores and holes of reservoir rocks before the subsurface injection process (Collins 1977), which is therefore sometimes called formation water or reservoir water. Hydrochemistry is useful for determining the reservoir nature and oil properties where the chemical compositions of these waters demonstrate several factors, including water origin (Bozdağ 2016; Ghalib 2017; Shah et al. 2021). Waterflooding, also known as water injection, is a process in which water is pumped into an oil reservoir to maintain reservoir pressure or move oil toward wells, thereby increasing output (Ghalib and Almallah 2017). Thus, oilfield water properties and water injection are tested to determine whether oilfield water is compatible or incompatible with being the injection water of the produced oil. The Piper diagram (Piper 1944) is employed in this study to classify waters using hydrochemical facies. It depicts MFW classification in Halfaya oilfield, with all water samples falling into the sodium chloride type (NaCl) t, except for HF-81, which is classified as a mix type (CaMgCl) type (Fig. 5).
Surface water samples are collected from different water types: TRW, PWF from Central Processing Facility in Halfaya oilfield, Southern Iraq (Table 2), GSW, MW, MKFW, and MODW. These water types are tested for chemical compatibility by mixing them with Mishrif formation oilfield water and then evaluating whether it is appropriate to be used as water injection in oil production. For this reason, their physical and chemical properties are determined.
A stiff diagram is used to determine all selected injection water qualities for simulation (Fig. 6). According to the diagram, all injection water types are Na and K predominant cations, whereas Cl is the predominant anion, and the water type is NaCl (Fig. 6).
Simulation of mineral scale quantity for Mishrif Oilfield water
Chemical compatibility of sample Tigris River water (TRW)
Table 4 illustrates the results of combining TRW with MFW at various mixing ratios under reservoir circumstances to calculate mineral SI and generate the equilibrium conditions of selected minerals. In all mixing ratios between TRW and MFW, the results reveal calcite, aragonite, dolomite, celestite, goethite, and strontium supersaturation. By contrast, the projected results show gypsum, anhydrite, and siderite undersaturation (Fig. 7). Moreover, mixing ratios (30–70%), (50–50%), (70–30%), and (90–10%) suggest that barite is close to the equilibrium zone, but only the mixing ratio (10–90%) depicts barite in the supersaturation zone (Table 4) (Fig. 7). Furthermore, only siderite is in the undersaturation state, and no precipitation is observed from the mixtures (10–90%), (30–70%), (50–50%), (70–30%), and (90–10%) of oilfield water to TRW (Table 3) (Fig. 7).
Chemical compatibility of sample producing water formation (PWF)
To find the chemical compatibility of PWF with the oilfield water of Mishrif reservoir, SI (kcal/mole) is calculated for nine mixtures at reservoir conditions. Table 5 shows the results of the SI of gypsum, anhydrite, and siderite in all mixing ratios in undersaturated states; moreover, the negative value indicates that these minerals are in equilibrium or undersaturated state and that no mineral precipitate occurs. Therefore, all these minerals occupy the undersaturated zone (Fig. 8). These SI values refer to that the celestite in all mixing ratios is in equilibrium zone or undersaturation with no mineral precipitate (Fig. 8). Other SI values refer to that the barite in mixing ratios (10–90%), (20–80%), (30–70%), (40–60%), (50–50%), and (60–40%) is in equilibrium zone, but that in mixing ratios (70–30%), (80–20%), and (90–10%) is in saturation state. As a result, mixing should be used to perform a periodic inhibitory therapy. The mixture ratios incompatible between the oilfield water and PWF are (10–90%), (20–80%), (30–70%), (40–60%), (50–50%), (60–40%), (70–30%), (80–20%), and (90–10%) for minerals dolomite, goethite, calcite, and aragonite; compatible waters for strontium mineral are in mixing ratios (10–90%), (20–80%), (30–70%), (40–60%), (50–50%), and (60–40%), whereas incompatible waters are in mixing ratios (70–30%), (80–20%), and (90–10%). Therefore, treatment is needed in these mixing ratios (Table 5) (Fig. 8).
