Abstract
The process of smart water injection into carbonate reservoirs has always faced many challenges. This study attempted to investigate this issue by examining two effective factors active ionic compounds in brine and active compounds in the oil phase. The potential for the reaction among three phases’ oil, rock, and brine in changing wettability requires the presence of active ionic compounds in the brine water and active compounds in the oil. These compounds in optimal concentrations are the driving force of the wettability alteration process. In the first step, the contact angle and the spontaneous imbibition process were performed on the outcrop samples and the limestone core to investigate the effect of the active compounds of smart water. The efficiency of calcium and divalent magnesium cations mainly depends on the sulfate ion concentration. However, reservoir physical condition and the presence of other effective compounds in the reactions network can be helpful in the determination of the essential active ions in the reaction. Finally, the optimal concentrations of these three ions lead to the formation of a stable water film and a change in the wettability of the rock, which leads to an increase in oil recovery. In this regard, cations in the presence of sulfate ions as much as the minimum concentration in seawater can have a positive function and have an acceptable efficiency compared to increased concentrations of sulfate ions in seawater. The cores were saturated with two oil samples for further investigation, and again, two tests of measuring contact angle and spontaneous imbibition were performed. The difference between imbibition rate and ultimate recovery illustrates that the carboxylic acid functional group in the original crude oil structure can facilitate displacement compared to oil-free acid components. Therefore, acidic components in crude oil affect the wettability alteration through electrostatic interaction with surface minerals and brine. Active components can act as a critical indicator in smart water injection processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the main mechanisms for recovery factor enhancement in carbonate reservoirs is known as wettability alteration. Wettability analysis in an oil–brine–rock three-phase system and its effects on oil recovery factor is so complex that it requires a more detailed description of the effective factors. The molecular interactions between three constituent phases give rise to many compounds with different chemical and physical properties (Drummond and Israelachvili 2004). Organic components in organic and aqueous solvents could easily be adsorbed on the carbonate rock surface and induce wettability alteration. Fatty acid could also be irreversibly absorbed from aqueous solutions to the rock surface. The surface wettability may become strongly oil wet as a result of these interactions. Electrostatic interactions between carbonate rock surface–brine system and oil–brine system make the brine layer thin and so unstable that adsorption of organic compounds to carbonate rock surface will be predictable. Therefore, the oil recovery factor will be significantly low in this condition (Hirasaki and Zhang 2004). In other words, oil extraction from carbonate reservoirs that have already become oil wet would be difficult due to the remarkable bonding energy between the heterogeneous carbonate surface and polar components in crude oil (Lager et al. 2008; Seccombe et al. 2008). This phenomenon can be problematic in the oil production process. So, it is necessary to enhance the recovery using novel methods considering the specific structure and condition of the carbonate reservoir. Water-based methods such as smart water are the most common treatment in reservoirs that use chemical additives to enhance wettability. The efficiency of a smart water injection process into the reservoir mainly depends on natural characteristics of the constituent formation such as lithology, initial wettability, porous media properties such as pore size distribution, and other physical and chemical conditions of the reservoir rock. A reaction on the rock surface can improve the water wetness of the carbonate reservoir rock, alter the distribution of phases in the pores, increase the oil phase mobility, and finally improve the oil recovery. Effective parameters such as temperature, initial wettability, polar groups, a relative abundance of crude oil, and active ionic compound content of formation water can clarify the smart water injection performance in oil recovery factor enhancement (Fathi et al. 2011).
