Abstract
In this work, the process of low salinity water injection (LSWI) into reservoirs at various salt concentrations was simulated in order to study the change in the oil recovery factor during oil production. The simulation results of the recovery factor were compared with the experimental data. The results demonstrated that the simulation data were in good agreement with the experimental results. In addition, the formation damage (rock permeability reduction) in carbonate core samples was evaluated through coreflood experiments during LSWI in the range of salt concentration and temperature of 1500–4000 ppm and 25–100 °C, respectively. In the worst scenario of LSWI, the rock permeability has reached about 83% of the initial value. Our previous correlation was used to predict the formation damage in LSWI. In this case, the R-squared value between predicted and experimental data of rock permeability ratios was more than 0.97. Furthermore, the recovery factor during LSWI was analyzed with and without the use of DTPMP scale inhibitor (diethylenetriamine penta (methylene phosphonic acid)), and various nanoparticles (TiO2, SiO2, Al2O3). The results of the coreflood experiments showed that the use of scale inhibitor provides an increase in the recovery factor by more than 8%. In addition, the highest recovery factor was observed in the presence of SiO2 nanoparticles at 0.05 wt.%. The oil displacement during LSWI in the porous media with SiO2 particles was better than TiO2 and Al2O3. The recovery factor in the presence of SiO2, TiO2, and Al2O3 with DTPMP was 72.2, 62.4, and 59.8%, respectively. Among the studied nanoparticles, the lowest values of the oil viscosity and interfacial tension (IFT) between oil and water were observed when using SiO2. Moreover, the contact angle was increased by increasing the brine concentration. The contact angle with the use of SiO2, TiO2, and Al2O3 at 0.05 wt.% was reduced by 11.2, 10.6, and 9.9%, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The modern approach to the economic development of oilfields requires the application of efficient technologies that increase oil recovery at a minimal cost. There are various methods of enhanced oil recovery, which are used for specific conditions. One of these methods, which has found wide application, is the low salinity water injection. This method has more advantages in terms of ease of operation, and environmental compatibility than other methods (Bartels et al. 2019; Katende and Sagala 2019; Tetteh et al. 2020; Xu et al. 2020). The main factors determining the mechanisms of the oil recovery for effective utilization of LSWI are the following: changing reservoir rock wettability, reducing IFT, plugging higher permeability pore channels, and redirecting the waterflood path to lower permeability pores to improve sweep efficiency (Hadia et al. 2013; Mohammadi et al. 2019; Taheriotaghsara et al. 2020).
The LSWI technology has been successfully tested in oilfield treatments. Many researchers confirmed an increase in oil production efficiency and recovery factor due to LSWI in the sandstone and carbonate reservoirs (Joonaki and Ghanaatian 2014; Nasralla et al. 2016; Shehata and Nasr-El-Din 2017; Olayiwola and Dejam 2020). The mechanism of the LSWI has been described as follows: IFT reduction, expansion of the electrical double layer, multicomponent ion exchange, and wettability change. However, the main factor is the change in wettability. In general, the greatest increase in oil recovery factor by LSWI occurs when the content of salts in the injection water is less than in the formation water (Al-Shalabi and Sepehrnoori 2016; Akkal et al. 2019). During the LSWI process, the clay swelling phenomenon can be observed, after which the clay particles can dissolve in brine, then migrate, settle, and get stuck in the pores (Sameni et al. 2015; Garcia-Olvera and Alvarado 2017). As a result of this sedimentation, the permeability of these pathways is reduced, and the brine is directed to the intact pathways, resulting in increased sweep efficiency and ultimate oil recovery (Abhishek et al. 2018; Chen et al. 2021; Mahboubi Fouladi et al. 2021; Mardashov et al. 2021). Zaheri et al. (2020) reported that the type and concentration of the ions in the brine play a vital role in the wettability changes and the ultimate oil recovery. Based on the experimental study, Su et al. (2018) observed that the maximum change in wettability is observed at the optimal salt concentrations.
Nanotechnology has attracted the attention of many scientists in different industries, including oil and gas, owing to its unique properties such as a very high specific surface area and small size. The ability of nanoparticles to penetrate into the smallest pores of reservoir rocks makes it possible to effectively develop huge oil reserves, the extraction of which is impossible using conventional technologies. The efficiency of oil displacement can be assessed at the nanoscale since the pore surface has a nanometer roughness, which determines the wetting properties of the formation rock samples (Asl et al. 2020; Sagala et al. 2020). Furthermore, there are various nanomaterials for utilization in the petroleum industry, in particular for enhanced oil recovery methods. The application of nanomaterials is ideal for increasing capillary pressure and oil recovery by increasing wettability. Nanoparticles with water form a nanodispersion or nanofluid that can be used as an oil displacement agent in the porous media (Nazari Moghaddam et al. 2015; Shojaei et al. 2015). Mansouri et al. (2019) reported that to maximize the oil recovery with LSWI, SiO2 nanoparticles should be used at an optimum concentration.
It is important to consider which parameters have a significant influence on the LSWI process using nanoparticles in order to enhance its efficiency. Some of them are as follows: size, type, concentration, and stability of nanoparticles, surface coating by nanoparticles, density, injection time and rate, temperature, rock wettability, and permeability (Hendraningrat et al. 2013; Roustaei and Bagherzadeh 2015; Ehtesabi et al. 2015; Peng et al. 2017; Sun et al. 2017; Eltoum et al. 2021; Pryazhnikov et al. 2022). The adsorption of nanoparticles on the surface of the reservoir rock affects its surface charge, thereby changing the wetting characteristics (Ali et al. 2019; Malgaresi et al. 2019). In addition, nanoparticles can increase productivity in heavy oil reservoirs. At the same time, the hydrophobic porous structure becomes hydrophilic due to the changes in the wettability of reservoir rocks, and as a result, the heavy oil can be displaced. Meanwhile, nanomaterials are used to reduce the heavy oil viscosity as a catalyst agent in the petroleum industry. These particles can be evenly dispersed in crude oil samples and significantly inhibit the formation of large viscoelastic networks, resulting in a decrease in oil viscosity (Taborda et al. 2017; Taheri-Shakib et al. 2018; Ke et al. 2022).
Due to the increase in the interfacial tension, oil and water can be immiscible fluids. The lower IFT values between the oil and brine can provide better miscibility. When the nanoparticles are injected into the reservoir, a reduction in the IFT occurs, which theoretically contributes to an increase in the oil recovery (Sadatshojaei et al. 2016). Moreover, the process of movement and retention of nanoparticles in the porous media depends on many parameters such as the size and shape of particles, injection rate, and concentration (Bera and Belhaj 2016; Nowrouzi et al. 2019). The main reasons for the retention of nanoparticles in the formation rocks are as follows: adsorption due to the Brownian motion of particles and the electrostatic interaction with the surface of pore channels, mechanical clogging of the pore channels, and gravitational subsidence due to the difference in density of liquid and particles (Yekeen et al. 2017; Rostami et al. 2020).