Chemical compatibility of sample Gulf seawater (GSW)
The SI (kcal/mole) of the mixture of the Mishrif formation oilfield water and sample GSW under reservoir conditions is calculated through nine mixtures. These SI values refer to that the siderite is undersaturated. By contrast, goethite, dolomite, calcite, aragonite, strontium, and celestite are saturated (Table 6) (Fig. 9). Barite, gypsum, and anhydrite are close to the equilibrium state under the saturated zone in all mixing ratios. The mixture ratios compatible between oilfield water and GSW are (10–90%), (20–80%), (30–70%), (40–60%), (50–50%), (60–40%), (70–30%), (80–20%), and (90–10%) for mineral siderite; they are compatible waters because no minerals precipitate and eventually no scale is formed (Table 6) (Fig. 9), whereas the mixture ratios mentioned above are incompatible for minerals goethite, dolomite, calcite, aragonite, strontium, and celestite; therefore, these minerals occupy the precipitation zone at these mixing ratios (Table 6) (Fig. 9). As a result, mixing should be used to perform a periodic inhibitory therapy, in addition to barite, gypsum, and anhydrite.
Chemical compatibility of sample Marshes waters (MW)
The results of mixing MW in MFW with varying mixing ratios under reservoir circumstances determine the SI of minerals and construct equilibrium conditions for the chosen minerals (Table 7). In general, the data demonstrate that siderite is undersaturated. By contrast, the projected results suggest that goethite, aragonite, dolomite, calcite, barite, strontium, and celestite are supersaturated (Fig. 10). Gypsum and anhydrite do not precipitate from any mixture of the oilfield with MW. Therefore, they are represented by a curve occupying equilibrium and undersaturated zones (Fig. 10). Goethite and dolomite SI show a supersaturation of (10, 20%), (30, 40%), (50, 60%), (70, 80%), and 90% of MW and MFW, respectively (Table 7) (Fig. 10). The mixture ratios compatible between the oilfield water and MW are (10–90%), (20–80%), (30–70%), (40–60%), (50–50%), (60–40%), (70–30%), (80–20%), and (90–10%) for mineral siderite; they are compatible waters because no minerals precipitate and eventually no scale is formed (Table 7) (Fig. 10). Meanwhile, the mixture ratios mentioned above are incompatible for minerals goethite, aragonite, dolomite, calcite, barite, strontium, and celestite. Thus, these minerals occupy the precipitation zone at these mixing ratios (Table 7) (Fig. 10). As a result, mixing should be used to perform a periodic inhibitory therapy, in addition to gypsum and anhydrite.
Chemical compatibility of sample Middle Kirkuk formation waters (MKFW)
To find the chemical compatibility of MKFW with the oilfield water of Mishrif reservoir, SI (kcal/mole) is calculated for nine mixtures at reservoir conditions. Table 8 illustrates the SI of gypsum, anhydrite, and celestite in all mixing ratios in the equilibrium state, including the negative values showing that these minerals are in equilibrium or undersaturated condition, with no mineral precipitates. Therefore, all these minerals occupy the undersaturated zone (Fig. 11). Goethite is in a clear saturation state for mixing ratios (10–90%), (20–80%), (30–70%), (90–60%), (50–50%), (60–40%), and (70–30%). Consequently, these mixtures occupy the precipitation zone (Fig. 11) and eventually represent incompatible waters. Mixing ratios (80–20%) and (90–10%) in equilibrium or undersaturated state appear as compatible waters. The result in (Table 8) shows that the dolomite is saturated in mixing ratios (10–90%), (20–80%), and (30–70%), whereas it is unsaturated in mixing ratios (50–50%), (60–40%), (70–30%), (80–20%), and (90–10%) (Fig. 11). These SI values refer to that the aragonite, calcite, and strontium (Fig. 11) are saturated in mixing ratios (10–90%), (20–80%), (30–70%), and (40–60%). However, they are unsaturated in mixing ratios (50–50%), (60–40%), (70–30%), (80–20%), and (90–10%). The curves of these minerals occupy an undersaturated zone, indicating that no scale is formed (Fig. 11).