In fact, a proper evaluation of the crude oil composition is a prerequisite for any successful process of low-salinity-water injection. The polarity of crude oil is related to the heteroatom content. Sulfur, nitrogen, and oxygen are the main heteroatoms of crude oil found in acidic and basic functional groups in crude oil, such as asphaltene and resins. These super-molecules can influence the initial wettability of rocks (Denekas et al. 1959). Fathi previously demonstrated that crude oil acidic constituents are effective in the initial wettability of rock surface. In this experimental investigation, core plugs saturated with synthetic oil made from water-soluble acids were more water wet. These results prove the claim that water-soluble acids can affect the stability of the primary water film in the reactions between oil and rock surface (Fathi et al. 2010c). Also, in another study, Austad et al. suggest that low-salinity-water injection process efficiency is mainly dependent on the initial wettability of rock and total acid number of crude oil (Austad et al. 2005). However, one cannot find a direct relationship between increasing acidic number and increasing recovery coefficient. Besides, there is another research by which the effect of amphiphilic water-soluble compounds in crude oil and their interactions with the ionic compounds of injected water was conducted to examine the change of wettability. The presence of acidic components soluble in water substantially reduces the efficiency of the wettability change than does low-salty freshwater. The amphiphilic water-soluble compounds lead to expanding electric-double-layer, coupling magnesium with the adsorbed compounds and interacting cationic components with acidic ones (Song et al. 2020). The effect of different formulations of smart water on the wettability alteration of carbonate rocks (limestone) was investigated by Awolayo et al. (2014). The results confirm that the contact angle decreases with increase in brine water exposure time. They also showed that the surface wettability varies from a strongly oil wet to a weakly oil-wet condition with sulfate concentration increase. They explained that as the concentration of sulfate ions increased, the water film on the rock surface became thicker and more stable. Therefore, the contact area between the oil droplets and the rock surfaces is reduced due to the electrostatic repulsion forces that lead to the repulsion of oil compounds from the rock surface, surface wettability alteration, and carboxylic acid functional group removal from the rock surface, after which the active ions of the injected water occupied the vacant active sites of the rock surface. In another study, researchers found that the water composition of water flooding is more important than its salinity. They state that if the water composition is designed to fit the conditions of the reservoir, it could improve oil recovery by up to 30% (Snosy et al. 2022). Bazhanova and Pourafshary designed Caspian Sea injection water as smart water for injection into carbonate tanks. They found that five times diluted seawater with a spiked concentration of calcium and sulfate is the most effective solution for the wettability alteration process (Bazhanova and Pourafshary 2020). Montazeri et al. also investigated wettability changes in carbonate rocks focusing on the role of individual ions in the fluid–solid interactions. They found that an increase in the concentration of SO42− ion would accelerate the reaction rate in the crude oil–brine–rock (CBR) system. In addition, Mg2+ divalent cation was more effective than Ca2+ cation in the wettability alteration process (Montazeri et al. 2020). Most studies generally have reported that application of low salinity water or designed water injection led to oil recovery enhancement in carbonate reservoirs (Austad et al. 2005; Yousef et al. 2011, 2010; Ligthelm et al. 2009; Puntervold et al. 2009; Webb et al. 2005; Winoto et al. 2012; Zahid et al. 2012). This study has tried to investigate the experiments in more realistic conditions and close to the reservoir conditions. In addition, there be a closer look at the interactions in the rock–oil and brine system and try to separate and study the effect of active ions separately in the interaction of rock, injection fluid, and oil. Therefore, in this study, while considering the effect of active ionic compounds on the rock surface, there is an attempt to study the factor of active ionic and active oil compounds in smart water separately.
Materials and methods
Crude Oils
This study selected a crude oil sample from the Iranian oil fields and used it as the original oil phase. Other oil phase samples are prepared from the original oil sample with standard methods considering the acidic components of crude oil. The crude oil with different properties has been chosen to investigate the effects of crude oil chemical constituents on the wettability alteration of carbonate rock. The specifications of the crude oil samples are given in Table 1.
Brines
All the water samples used in this study have been synthesized. For this reason, the required amount of sodium chloride, sodium sulfate, magnesium chloride, calcium chloride, potassium chloride, and sodium bicarbonate salts purity > 99% were purchased from Merck Co and were dissolved in deionized water based on available information about real seawater (Persian Gulf) and formation water (Iranian oil fields) constituent ions and concentration, while other samples of synthesized waters are prepared in the same way as required. In this study, synthetic waters have been formulated to investigate the effects of various active ions. Also, we tried to keep the two factors of ionic strength and pH constant. The physical and chemical properties of smart water used in this study are presented in Table 2.
Reservoir limestone cores
Four identical core plugs with similar characteristics have been prepared from natural calcite reservoir rock and labeled as core 1 to core 4. To confirm the similarity of rock properties, three index parameters such as absolute permeability, porosity, and average pore size are measured. The specifications of core plugs are given in Table 3. According to Figure 1, CT scans were taken to investigate the probable existence of any heterogeneity, such as micro-fractures in the rock samples. To gain an insight into the constituting elements of these cores, X-ray diffraction (XRD) and SEM coupled with EDX analysis was performed, and the results are presented in Table 4.
Outcrop calcite cores
Also, due to limitations in access to the real reservoir rock, some samples from calcite outcrops with similar properties to real ones were used to determine the effect of fluid injection and oil composition on wettability. The outcrop samples have specifications such as average porosity of 30%, average permeability of 1 mD, and an average pore volume of 5 cc.