LSWI, like any technology, has disadvantages such as the dependence of stability on the temperature and brine salinity. The presence of ions in the brines can lead to salt perception and formation damage, whereby the recovery factor can be decreased (Mahmoud 2014; Sheng 2014; Khormali et al. 2016, 2018; Moghadasi et al. 2019). In the oilfield practice, the injection of scale inhibitors during the waterflooding is applied to reduce scale precipitation in the near-wellbore region and equipment. The majority of scale inhibitors are the following phosphorus compounds: inorganic polyphosphates, organic phosphate esters, phosphonates, and aminophosphates (de Morais et al. 2021; Khormali et al. 2021a; Mady et al. 2021). The squeeze lifetime of scale inhibitors is dependent on the ion content of the water, temperature, pH, and the adsorption capacity of the reservoir rocks (BinMerdhah 2010; Golsefatan et al. 2016; Jarrahian et al. 2020; Khormali et al. 2021b). Safari et al. (2020) reported the scale inhibition effect of nano-glass flakes and nanosilica during smart waterflooding, which could effectively reduce the amount of salt precipitation. They concluded that the mechanism of scale inhibition is based on the adsorption of nanoparticles on the surface of cations.
A review of past work in describing the performance of LSWI shows that, despite the wide range of laboratory tests, the simultaneous use of nanoparticles and scale inhibitor in LSWI for increasing the oil recovery has been little studied. This work aims to simulate the LSWI at various concentrations of brines and compare the simulation results of changes in the recovery factor with the experimental results. The changes in the permeability of carbonate core samples during LSWI will be investigated. Also, our previous correlation will be used to determine the changes in the permeability of core samples due to scale precipitation during LSWI. Moreover, it is planned to assess the influence of the presence of the scale inhibitor and various nanoparticles on the recovery factor under various conditions. For this purpose, an optimal concentration of the nanoparticles will be determined. The final objective is to analyze the effect of nanoparticles on IFT between oil and brine, oil viscosity, and contact angle.
Material and methods
Material (oil, water, core samples, nanoparticles, scale inhibitor)
The crude oil used in the current study was from a Russian oilfield. The API and viscosity of the used oil at room temperature were 28, and 0.03 Pa.s, respectively. The asphaltene content of the applied crude oil was about 9.5 wt.%. The crude oil sample was degassed, dehydrated, and filtered through a filter with a pore size of 0.45 µm.
Five various brines were prepared in order to evaluate the LSWI process and determine the effect of water salinity on the recovery factor and formation damage. The ionic concentration of these brines is depicted in Table 1. The brines were prepared by dissolving the required salts in distilled water.
The core samples used in this study were taken from a carbonate oil reservoir in Russia. The properties of the studied core samples are presented in Table 2.
In this work, the following nanoparticles from TECNAN Company with a size of 20 nm were used for increasing oil recovery during LSWI: silicon dioxide (nano-SiO2), titanium dioxide (nano-TiO2), aluminum oxide (nano-Al2O3).
DTPMP (diethylenetriamine penta (methylene phosphonic acid)) as a scale inhibitor at 25 ppm was used to control the salt precipitation and increase the recovery factor. This inhibitor is widely used for scale prevention in the petroleum industry.
Simulation of recovery factor at various salt concentrations in water
To analyze the effect of the LSWI process on the change in recovery factor, the simulation of water injection into the reservoir was performed using the Eclipse 100 black oil simulator. Simulation of the LSWI process in the Eclipse is performed through salt distribution by solving the mass balance equation in the model. According to the simulation assumption, salt exists only in water and is modeled by solving the mass balance equation for salt between two grid blocks. To simulate the LSWI process in this program, the LOWSALT keyword in the RUNSPEC section is used. This keyword provides the ability to select the salinity (concentration) of the water and allows the user to enter separate salinity-dependent sets of saturation (two various sets). Also, the salinity level of the injection water is determined using the WSALT keyword. Furthermore, this model adds a separate salt phase to the existing phase and solves a mass conservation equation for each block in the reservoir model. More detailed information on LSWI modeling by the Eclipse program at various water concentrations is presented in previous works (Knut 2012; Xie et al. 2015; Qiao et al. 2016; Olabode et al. 2020; Al-Ibadi et al. 2021). In this work, in the used model, the minimum distance from the ground to the first layer of the reservoir was 2500 m, and in the deepest part of the reservoir was 3100 m. The reservoir model included nine producers and two injection wells completed at the top of the reservoir. The model used for the reservoir fluid was a black oil model with three phases of water, gas, and oil, in which the reservoir was saturated, and some gas is dissolved in the oil. Efforts have been made to maintain the distance of the injection wells from the production wells to prevent wellbore breakdown under fluid injection. In this case, the rock permeability along the x-axis, y-axis, and z-axis was 26, 26, and 5 mD, respectively. The rock porosity was 18%. The depths of gas-oil and water–oil contacts were 2610, and 2920 m. The gas, oil, and formation water densities were 0.9, 860, and 1035 kg/m3, respectively, and the initial reservoir pressure at the datum depth was 25 MPa. In this model, conventional flooding was first performed as a secondary recovery method for 100 days. Then, the low salinity water injection process was carried out using the brines with salt concentrations of 1500, 2500, and 4000 ppm for the next 100 days. The properties of rocks and fluids used in the modeling are shown in Table 3. The simulation of this work was completed to investigate the influence of TDS on the oil recovery factor. Thus, the field oil recovery (FOE) was determined during production. It should be noted that the modeling was carried out in the absence of the scale inhibitors and nanoparticles. Finally, the simulation results were compared with the experimental results in order to evaluate the accuracy of the LSWI modeling.
Coreflood experiments for determination of formation damage and recovery factor
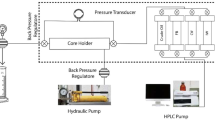
In the current study, the changes in permeability caused by the salt precipitation during the LSWI process in the carbonate cores were evaluated at various salt concentrations and temperatures. In addition, the recovery factor after the LSWI process with and without DTPMP and nanoparticles was determined. For this purpose, a coreflood apparatus was utilized to analyze formation damage and recovery factor. The used coreflood apparatus consisted of a coreholder, containers for brines, and an oven, which could provide the desired reservoir conditions. An injection rate of 0.5 mL/min was applied in the experiments.