Chemical compatibility of sample main outfall drain water (MODW)
Table 9 and Fig. 12 display the results of mixing MODW in MFW with various mixing ratios under reservoir circumstances resulting in the determination of SI and the construction of equilibrium conditions for the chosen minerals. The results demonstrate that goethite and dolomite are supersaturated in general, in mixtures of 10, 20, 30, 40, 50, 60, 70, 80, and 90% oilfield water by 90, 80, 70, 60, 50, 40, 30, 20, and 10% MDW, respectively. Scales may precipitate according to this theory (Fig. 12). In addition, the results for aragonite, strontium, and calcite in saturation show all mixing ratios, but low ones. As a result, mixing should be used to perform a periodic inhibitory therapy. Table 8 provides the results of the SI of celestite and barite in all mixing ratios in the equilibrium zone or undersaturated zone and no mineral precipitates (Fig. 12). These SI values refer to the siderite, anhydrite, and gypsum undersaturated in mixing ratios (10–90%), (20–80%), (30–70%), (40–60%), (50–50%), (60–40%), (70–30%), (80–20%), and (90–10%). However, due to their low saturation ratios, siderite, anhydrite, and gypsum scales do not require treatment. Nevertheless, goethite, dolomite, aragonite, strontium, and calcite scales should be treated regularly.
Findings
This study uses the mathematical model of chemical compatibility to mix six types of injection water sources in different ratios with MFW detected in surface water injection. The following items can be concluded and highlighted:
-
The best water types used for injection are MKFW, followed by MODW, and then GFW, which reveals compatibility with formation water
-
The findings of the scale prediction model for the Mishrif formation utilizing TRW for injection suggest that the most prevalent oilfield scales characterized by carbonate and sulfate minerals have a chance to precipitate. Siderite, anhydrite, and gypsum do not precipitate. Celestite and barite, meanwhile, are extremely rare
-
MW injections produce the same findings as in the GSW prediction model. The principal precipitated scales are carbonate and sulfate minerals, whereas other projected scales are not expected to present serious issues because they dissolve under reservoir conditions
-
When mixing the water formation of Mishrif reservoir with MKFW for injection, the findings of the scale estimate model indicate that sulfate and carbonate minerals are the principal precipitated scales, only for the mixing ratios of 10, 20, and 30%; the rest of the mixing ratios are not precipitated scales
-
Scaling simulation findings demonstrate supersaturation and the possibility of precipitating aragonite, calcite, dolomite, goethite, and strontianite minerals when mixing MDW with various ratios. Other predicted scales are not anticipated to present serious issues under reservoir conditions
-
Given the findings, MWF is incompatible with TRW and PWF
-
All scale-predicting models of surface water injection indicate no compatibility with the formation water; thus, a chemical inhibitor treatment by mixing is considered to avoid or diminish barite and calcite scale precipitation
-
This study is a step toward improving oil production by detecting the appropriate water types for injection processes. It provides a further understanding of the serious issues in the water injection processes that aim at reducing corrosion and other issues.
Advantages and disadvantages
The advantages of this research are given as follows:
-
Through this methodology, the best water to enhance oil production was identified in its compatibility to prevent sedimentation resulting from the injection. The sedimentation leads to the closing of the pores and permeability, thus decreasing oil production instead of enhancing it.
-
Obtaining the above results at the lowest possible cost.
-
Ease of applying the study method through simulation through specialized programs such as PHREEQC due to its unique and comprehensive capabilities and accessibility.
-
Different injection sources were used that are available in the region, considering the economic feasibility of its proximity to the oil production field.