Reservoir limestone core preparation
First, the limestone core was flooded with toluene and methanol to remove initial crude oil until a clear/transparent effluent solution. The methanol was displaced by flooding three pore volumes (PV) with heptane. Dionysus water was injected to remove formation water (FW). So, dry rock samples were saturated with synthetic formation water under vacuum conditions and then placed under the pressure of 300 psi of the same brine for 24 hours. The core samples were then flooded with oil using HPHT core flood apparatus under constant flow conditions to the point that no brine was observed in the outlet (irreducible water saturation establishment). In these experiments, rock sample characteristics are mentioned in Table 3 with the same formation water saturation. The rock samples were aged for 40 days at 90 c with a different oil.
Changes oil composition
In this study, oil samples with different content of acidic groups were prepared. The initially selected oil sample with a medium level of acidic constituents was exposed to silica gel to reduce or eliminate the acidic or basic compounds. The optimal concentration of silica gel was 10% wt. The oil samples’ acidic and basic component content is measured by titration and potentiometric methods. Acidic and basic values were based on ASTM D664 and ASTM D2896 guidelines, respectively. The Fourier transform infrared (FTIR) spectroscopy was adopted to determine and evaluate the polar functional groups of prepared oil samples and their effect on total acid and base numbers. The FTIR spectra of samples with various acidic compounds content are presented in Figure 2.
Contact angle measurement
Contact angle measurements are performed using the sessile drop method and a drop shape analysis system, as shown in Figure 3. The outcrops were purged with toluene and methanol and then dried. The dried carbonate plates were placed in a vacuum chamber for 4 hours and saturated with the formation water. To ensure 100% saturation, the sample was placed under formation water flow with a pressure of 300 psi for a while. The carbonate rock plates were held in oil for 30 days at 90 °C temperature. Then, the excess oil was removed, and the initial contact angle of the oil-aged rock samples was measured. Then, the rock plates were dipped into the different brine-filled chambers, and the contact angle of oil droplets at the rock’s surface was measured at specified time intervals. It was observed that the contact angle first changed gradually and then eventually stabilized. Images were taken at different times. To improve the accuracy of the experiment, the number of carbonate slides increased, and the experiment was repeated under the same conditions at two different time intervals.
Spontaneous imbibition tests
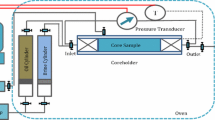
In order to perform spontaneous imbibition tests in more realistic reservoir conditions, a setup was designed according to Figure 4 that can control the temperature and pressure during this process for a long time and also can inject smart water continuously. This setup is designed from a cell with dimensions (42*250 mm) to hold the core during the process with a pressure tolerance of 4000 psi. The oven is used to maintain temperature uniformity in the long run. At the end of the aging period, the core plug was placed in an Amott cell filled with the selected injection fluid at 90 °C. When no more oil is recovered, the formulation of smart water can be changed until the optimum condition is reached. The produced oil content was constantly observed and recorded over time. When no more oil was recovered at the output, the core plug was placed in centrifuge apparatus to recover residual.
Results and discussion
In this study, an experimental procedure has been designed. The effect of different ions on the wettability alteration process can be analyzed considering the mechanism of ion-exchange and crude oil constituents’ structure. So, the experiments are designed in two parts to cover the purpose of each section separately. This fact must also be considered that the surface of rock from different origins can have different initial wettability and, therefore, different behavior in contact with water containing active and inactive ions, depending on the reservoir thermodynamic conditions and some other influential factors.
Measurement of the contact angle
Figure 5 shows the high-resolution images of the oil droplet placed on the carbonate surface to specify the measure of the contact angles. The contact angle measurements are performed through immersion of rock sample in the fluid phase with higher density, i.e., the aqueous phase, and placing an oil droplet on it. The contact angle measurement is made by matching the oil/brine profile, determining the baseline on the carbonate rock surface, and specifying the tangent line on the three-phase contact point. The arithmetic means of the right and left captured angles of the stable oil drop on the surface of the carbonate sample have been introduced as the final contact angle. The measurement approach in these experiments is based on the Young Laplace method. A high-resolution camera, a large number of rock samples being tested, and repetition of tests in two time periods cause the measurement error to be negligible. First, the contact angle measurement was started on formation water (FW) aged rock samples (Outcrop), and the results were considered the initial rock wettability (θi). In the second step, the rock samples were aged under the above-mentioned conditions in crude oil. Then, contact angle of oil-aged rock samples was measured, referred to as original wettability values (θ0). In the third step, the contact angle of samples was measured after at least 16 days under the same condition. The results would be known as the final contact angle (θf). The wettability alteration index (WAI) was defined and calculated for the samples as follows Rashid et al. (2015). WAI ranged from 0 to 1. A WAI value of around zero indicates that no wettability alteration occurs, while a WAI close to one implies complete wettability reversal.