Prior to the LSWI process, the core samples were saturated with oil by the coreflood apparatus. For this purpose, a certain volume of the oil was passed through the samples, and their pores were filled with it. To determine the recovery factor, the brines were injected into the core samples. The amounts (volumes) of displaced water and oil were first collected in beakers and then measured. Finally, the oil recovery factor (RF) was calculated as follows:
where Vf is the volume of displaced oil, mL; Vi is the initial volume of injected oil, mL.
The following tests were carried out using the coreflood apparatus:
1. Injection of brines No. 1, 3, and 4 (1500, 2500, and 4000 ppm) into the core samples for 10 PV (pore volume injected) at 60 °C without the use of the scale inhibitor and nanoparticles to assess the influence of TDS of water on the formation damage. The permeability was determined using the Darcy equation (Fig. 4a).
2. Injection of brines No. 3 (2500 ppm) into the core samples for 10 PV at temperatures of 25, 60, and 100 °C without the use of the scale inhibitor and nanoparticles to determine the effect of temperature on the formation damage during the LSWI process (Fig. 4b).
3. Determination of the recovery factor after injection of 5 PV of all brines into the cores with and without the use of DTPMP (25 ppm) in the absence of nanoparticles at 60 °C to examine the influence of scale inhibitor presence on the recovery factor of LSWI process (Fig. 6).
4. Injection of brine No. 3 (2500 ppm) into the core samples with the addition of SiO2, TiO2, and Al2O3 nanoparticles to it at different dosages (between 0.01 and 0.2 wt.%) without the use of the scale inhibitor at 60 °C to analyze the effect of type and content of nanoparticles on the recovery factor after 5 PV (Fig. 7).
5. Injection of brine No. 3 (2500 ppm) at 60 °C for 3 PV in the following four cases: SiO2 (0.05 wt.%) and DTPMP (25 ppm); SiO2 (0.05 wt.%); DTPMP (25 ppm); blank (Fig. 8)
6. Injection of brine No. 3 (2500 ppm) at 60 °C for 3 PV in the following four cases: SiO2 and DTPMP; TiO2 and DTPMP; Al2O3 and DTPMP; DTPMP. All nanoparticles and the scale inhibitor were used at concentrations of 0.05 wt.% and 25 ppm, respectively (Fig. 9).
IFT measurement
The IFT between oil and brine was examined using a tensiometer. Measurement of IFT between brine No. 3 (2500 ppm) and oil was carried out in the range of nanoparticle concentrations between 0 and 0.1 wt%.
Oil viscosity determination
A viscometer was applied to analyze the effect of nanoparticles on oil viscosity reduction. The experiments were conducted in a temperature range between 20 and 80 °C. These experiments were performed in the presence and absence of SiO2, TiO2, and Al2O3 nanoparticles. The nanoparticles were added to the injection fluid at a concentration of 0.05 wt.%.
Contact angle measurement
Contact angle measurement was completed using a Kruss apparatus under room conditions, the mechanism of which was based on the pendant drop method. In this apparatus, the presence of a high-pressure piston pump (manual type) allowed accurate measurement of the drop in the system. Meanwhile, the diameter of the needle in the measurement was selected depending on the application. In this experiment, the contact angle was first studied in the absence of nanoparticles using all brines in order to assess the influence of TDS on the changes in contact angle. Then, the contact angle was measured using brine No. 3 (with a TDS of 2500 ppm) in the presence of SiO2, TiO2, and Al2O3 nanoparticles at 0.05 wt.%. The prepared samples for the evaluation of the contact angle are shown in Fig. 1.
Results and discussion
Results of LSWI process simulation and the comparison between the simulation and experimental data
Figure 2 depicts the simulation results of LSWI in the reservoir at various concentrations of the brines without the use of nanoparticles and DTPMP. As demonstrated in this figure, during the conventional waterflooding (in the first period of production), the recovery factor was increased over time. In this case (without LSWI), the recovery factor reached 43% after 75 days of production. Further, the recovery factor was not changed over time with conventional waterflooding due to the low performance of this technique, and formation damage during the injection due to salt precipitation. Therefore, after 100 days of production, the low salinity water injection was carried out with TDS of 1500, 2500, and 4000 ppm in order to increase the recovery factor. Figure 2 demonstrates that the recovery factor was enhanced at all concentrations using LSWI. After 100 days of production using LSWI, the recovery factor for brines with TDS of 4000, 2500, and 1500 ppm has reached 52, 55, and 57%, respectively. Thus, for LSWI at 4000, 2500, and 1500 ppm, an increase in the recovery factor by about 9, 12, and 14% was observed. This enhancement in the recovery factor can be attributed to the changes in wettability and the reduction in interfacial tension (Katende and Sagala 2019; Liu and Wang 2020; Tetteh et al. 2020). Moreover, a lower oil recovery factor at higher water salinities indicates that the water injection with lower TDS is more efficient. The decrease in recovery factor with an increase in the brine concentration may be associated with the changes in rock permeability due to scale formation in the pores of reservoir rocks (Khormali et al. 2021a). Therefore, formation damage analysis and the application of the scale inhibitor have been studied experimentally and presented in the next sections.
Figure 3 represents the comparison of the simulation results of the recovery factor changes with the experimental data. To this end, the coreflood tests were completed in the long core samples (carbonate type) for 10 PV (first 5 PV with conventional waterflooding, second 5 PV with LSWI at various values of TDS) in the absence of DTPMP and nanoparticles at 60 °C. In this case, PV was calculated as follows (Khormali et al. 2018):
where q is the injection rate, mL/min; t is the injection time, min; d is the diameter of the core samples, cm; L is the length of the core samples, cm; ϕ is the rock porosity.
As can be seen from Fig. 3, the simulation data on oil recovery factor during the LSWI process were in good agreement with experimental data in all studied TDS. In this case, the R-squared value between simulation and experimental data was 0.9885. The simulation and experimental results have confirmed that a greater increase in recovery factor can be achieved at lower salinities. The mechanism of enhancement in oil recovery factor by LSWI can be related to the wettability change to the water-wet condition, increase in pH value under the influence of calcium carbonate dissolution, and multi-component ion exchanges (Katende and Sagala 2019; Liu and Wang 2020; Tetteh et al. 2020). Although the results of simulation and experiments showed the enhancement in oil recovery factor by the LSWI process, the formation damage and a decrease in the production rate due to salt precipitation could occur at higher salinities. Thus, in the next sections, to analyze the adverse influence of scaling on rock permeability, the amount of formation damage due to salt precipitation during LSWI has been evaluated by an experimental study. At the same time, the scale inhibitor and nanoparticles have also been used in LSWI to reduce formation damage and increase the recovery factor.