The potential limitations and disadvantages include:
-
As a potential limitations of this research, the results of this study cannot be generalized to all other oil reservoirs with different geological composition and various reservoir conditions even though the methodology of this study can be globally used. In the event of employing a certain reservoir conditions and different injection source to get results of this certain reservoir, it would definitely differ from our results of the studied reservoir in the current study.
-
Intricacy of the reaction conditions that differ with each type of water used.
-
The results demonstrated that the marine water is appropriate for injection. Yet, it is infeasible to use due to requiring costly transportation facilities from the source (the Gulf) to the far oilfields.
-
Some of the injected water requires potentially costly treatment before the injection.
-
This study has yet to do experimental mixing between injected and oilfield waters, which is recommended for future research
Conclusions
This study uses the mathematical model of chemical compatibility to mix six injection water sources in different ratios with Mishrif formation water detected in the surface water injection. The research concludes the following:
-
The results showed that most of the suggested water for injection is sodium chloride.
-
Given the findings, the formation water of the Mishrif reservoir is incompatible with the Tigris River water and producing water formation.
-
A scale is deposited during mixing. The number of precipitated salts varies depending on the mixing ratios.
-
The precipitated salts lead to many problems in the oil reservoirs by preventing fluid movement in the reservoir due to locking the pore spaces and fractures.
-
To reach formation water compatibility, implementing the chemical inhibition treatment through mixing should be taken into consideration to avoid scale precipitation.
-
This study's geochemical modeling method is a step toward better understanding scaling issues by efficiently detecting the best injection water with the lowest possible cost, which enhances oil production.
Abbreviations
- ARN::
-
A family of tetrameric acids
- GSW::
-
Gulf seawater
- HF::
-
Halfaya oilfield
- IAP::
-
Product of the ionic activity in dissolution reaction
- Ksp::
-
Equilibrium constant of the mineral dissolution at a specific temperature
- MFW::
-
Mishrif formation water
- MKFW::
-
Middle Kirkuk formation water
- MODW::
-
Main outfall drain water
- MW::
-
Marshes water
- PWF::
-
Producing water formation
- TRW::
-
Tigris river water
- SI::
-
Saturation index
References
Aghli G, Moussavi-Harami R, Mohammadian R (2020) Reservoir heterogeneity and fracture parameter determination using electrical image logs and petrophysical data (a case study, carbonate Asmari Formation, Zagros Basin, SW Iran). Pet Sci 17:51–69
Al-Asadi SA, Al Hawash AB, Alkhlifa N-HA, Ghalib HB (2019) Factors affecting the levels of toxic metals in the Shatt Al-Arab River, Southern Iraq. Earth Syst Environ 3:1–13
Al-Atabi AN (2009) Hydrogeochemistry of Yamama Formation reservoir water and the over pressure estimation in selected oil wells—Southern Iraq fields. Unpub. PhD Thesis, M. Sc. Thesis, College of Science, University of Basrah
Alkersan H (1975) Deposition environment and geological history of the Mishrif Formation in southern Iraq. In: 9th Arab Petroleum Congress, Dubai, UAE, Paper 121(section B-3), pp. 1e18
Al-Khafaji JL (2003) Hydrochemistry and hydrodynamics of oil-field brines of cretaceous reservoirs in oil-fields-south Iraq. PhD Thesis, Ph. D. Thesis, College of Science, Baghdad University
Allen DM, Suchy M (2001) Geochemical evolution of groundwater on Saturna Island, British Columbia. Can J Earth Sci 38:1059–1080