Permeability changes due to LSWI
One of the problems of the LSWI process is the possibility of salt precipitation in the porous media, and formation damage. For this reason, the reduction in rock permeability during LSWI was evaluated at various concentrations and temperatures in the absence of nanoparticles and DTPMP. It should be noted that the changes in the permeability due to scale formation in LSWI are less than in conventional waterflooding because of lower water salinity (Tetteh et al. 2020). The results obtained from the experiments are shown in Fig. 4. The Kd/Ki parameter represents the ratio of reduced to original (initial) values of the permeability during coreflood tests. As presented in Fig. 4a, the formation damage due to scaling was increased by increasing the brine TDS at 60 °C. In this case, the rock permeability with the use of brines No. 1 (1500 ppm), No. 3 (2500 ppm), and No. 4 (4000 ppm) was decreased by 5.8, 11.9, and 17.1% after 10 PV. The justification for this behavior is that by increasing water salinity the supersaturation of the brines with ions is increased and leads to the crystallization and precipitation of salts in the core samples. In addition, Fig. 4b depicts the effect of temperature on the formation damage during LSWI using brine No. 3 (2500 ppm). As the temperature increased, the formation damage was increased since the solubility of the formed salts was decreased by increasing the temperature. The used brines were prone to the formation and deposition of CaCO3 and CaSO4 as they contained calcium, sulfate, and bicarbonate ions. Therefore, an effective scale inhibitor should be used in the LSWI to reduce formation damage and enhance oil recovery.
To predict the formation damage due to scaling during the LSWI process, we used some correlations. Then, the values of rock permeability ratios (Kd/Ki) determined by the correlation were compared with laboratory data obtained in this work. Among the studied correlations, our previous correlation (Khormali et al. 2018) had the highest accuracy in predicting rock permeability changes. Thus, this correlation was used for formation damage prediction. This model is demonstrated as follows (Khormali et al. 2018):
where ϕ is the rock porosity; A is the area of the core samples, cm2; T is the temperature, °C; C is the supersaturation index; q is the injection rate, mL/min.
The Kd/Ki values under different conditions (various values of PV, T, C, q) were determined by the coreflood tests and compared with the predicted data using Eq. 3. The results of the analysis are shown in Fig. 5. As presented in the figure, the agreement between the experimental data of formation damage during LSWI and the data predicted by the correlation is very good. In this case, the R-squared value between predicted and experimental data was more than 0.97. Thus, this model can predict the degree of change in rock permeability changes due to scaling during the LSWI process and provide the necessary information about adding scale inhibitors to the injection fluid to increase the ultimate oil recovery. Moreover, the proposed model was applied for the ranges of PV and Kd/Ki between 0 and10 PV, and 80–100%, respectively. Therefore, this model can be utilized for the prediction of permeability changes during LSWI at PV less than 10 and Kd/Ki more than 80%.
Effect of water salinity on the recovery factor in the presence and absence of DTPMP
In the previous section, it was concluded that salt precipitation caused formation damage and decreased rock permeability during the LSWI process. Thus, the scale inhibitor (DTPMP) was added to the injection fluid to increase the performance of the LSWI. Figure 6 illustrates the changes in the recovery factor with and without the use of DTPMP at various salt concentrations. These experiments were completed in the absence of nanoparticles. As shown in the figure, the recovery factor was reduced by increasing the water salinity in the absence of DTPMP. After adding the chemical inhibitor to the brine, the recovery factor was increased by about 8% at all salt concentrations. At the same time, the change in the water salinity did not influence the recovery factor in the presence of DTPMP. This confirms that the used scale inhibitor can effectively prevent salt appreciation in all studied brines. Also, DTPMP effectively inhibited the formation of inorganic deposits at a low dosage (25 ppm). The used reagent chemically interacted with salt crystals and significantly reduced their growth rate. DTPMP acted at the macro level, enveloping already formed microcrystals. Such microcrystals could not be precipitated in the pores of the reservoir rocks. As a result, the microdispersed particles of mineral salts constantly remained suspended in the flow. It should be noted that DTPMP could prevent salt precipitation after crystallization.
Effect of nanoparticles on the effectiveness of the LSWI process
As mentioned above, many parameters affect the efficiency of oil displacement in the porous media when using nanoparticles (Peng et al. 2017; Eltoum et al. 2021; Pryazhnikov et al. 2022). In this section, the influence of the concentration and type of nanoparticles, as two main parameters, on the oil recovery factor was evaluated. To determine the influence of the presence of nanoparticles on the oil recovery factor during LSWI, brine No. 3 (2500 ppm) was pumped into the cores for 5 PV. The dosage of nanoparticles (SiO2, TiO2, Al2O3) was 0.01–0.2 wt.%. These experiments were completed at 60 °C in the absence of the scale inhibitor. Figure 7 depicts the obtained results. As shown in this figure, the addition of nanoparticles to the injection fluid in LSWI significantly improved the oil recovery factor. At the same time, it was observed that increasing the dosage of nanoparticles in the fluid up to 0.05 wt.% has continuously increased the recovery factor. Moreover, the results demonstrate that the use of nanoparticles at high concentrations (more than 0.05 wt.%) could not increase the oil recovery factor. On the other hand, it is extremely difficult to make a homogeneous dispersion at high concentrations of nanoparticles. Therefore, we can conclude that oil recovery is not necessarily improved with a growth in the nanoparticle dosage in the injection fluid during LSWI. There is an optimal concentration of nanoparticles, at which they have the greatest efficiency. The optimal content of all nanoparticles was 0.05 wt.%. Among the studied nanoparticles, SiO2 had the highest performance for enhancing oil recovery. The recovery factor at 0.05 wt.% of SiO2, TiO2, and Al2O3 was 67.4, 59.1, and 57.5%, respectively. Thus, the oil displacement during LSWI in the porous media with SiO2 particles was better than with TiO2 and Al2O3. The adsorption of nanoparticles on the rock surface affected its surface charge, thereby changing the wetting characteristics. In this case, the main reason for the change in the recovery factor is the pressure in the wedge film of nanoparticles, which began to increase when the nanoparticles were placed between the surface of the core samples and oil.
Figure 8 demonstrates the results of recovery factor changes during LSWI in coreflood tests with the carbonate core samples for 3 PV in the following four different cases: with SiO2 (0.05 wt.%) and DTPMP (25 ppm), with SiO2 (0.05 wt.%), with DTPMP (25 ppm), and blank. As presented in the figure, the highest recovery factor was observed in the case of simultaneous application of DTPMP and SiO2 nanoparticles. In this case, the recovery factor has reached 72.2% after 1 PV. The further injection of the fluid into the core samples could not change the recovery factor. As can be seen from Fig. 8, the recovery factor in the presence of SiO2 without DTPMP after 1 PV was 64.1%, which is 8.1% less than in the case of simultaneous application of DTPMP and SiO2. Therefore, the presence of an effective scale inhibitor significantly could enhance oil recovery by preventing salt precipitation in the porous media during LSWI. In addition, the recovery factor in the presence of SiO2 nanoparticles was higher than in their absence. Thus, it can be concluded that the application of nanoparticles together with a scale inhibitor in the LSWI process can considerably improve oil recovery by minimizing the formation damage.
Figure 9 presents the effect of various nanoparticles (0.05 wt.%) on the recovery factor during LSWI in the presence of DTPMP at 60 °C. As shown in the figure, the addition of all nanoparticles to the injection fluid enhanced the recovery factor. The maximum oil recovery was observed with the use of SiO2. This is associated with the fact that the properties of nanoparticles were retained as long as their size dispersed in the solution corresponded to the nanoscale. Furthermore, it should be taken into account that the better dispersion of nanoparticles in the porous media could increase their stability, and as a result, maintain the efficiency of the adsorption phenomenon. Moreover, the stability of nanoparticles can be considered a key factor in evaluating their effectiveness in enhancing oil recovery. The stability time of the nanoparticles should be such that these particles can act on the core surface for a sufficient time. If the stability time of the nanoparticles is short, then after a while they stick together. As a result, the influence of nanoparticles on the surface of the porous media is significantly reduced and can lead to formation damage. Also, nanoparticles are very sensitive to the salinity of the brines, and an increase in water salinity can reduce their stability. As shown in Fig. 9, the stability of Al2O3 and TiO2 was less than SiO2. SiO2 nanoparticles were less affected by water salinity. Therefore, it can be concluded that the type and amount of the electric charge on the surface of nanoparticles, which determine their stability, strongly depend on the degree of water salinity.
Effect of nanoparticles on IFT and oil viscosity
As observed in the previous sections, the content of nanoparticles in the fluid during LSWI plays a vital role in the oil recovery process: the higher the content of nanoparticles (up to an optimal concentration), the greater their retention. Furthermore, the high content of nanoparticles contributes to an increase in electrostatic repulsion and a reduction in IFT. On the other hand, reducing the interfacial tension leads to a decrease in capillary pressure in the rock pores. This mechanism can be considered one of the main parameters affecting the oil production process. It is important to note that the decrease in IFT is associated with a reduction in surface free energy. At the same time, large oil droplets become smaller, facilitating their migration through the porous media. IFT is a thermodynamic characteristic at the interface between two phases, in which the temperature, the volume of the system, and the chemical potentials of all components remain constant in both phases.
Figure 10 illustrates the experimental results of the changes in the IFT between oil and brine in the presence of nanoparticles at various dosages. As depicted in this figure, the IFT values were decreased by increasing the nanoparticle dosage. The greatest decrease in IFT occurred in the presence of SiO2 nanoparticles. By increasing the dosage of nanoparticles up to 0.05 wt.%, the IFT values were decreased from 25.3 to 7.7, 10.5, and 11.6 mN/m in the presence of SiO2, TiO2, and Al2O3, respectively. At concentrations higher than 0.05 wt.%, IFT was not changed significantly by increasing the nanoparticle dosage in the fluid. Thus, the optimal concentration for all studied nanoparticles was obtained to be 0.05 wt.%. This concentration was also determined in the coreflood tests. Moreover, the nanoparticles are placed at the interface between two phases, which reduces the interfacial tension. The adsorption of surfactants at the interface occurs due to their hydrophilic head and hydrophobic tail. In addition, the adsorption of nanoparticles occurs by maintaining the interface at the lowest energy level in the particle–fluid system. This is done by directing these particles to change the surface free energy by removing the liquid–liquid interface and replacing it with a particle-liquid interface.
Viscosity plays a decisive role in oil production and transportation. The high viscosity of an oil is due to the presence of colloidal particles in its composition, which are formed by asphaltenes, resins, and other high molecular weight organic compounds. Figure 11 presents the changes in oil viscosity with and without the use of the nanoparticles at different temperatures. The nanoparticles were added to the fluid at 0.05 wt.%. As shown in this figure, all nanoparticles reduced the dynamic viscosity of the oil. Thus, it has been shown that the viscosity and rheological properties of the oil can be controlled using nanoparticles. Figure 11 depicts that the reduction in oil viscosity with SiO2 was greater than with TiO2 and Al2O3 nanoparticles. The mechanism of oil viscosity reduction in the presence of studied nanoparticles is a topic for our future work that will be evaluated by conducting additional experiments through analysis of the composition of the oil before and after treatment with nanoparticles.
Results of contact angle measurement
One of the most important parameters influencing the displacement and production of oil in the reservoir is wettability. The phase behavior of formation fluids and displacing agents in the porous media, which determines the ultimate oil recovery, depends on wettability. Thus, the assessment and prediction of the changes in the wettability of reservoir rocks can play a key role in optimizing oil recovery techniques, especially in the LSWI process. An incorrect assumption about the nature of the wettability can lead to irreversible damage and complicate the oilfield development. The main characteristic in the experimental evaluation of rock wettability is the contact angle. For this reason, the changes in the contact angle during LSWI were analyzed in this work. Table 4 presents the contact angle values (wetting angle) at various salt concentrations in the absence of nanoparticles and DTPMP. As presented in the table, the contact angle was increased by increasing ion concentration in brines. Consequently, the hydrophilicity of the studied carbonate rocks was decreased due to an increase in the water salinity. This behavior is associated with an increase in the possibility of salt crystallization.
Table 5 presents the changes in the contact angle in the presence of SiO2, TiO2, and Al2O3 at various concentrations. As presented in this table, the contact angle was decreased by increasing the dosage of the nanoparticles, which is related to the growth in the electrostatic repulsion force between them. This reduction in the contact angle can improve and enhance oil production under reservoir conditions. Among the studied nanoparticles, the SiO2 particles had the highest efficiency in reducing the contact angle. The contact angle with the use of SiO2, TiO2, and Al2O3 nanoparticles at a concentration of 0.05 wt.% was 53.8, 54.2, and 54.6, which provided a decrease of 11.2, 10.6, and 9.9% compared to the blank case (Table 4), respectively. Therefore, the application of SiO2 nanoparticles can be more effective than TiO2 and Al2O3 for oil recovery in LSWI in carbonate reservoirs.