Almalikee HS, Sen S (2021) Present-day stress field and stress path behaviour of the depleted Mishrif reservoir from the super-giant Zubair oilfield, Iraq–A geomechanical case study. J Afr Earth Sc 184:104381
Al-Mallah IA, Al-Qurnawi WS, Ghalib HB et al (2022) Evaluation of groundwater quality in the Dibdibba aquifer using hydrogeochemical and isotope techniques (Basrah Province, Iraq). Acta Geochimica 41:1–16
Alnajar AD, Refai RT (2022) Prediction Of calcium carbonate and calcuim sulfate scale deposits in mabruk oil field, Libya. The 1st International Conference of the Faculties of Sciences
Al-Qurnawi WS, Ghalib HB, Alabadi MA, Hawash ABA (2022) Corrosion-scaling potentially of domestic water pipelines and evaluate the applicability of raw water sources in Basrah, IRAQ Iraqi. J Sci 63:2089–2102
Al-Samhan M, Alanezi K, Al-Fadhli J et al (2020) Evaluating scale deposition and scale tendency of effluent water mix with seawater for compatible injection water. J Petrol Explor Prod Technol 10:2105–2111
Ameen MS (1992) Effect of basement tectonics on hydrocarbon generation, migration, and accumulation in northern Iraq. AAPG Bull 76:356–370
APHA (2017) Standard methods for the examination of water and wastewater. American Public Health Association, Washington
Aqrawi AA (1998) Paleozoic stratigraphy and petroleum systems of the western and southwestern deserts of Iraq. GeoArabia 3:229–248
Aqrawi AA, Goff JC, Horbury AD, Sadooni FN (2010) The petroleum geology of Iraq. Scientific press
Avseth P, Mukerji T, Mavko G, Dvorkin J (2010) Rock-physics diagnostics of depositional texture, diagenetic alterations, and reservoir heterogeneity in high-porosity siliciclastic sediments and rocks—a review of selected models and suggested work flows. Geophysics 75:75A31-75A47
Azzam IN, Taher AK (1993) Sequence stratigraphy and source rock potential of Middle Cretaceous (upper Wasia Group) in west Abu Dhabi. In: Middle East oil show. OnePetro, p 13
Bonto M, Eftekhari AA, Nick HM (2022) Electrokinetic behavior of artificial and natural calcites: A review of experimental measurements and surface complexation models. Adv Coll Interface Sci 301:31. https://doi.org/10.1016/j.cis.2022.102600
Bozdağ A (2016) Hydrogeochemical and isotopic characteristics of Kavak (Seydişehir-Konya) geothermal field, Turkey. J Afr Earth Sc 121:72–83
Buday T, Jassim SZ (1987) The regional geology of Iraq, tectonism, magmatism and metamorphism, vol 2. Publication of GEOSURV, Baghdad, p 352
Collins AG, Wright CC (1985) Enhanced oil recovery injection waters. In: Developments in petroleum science. Elsevier, pp 151–221
Collins A (1977) Enhanced-oil-recovery injection waters. In: SPE international oilfield and geothermal chemistry symposium. OnePetro
Dunnington HV (1958) Generation, migration, accumulation, and dissipation of oil in northern Iraq: Middle East. American Association of Petroleum Geologists, Tulsa
El-Hattab MI (1982) GUPCO’s experience in treating Gulf of Suez seawater for waterflooding the El Morgan oil field. J Petrol Technol 34:1449–1460
Faure G (1998) Principles and application of geochemistry, 2nd edn. Prentice HallInc, Hoboken, p 600
Frenier WW (2018) Chemical and mechanical methods for pipeline integrity. Soc Petrol Eng. https://doi.org/10.2118/9781613994962
Gao J, Changbing T, Weimin Z (2013) Characteristics and genesis of carbonate reservoir of the Mishrif Formation in the Rumaila oil field, Iraq. Acta Petrolei Sinica 34:843–852
Gedamy YR, El-Fadl MMA, Fakharany M, Mahmoud MA (2011) Geochemical evidences for possible water seepage from Lake Nasser to the groundwater in the area between Abu Simbel and Tushka. Egypt J Pure Appl Sci 49:12
Ghalib HB (2017) Groundwater chemistry evaluation for drinking and irrigation utilities in east Wasit province, Central Iraq. Appl Water Sci 7:3447–3467. https://doi.org/10.1007/s13201-017-0575-8
Ghalib HB, Almallah IA (2017) Scaling simulation resulting from mixing predicted model between Mishrif formation water and different waters injection in Basrah oil field, southern Iraq. Model Earth Syst Environ 3:1557–1569. https://doi.org/10.1007/s40808-017-0384-y
Ghalib HB, Sogut AR (2014) Environmental isotopic characterization of groundwater and surface water in northeast Missan Province, South Iraq. Acta Geol Sin-Engl Ed 88:1227–1238. https://doi.org/10.1111/1755-6724.12285
Ghalib HB (2014) Geochemical modeling characteristics of groundwater in northeast Missan (southern Iraq). PhD Thesis, Selcuk University, 203
Henkel HO (1953) Surface and underground disposal of chemical wastes at Victoria, Texas. Sew Ind Wastes 25:1044–1049
Hussain SA, Han F-Q, Han W et al (2019) Climate change impact on the evolution of the saline lakes of the Soan-Sakaser Valley (Central Salt Range; Pakistan): evidences from hydrochemistry and water (δD, δ18O) and chlorine (δ37Cl) stable isotopes. Water 11:912
Jumaah HA (2021) Modified Archie’s parameters for estimating water saturation for carbonate reservoir in north of Iraq. J Petrol Explor Prod Technol 11:3689–3697
Liang J, Jin L, Li W, et al (2019) Material selection for water injection system for a giant oil field, Iraq. In: SPE Kuwait Oil & Gas Show and Conference. OnePetro
Lindlof JC, Stoffer KG (1983) A case study of seawater injection incompatibility. J Petrol Technol 35:1256–1262
Liu X, Jungang L, Qianya Z et al (2009) The analysis and prediction of scale accumulation for water-injection pipelines in the Daqing Oilfield. J Petrol Sci Eng 66:161–164
Lu P, Luo P, Wei W, Zhu C (2022) Effects of gas saturation and reservoir heterogeneity on thermochemical sulfate reduction reaction in a dolomite reservoir, Puguang gas field China. Marine Petrol Geol 135:105402
Lutnaes BF, Brandal Ø, Sjöblom J, Krane J (2006) Archaeal C 80 isoprenoid tetraacids responsible for naphthenate deposition in crude oil processing. Org Biomol Chem 4:616–620
Merdhah AB, Yassin AM (2007) Scale formation in oil reservoir during water injection at high-salinity formation water. J Appl Sci 7:3198–3207
Merdhah AB, Yassin AM (2008) Formation damage due to scale formation in porous media resulting water injection. System 14:15
Mitchell RW, Grist DM, Boyle MJ (1980) Chemical treatments associated with North Sea projects. J Petrol Technol 32:904–912
Moghadasi J, Jamialahmadi M, Müller-Steinhagen H, et al (2003) Scale formation in Iranian oil reservoir and production equipment during water injection. In: International symposium on oilfield scale. OnePetro
Moghadasi J, Müller-Steinhagen H, Jamialahmadi M, Sharif A (2007) Scale deposits in porous media and their removal by edta injection
Murtaza M, Alarifi SA, Rasm MY et al (2022) Single step calcium sulfate scale removal at high temperature using tetrapotassium ethylenediaminetetraacetate with potassium carbonate. Sci Rep 12:1–17
Muther T, Qureshi HA, Syed FI et al (2021) Unconventional hydrocarbon resources: geological statistics, petrophysical characterization, and field development strategies. J Petrol Explor Prod Technol 12:1–26
Oddo JE, Sloan KM, Tomson MR (1982) Inhibition of CaCO3 precipitation from brine solutions: a new flow system for high-temperature and-pressure studies. J Petrol Technol 34:2409–2412
Olajire AA (2015) A review of oilfield scale management technology for oil and gas production. J Petrol Sci Eng 135:723–737
Owen RMS, Nasr SN (1958) Stratigraphy of the Kuwait-Basra Area: Middle East
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (Version 2): a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. Water Res Investig Rep 99:312
Paulo J, Mackay EJ, Menzies N, Poynton N (2001) Implications of brine mixing in the reservoir for scale management in the alba field. In: International symposium on oilfield scale. OnePetro