Conclusions
An in-depth study of scientific sources indicates that little research has been conducted on the simultaneous use of nanoparticles and scale inhibitors in LSWI for increasing oil recovery. Therefore, in this work, the process of low salinity water injection in the presence of DTPMP scale inhibitor and various nanoparticles (SiO2, TiO2, and Al2O3) was investigated. The main findings can be concluded as follows:
-
1.
The simulation results showed that the recovery factor was increased by decreasing the water salinity. A growth of 9, 12, and 14% in recovery factor was achieved after LSWI at 4000, 2500, and 1500 ppm.
-
2.
Formation damage due to salt precipitation during LSWI was evaluated at different brine concentrations (1500–4000 ppm), and temperatures (25–100 °C). At the end of LSWI process, the rock permeability was reduced by about 17%, which indicates the need to add a scale inhibitor to the injection fluid to prevent formation damage and increase oil recovery.
-
3.
The results of the coreflood experiments showed that the recovery factor in the presence of DTPMP was increased by about 8% compared to the blank case. Also, the increase in the water salinity did not affect the recovery factor in the presence of a scale inhibitor.
-
4.
The maximum recovery factor was observed at a concentration of 0.05 wt.% of the nanoparticles. The simultaneous use of nanoparticles and DTPMP significantly improved oil recovery in the LSWI process. The recovery factor in the blank case was 33.5%. This value in the presence of SiO2, TiO2, and Al2O3 together with DTPMP was 72.2, 62.4, and 59.8%, respectively.
-
5.
The IFT values between brine and oil were decreased by increasing the concentration of the nanoparticles until it reached 0.05 wt.%. The experimental results showed that the lowest values of the oil viscosity and IFT between oil and water were observed in the presence of SiO2 compared to TiO2 and Al2O3.
-
6.
The contact angle in the presence of SiO2, TiO2, and Al2O3 nanoparticles at an optimal concentration of 0.05 wt.% was decreased by 11.2, 10.6, and 9.9% compared to the blank case, respectively.
Abbreviations
- LSWI:
-
Low salinity water injection
- DTPMP:
-
Diethylenetriamine penta (methylene phosphonic acid)
- FOE:
-
Field oil recovery (recovery factor)
- IFT:
-
Interfacial tension, mN/m
- TDS:
-
Total dissolved solids, mg/L
- PV:
-
Pore volumes injected, dimensionless
- RF:
-
Recovery factor,
- A :
-
Area of the core samples, cm2
- C :
-
Supersaturation index, dimensionless
- D :
-
Diameter of the core samples, cm
- K d /K i :
-
Ratio of damaged to initial permeability, dimensionless
- L :
-
Length of the core samples, cm
- Q :
-
Injection rate, mL/min
- T :
-
Temperature, °C
- T :
-
Injection time, min
- V f :
-
Volume of displaced oil, mL
- V i :
-
Initial volume of injected oil, mL
- ϕ :
-
Rock porosity, dimensionless
References
Abhishek R, Hamouda AA, Murzin I (2018) Adsorption of silica nanoparticles and its synergistic effect on fluid/rock interactions during low salinity flooding in sandstones. Colloids Surf A Physicochem Eng Asp 555:397–406. https://doi.org/10.1016/j.colsurfa.2018.07.019
Akkal R, Ramézani H, Khodja M, Azzi S (2019) Influence of the clay content and type of algerian sandstone rock samples on water-oil relative permeabilities. Energy Fuels 33(9):9330–9341. https://doi.org/10.1021/acs.energyfuels.9b01584
Ali JA, Kolo K, Manshad AK, Stephen KD (2019) Potential application of low-salinity polymeric-nanofluid in carbonate oil reservoirs: IFT reduction, wettability alteration, rheology and emulsification characteristics. J Mol Liq 284:735–747. https://doi.org/10.1016/j.molliq.2019.04.053
Al-Ibadi H, Stephen KD, Mackay E (2021) An analysis of numerically induced pulses in simulations of low-salinity waterflooding and their reduction by flow upscaling. SPE J 26(2):897–917. https://doi.org/10.2118/192074-PA
Al-Shalabi EW, Sepehrnoori K (2016) A comprehensive review of low salinity/engineered water injections and their applications in sandstone and carbonate rocks. J Pet Sci Eng 139:137–161. https://doi.org/10.1016/j.petrol.2015.11.027
Asl HF, Zargar G, Manshad AK, Takassi MA, Ali JA, Keshavarz A (2020) Effect of SiO2 nanoparticles on the performance of L-Arg and L-Cys surfactants for enhanced oil recovery in carbonate porous media. J Mol Liq 300:112290. https://doi.org/10.1016/j.molliq.2019.112290
Bartels WB, Mahani H, Berg S, Hassanizadeh SM (2019) Literature review of low salinity waterflooding from a length, and time scale perspective. Fuel 236:338–353. https://doi.org/10.1016/j.fuel.2018.09.018
Bera A, Belhaj H (2016) Application of nanotechnology by means of nanoparticles and nanodispersions in oil recovery - a comprehensive review. J Nat Gas Eng 34:1284–1309. https://doi.org/10.1016/j.jngse.2016.08.023
BinMerdhah AB (2010) Inhibition of calcium sulfate and strontium sulfate scale in waterflood. SPE Prod Oper 25(4):545–552. https://doi.org/10.2118/141168-PA
Chen Q, Otaibi M, Ayirala S, Yousef A (2021) The prospects and potential opportunities of low salinity water flooding for offshore applications in sandstones. J Pet Sci Eng 199:108260. https://doi.org/10.1016/j.petrol.2020.108260
de Morais SC, Bezerra BG, Castro BB, Balaban RDC (2021) Evaluation of polyelectrolytic complexes based on poly (epichlorohydrin-co-dimethylamine) and poly (4-styrene-sulfonic acid-co-maleic acid) in the delivery of polyphosphates for the control of CaCO3 scale in oil wells. J Mol Liq 339:116757. https://doi.org/10.1016/j.molliq.2021.116757
Ehtesabi H, Ahadian MM, Taghikhani V (2015) Enhanced heavy oil recovery using TiO2 nanoparticles: investigation of deposition during transport in core plug. Energy Fuels 29(1):1–8. https://doi.org/10.1021/ef5015605
Eltoum H, Yang YL, Hou JR (2021) The effect of nanoparticles on reservoir wettability alteration: a critical review. Pet Sci 18:136–153. https://doi.org/10.1007/s12182-020-00496-0