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. EOS Trans Am Geophys Union 25:914–928
Pitzer KS, Press CRC (1991) Activity coefficients in electrolyte solutions. CRC Press Boca Raton, FL
Plummer LN (1992) Geochemical modeling of water-rock interaction; Past, present, future. In: International symposium on water-rock interaction. pp 23–33
Rahman NM, Obathani OH (2019) An investigation into apparent differences between injection transmissibility and falloff transmissibility in oil reservoirs subject to water injection. In: SPE middle east oil and gas show and conference. OnePetro, p 10
Shah M, Sircar A, Shah V, Dholakia Y (2021) Geochemical and Geothermometry study on hot-water springs for understanding prospectivity of low enthalpy reservoirs of Dholera Geothermal field, Gujarat India. Solid Earth Sciences 6(3):297–312
Shuler PJ, Freitas EA, Bowker KA (1991) Selection and application of BaSO4 scale inhibitors for a CO2 flood, Rangely Weber Sand Unit, Colorado. SPE Prod Eng 6:259–264
Taheri A, Zahedzadeh M, Masoudi R, et al (2011) Simulation and experimental studies of mineral scale formation effects on performance of Sirri-C oil field under water injection
Tetteh JT, Barimah R, Korsah PK (2022) Ionic interactions at the crude oil–brine–rock interfaces using different surface complexation models and DLVO theory: application to carbonate wettability. ACS Omega 7:7199–7212
Van Buchem FS, Razin P, Homewood PW et al (2002) Stratigraphic organization of carbonate ramps and organic-rich intrashelf basins: Natih Formation (middle Cretaceous) of northern Oman. AAPG Bull 86:21–53
Varfolomeev M, Rezaei Koochi M, Yuan C, et al (2022) Feasibility of ion-modified water for low salinity water flooding: a case study for ultra-high-salinity carbonate reservoir in Akanskoe Oilfield Tatarstan, Russia. In: SPE Conference at Oman Petroleum & Energy Show, OnePetro, 11
Vetter OJ, Farone WA, Veith E, Lankford S (1987) Calcium carbonate scale considerations: a practical approach. In: SPE production technology symposium. OnePetro
Warsinger DM, Swaminathan J, Guillen-Burrieza E, Arafat HA (2015) Scaling and fouling in membrane distillation for desalination applications: a review. Desalination 356:294–313
Wilkin RT, DiGiulio DC (2010) Geochemical impacts to groundwater from geologic carbon sequestration: controls on pH and inorganic carbon concentrations from reaction path and kinetic modeling. Environ Sci Technol 44:4821–4827
Yan Z, Hu R, Li F et al (2021) Scaling tendency prediction in water injection well of K2 formation of C68 block. E3S Web Conf 300:02012. https://doi.org/10.1051/e3sconf/202130002012
Yuan MD, Todd AC (1991) Prediction of sulfate scaling tendency in oilfield operations (includes associated papers 23469 and 23470). SPE Prod Eng 6:63–72
Zhong Y, Zhou L, Tan X et al (2018) Characteristics of depositional environment and evolution of Upper Cretaceous Mishrif Formation, Halfaya oil field, Iraq based on sedimentary microfacies analysis. J Afr Earth Sc 140:151–168
Acknowledgements
The authors want to express their appreciation to the Missan Oil Company for their permission and data providing. Special thanks to Editor and the reviewers for their critical comments and constructive reviews which improved the manuscript.
Funding
This current research was not supported by any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghalib, H.B., Al-Hawash, A.B., Muttashar, W.R. et al. Determining the effect of mineral scaling formation under different injection water sources on the performance of Mishrif carbonate reservoir in Halfaya oilfield, Southern Iraq. J Petrol Explor Prod Technol 13, 1265–1282 (2023). https://doi.org/10.1007/s13202-023-01614-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-023-01614-5