Garcia-Olvera G, Alvarado V (2017) Interfacial rheological insights of sulfate-enriched smart-water at low and high-salinity in carbonates. Fuel 207:402–412. https://doi.org/10.1016/j.fuel.2017.06.094
Golsefatan AR, Safari M, Jamialahmadi M (2016) Using silica nanoparticles to improve DETPMP scale inhibitor performance as a novel calcium sulfate inhibitor. Desalination Water Treat 57(44):20800–20808. https://doi.org/10.1080/19443994.2015.1119742
Hadia NJ, Ashraf A, Tweheyo MT, Torsaeter O (2013) Laboratory investigation on effects of initial wettabilities on performance of low salinity waterflooding. J Pet Sci Eng 105:18–25. https://doi.org/10.1016/j.petrol.2013.03.014
Hendraningrat L, Li S, Torsater O (2013) Effect of some parameters influencing enhanced oil recovery process using silica nanoparticles: an experimental investigation. In: paper presented at the spe reservoir characterization and simulation conference and exhibition, Abu Dhabi, UAE https://doi.org/10.2118/165955-MS
Jarrahian K, Sorbie K, Singleton M, Boak L, Graham A (2020) Building a fundamental understanding of scale-inhibitor retention in carbonate formations. SPE Prod Oper 35(1):85–97. https://doi.org/10.2118/193635-PA
Joonaki E, Ghanaatian S (2014) The application of nanofluids for enhanced oil recovery: effects on interfacial tension and coreflooding process. Pet Sci Technol 32(21):2599–2607. https://doi.org/10.1080/10916466.2013.855228
Katende A, Sagala F (2019) A critical review of low salinity water flooding: mechanism, laboratory and field application. J Mol Liq 278:627–649. https://doi.org/10.1016/j.molliq.2019.01.037
Ke H, Yuan M, Xia S (2022) A review of nanomaterials as viscosity reducer for heavy oil. J Dispers Sci Technol 43(9):1271–1282. https://doi.org/10.1080/01932691.2020.1851246
Khormali A, Sharifov AR, Torba DI (2018) Increasing efficiency of calcium sulfate scale prevention using a new mixture of phosphonate scale inhibitors during waterflooding. J Pet Sci Eng 164:245–258. https://doi.org/10.1016/j.petrol.2018.01.055
Khormali A, Bahlakeh G, Struchkov I, Kazemzadeh Y (2021a) Increasing inhibition performance of simultaneous precipitation of calcium and strontium sulfate scales using a new inhibitor—laboratory and field application. J Pet Sci Eng 202:108589. https://doi.org/10.1016/j.petrol.2021.108589
Khormali A, Moghadasi R, Kazemzadeh Y, Struchkov I (2021b) Development of a new chemical solvent package for increasing the asphaltene removal performance under static and dynamic conditions. J Pet Sci Eng 206:109066. https://doi.org/10.1016/j.petrol.2021.109066
Khormali A, Petrakov DG, Farmanzade AR (2016) Prediction and inhibition of inorganic salt formation under static and dynamic conditions–Effect of pressure, temperature, and mixing ratio. Int J Technol 7(6):943–951. https://doi.org/10.14716/ijtech.v7i6.2871
Knut EK (2012) Simulation of low salinity waterflooding in a synthetic reservoir model and frøy field reservoir model. Dissertation, Norwegian University of Science and Technology
Liu F, Wang M (2020) Review of low salinity waterflooding mechanisms: Wettability alteration and its impact on oil recovery. Fuel 267:117112. https://doi.org/10.1016/j.fuel.2020.117112
Mady MF, Rehman A, Kelland MA (2021) Synthesis and study of modified polyaspartic acid coupled phosphonate and sulfonate moieties as green oilfield scale inhibitors. Ind Eng Chem Res 60(23):8331–8339. https://doi.org/10.1021/acs.iecr.1c01473
Mahboubi Fouladi M, Hassani K, Rostami B, Pourafshary P (2021) Experimental studies of low salinity water flooding in sandstone porous media: effects of the presence of silica and kaolinite. Energy Sources Recovery Util Environ Eff. https://doi.org/10.1080/15567036.2020.1859019
Mahmoud MA (2014) Evaluating the damage caused by calcium sulfate scale precipitation during low- and high-salinity-water injection. J Can Pet Technol 53(3):141–150. https://doi.org/10.2118/164634-PA
Malgaresi GVC, Zhang H, Chrysikopoulos CV, Bedrikovetsky P (2019) Cotransport of suspended colloids and nanoparticles in porous media. Transp Porous Media 128(1):153–177. https://doi.org/10.1007/s11242-019-01239-5
Mansouri M, Nakhaee A, Pourafshary P (2019) Effect of SiO2 nanoparticles on fines stabilization during low salinity water flooding in sandstones. J Pet Sci Eng 174:637–648. https://doi.org/10.1016/j.petrol.2018.11.066
Mardashov D, Duryagin V, Islamov S (2021) Technology for improving the efficiency of fractured reservoir development using gel-forming compositions. Energies 14(24):8254. https://doi.org/10.3390/en14248254
Moghadasi R, Rostami A, Hemmati-Sarapardeh A, Motie M (2019) Application of nanosilica for inhibition of fines migration during low salinity water injection: experimental study, mechanistic understanding, and model development. Fuel 242:846–862. https://doi.org/10.1016/j.fuel.2019.01.053
Mohammadi S, Kord S, Moghadasi J (2019) An experimental investigation into the spontaneous imbibition of surfactant assisted low salinity water in carbonate rocks. Fuel 243:142–154. https://doi.org/10.1016/j.fuel.2019.01.074
Nasralla RA, Sergienko E, Masalmeh SK, van der Linde HA, Brussee NJ, Mahani H, Suijkerbuijk BM, Al-Qarshubi IS (2016) Potential of low-salinity waterflood to improve oil recovery in carbonates: demonstrating the effect by qualitative coreflood. SPE J 21(5):1643–1654. https://doi.org/10.2118/172010-PA
Nazari Moghaddam R, Bahramian A, Fakhroueian Z, Karimi A, Arya S (2015) Comparative study of using nanoparticles for enhanced oil recovery: wettability alteration of carbonate rocks. Energy Fuels 29(4):2111–2119. https://doi.org/10.1021/ef5024719
Nowrouzi I, Manshad AK, Mohammadi AH (2019) Effects of concentration and size of TiO2 nano-particles on the performance of smart water in wettability alteration and oil production under spontaneous imbibition. J Pet Sci Eng 183:106357. https://doi.org/10.1016/j.petrol.2019.106357
Olabode O, Alaigba D, Oramabo D, Bamigboye O (2020) Modelling low-salinity water flooding as a tertiary oil recovery technique. Model Simul Eng. https://doi.org/10.1155/2020/6485826
Olayiwola SO, Dejam M (2020) Synergistic interaction of nanoparticles with low salinity water and surfactant during alternating injection into sandstone reservoirs to improve oil recovery and reduce formation damage. J Mol Liq 317:114228. https://doi.org/10.1016/j.molliq.2020.114228
Peng B, Zhang L, Luo J, Wang P, Ding B, Zeng M, Cheng Z (2017) A review of nanomaterials for nanofluid enhanced oil recovery. RSC Adv 7(51):32246–32254. https://doi.org/10.1039/C7RA05592G
Pryazhnikov MI, Minakov AV, Pryazhnikov AI, Denisov IA, Yakimov AS (2022) Microfluidic study of the effect of nanosuspensions on enhanced oil recovery. Nanomaterials 12(3):520. https://doi.org/10.3390/nano12030520
Qiao C, Johns R, Li L (2016) Modeling low-salinity waterflooding in chalk and limestone reservoirs. Energy Fuels 30(2):884–895. https://doi.org/10.1021/acs.energyfuels.5b02456
Rostami P, Sharifi M, Aminshahidy B, Fahimpour J (2020) Enhanced oil recovery using silica nanoparticles in the presence of salts for wettability alteration. J Dispers Sci Technol 41(3):402–413. https://doi.org/10.1080/01932691.2019.1583575
Roustaei A, Bagherzadeh H (2015) Experimental investigation of SiO2 nanoparticles on enhanced oil recovery of carbonate reservoirs. J Pet Explor Prod Technol 5:27–33. https://doi.org/10.1007/s13202-014-0120-3
Sadatshojaei E, Jamialahmadi M, Esmaeilzadeh F, Ghazanfari MH (2016) Effects of low-salinity water coupled with silica nanoparticles on wettability alteration of dolomite at reservoir temperature. Pet Sci Technol 34(15):1345–1351. https://doi.org/10.1080/10916466.2016.1204316
Safari S, Rahimi A, Lah RM, Gholami R, Khur WS (2020) Sustaining sulfate ions throughout smart water flooding by nanoparticle based scale inhibitors. J Mol Liq 310:113250. https://doi.org/10.1016/j.molliq.2020.113250
Sagala F, Hethnawi A, Nassar NN (2020) Integrating silicate-based nanoparticles with low-salinity water flooding for enhanced oil recovery in sandstone reservoirs. Ind Eng Chem Res 59(37):6225–16239. https://doi.org/10.1021/acs.iecr.0c02326
Sameni A, Pourafshary P, Ghanbarzadeh M, Ayatollahi S (2015) Effect of nanoparticles on clay swelling and migration. Egypt J Pet 24(4):429–437. https://doi.org/10.1016/j.ejpe.2015.10.006
Shehata AM, Nasr-El-Din HA (2017) The role of sandstone mineralogy and rock quality in the performance of low-salinity waterflooding. SPE Res Eval Eng 20(1):87–106. https://doi.org/10.2118/181754-PA
Sheng JJ (2014) Critical review of low-salinity waterflooding. J Pet Sci Eng 120:216–224. https://doi.org/10.1016/j.petrol.2014.05.026
Shojaei MJ, Ghazanfari MH, Masihi M (2015) Relative permeability and capillary pressure curves for low salinity water flooding in sandstone rocks. J Nat Gas Eng 25:30–38. https://doi.org/10.1016/j.jngse.2015.04.023
Su W, Liu Y, Pi J, Chai R, Li C, Wang Y (2018) Effect of water salinity and rock components on wettability alteration during low-salinity water flooding in carbonate rocks. Arab J Geosci 11:260. https://doi.org/10.1007/s12517-018-3611-6
Sun X, Zhang Y, Chen G, Gai Z (2017) Application of nanoparticles in enhanced oil recovery: a critical review of recent progress. Energies 10(3):345. https://doi.org/10.3390/en10030345
Taborda EA, Franco CA, Ruiz MA, Alvarado V, Cortes FB (2017) Experimental and theoretical study of viscosity reduction in heavy crude oils by addition of nanoparticles. Energy Fuels 31(2):1329–1338. https://doi.org/10.1021/acs.energyfuels.6b02686
Taheriotaghsara M, Eftekhari AA, Nick HM (2020) Adsorption- and diffusion-controlled wettability change in modified salinity water flooding. Energy Fuels 34(11):13767–13781. https://doi.org/10.1021/acs.energyfuels.0c02493
Taheri-Shakib J, Shekarifard A, Naderi H (2018) Heavy crude oil upgrading using nanoparticles by applying electromagnetic technique. Fuel 232:704–711. https://doi.org/10.1016/j.fuel.2018.06.023
Tetteh JT, Brady PV, Barati Ghahfarokhi R (2020) Review of low salinity waterflooding in carbonate rocks: mechanisms, investigation techniques, and future directions. Adv Colloid Interface Sci 284:102253. https://doi.org/10.1016/j.cis.2020.1022533
Xie Q, Ma D, Wu J, Liu Q, Jia N, Luo M (2015) Low salinity waterflooding in low permeability sandstone: coreflood experiments and interpretation by thermodynamics and simulation. In: paper presented at the spe asia pacific enhanced oil recovery conference, Kuala Lumpur, Malaysia https://doi.org/10.2118/174592-MS
Xu ZX, Li SY, Li BF, Chen DQ, Liu ZY, Li ZM (2020) A review of development methods and EOR technologies for carbonate reservoirs. Pet Sci 17:990–1013. https://doi.org/10.1007/s12182-020-00467-55
Yekeen N, Manan MA, Idris AK, Samin AM, Risal AR (2017) Experimental investigation of minimization in surfactant adsorption and improvement in surfactant-foam stability in presence of silicon dioxide and aluminum oxide nanoparticles. J Pet Sci Eng 159:115–134. https://doi.org/10.1016/j.petrol.2017.09.021
Zaheri SH, Khalili H, Sharifi M (2020) Experimental investigation of water composition and salinity effect on the oil recovery in carbonate reservoirs. Oil Gas Sci Technol 75:21. https://doi.org/10.2516/ogst/2020010
Acknowledgements
This work was supported by the Ministry of Science and Higher Education of the Russian Federation under agreement No. 075-15-2021-931 within the framework of the development program for a world-class Research Center “Efficient development of the global liquid hydrocarbon reserves.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the co-authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khormali, A., Koochi, M.R., Varfolomeev, M.A. et al. Experimental study of the low salinity water injection process in the presence of scale inhibitor and various nanoparticles. J Petrol Explor Prod Technol 13, 903–916 (2023). https://doi.org/10.1007/s13202-022-01583-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-022-01583-1