The results indicate that carbonate rock surface contact angle declines over time, especially at the exposed surface to the injected water. Thus, exposure of smart water to carbonate rock surface can influence initial wetting conditions considerably and alter it to a more water-wet condition. Altogether, smart water mechanism could be explained by the observed surface wettability alteration caused by the changes in surface charge or electrostatic forces.
The effect of changes in the ionic compounds of smart water
In this section, the carbonate core plugs aged in crude oil (A) are placed in the vicinity of smart water with different compositions. The results of experiments only represent the effect of ion concentration on performance and rate of surface wettability alteration in the vicinity of smart water because of similar experimental and physical conditions. Figure 6 shows the results of the measured contact angles for each composition of smart water. In these experiments, the contact angle of an oil droplet on a carbonate surface in the presence of formation water has been introduced as an index of measurement. Long-term exposure to aqueous phases such as seawater with twice the sulfate ion concentration induces the most significant alteration in surface wettability. Besides, Figure 7 demonstrates measured contact angles according to WAI for various compositions of smart water. It can be seen that seawater with twice the concentration of the sulfate ion causes the most significant decrease in the contact angle with the carbonate surface. Although the rate of change is higher in the beginning steps of the experiment, contact angle decrease does not occur in a relatively short time. As shown in Figure 5, the wettability alteration of carbonate surface during 16 days in seawater with twice the sulfate ion concentration is evident. Moreover, the most minor changes were reported in plugs in the vicinity of seawater without ionic composition manipulation. It is worth mentioning that an increase of more than two times in the concentration of sulfate ions has less influence on the wettability alteration process, which is completely following the results of previous studies. Published experimental data indicate that reaction which causes desorption of acidic oil compounds from the rock surface might alter the wettability of carbonate rocks toward the higher water-wet condition (Austad et al. 2005; Hiorth et al. 2010; Myint and Firoozabadi 2015; Purswani et al. 2017). Experimental results clearly show that the effect of Ca2+ on the wettability alteration is greater than that of Mg2+, which can be related to the different hydration energy of calcium and magnesium at 90 °C. In fact, magnesium ions can replace calcium ions on rock surfaces and improve the desorption conditions of carboxylic groups. High temperature and high density of positive charges are essential to make the reaction as efficient as possible. The spontaneous imbibition test was also exploited to confirm the results obtained from the wettability alteration measurement and replication evaluation of gathered data. Reactions from the beginning of smart water injection on the calcite surface are shown as reactions (1)–(5) in Table 5.
Change the oil composition
In this experiment, carbonate core plugs were aged with a base crude oil A and a synthetic crude oil A.S. This process aims to expose carbonate plugs to crude oil with different degrees of acidity number. Then, the plugs are exposed to smart water in two separate containers. The sulfate ion concentration in smart water is twice that of seawater. The results can be seen in Figs. 8 and 9. The initial contact angle of carbonate core plugs was measured before being exposed to smart water. After a specific period of time, the contact angle was measured again to examine whether induced changes in the contact angle toward water wet or a neutral wet. The results show that the smart water injection process cannot be adequately effective in core plug samples aged with non-acidic crude oil. However, the initial wettability of these plugs was less oil wet than ones exposed to original oil. Besides, the difference in the mechanism of contact angle changes during a specific period of time can be observed in Fig. 10. Carboxylic acids are strongly adsorbed to the carbonate surface as single monolayers. Then, their aliphatic chains will make them stable by interactions with a closed hydrophobic layer or polymer structures in crude oil, implying that this interaction will shift the wettability toward more oil wet depending on their concentration in crude oil. However, it should be noted that complex acidic and basic compounds can be formed due to ionic interactions with the formation water in the vicinity of the rock surface. Acidic compounds are more reactive in the vicinity of carbonate surfaces than basic compounds one. So, they can be more effective in the wettability alteration process (Austad et al. 2012; Fathi et al. 2010b). The results of FTIR spectroscopy are shown in Fig. 2. The significant difference between compositions of heterotopic constituents of two crude oil samples is evident. Thus, it can be assumed that the polar compounds needed to participate in the surface complex reaction are not sufficient for treated oil. Likewise, the network of reactions and the interactions available in the oil–water are discussed hereunder. These reactions interact between carboxylic acid groups and phenols with water cations, leading to the decomposition and repulsion of carboxylic acid groups. Based on the FTIR analysis, the acidic compounds-free oil has lower heteroatoms like sulfur, nitrogen, and oxygen, so it cannot readily react with smart water cations to excrete carboxylic groups. This results in lacking the wettability change of the aged surfaces related to this type of oil. The reaction network consists of reactions in the aqueous-phase and surface complex reactions at the interface between oil–water–rock, oil–rock, and water–rock. Reactions at the rock–water interface include sulfate adsorption and carboxylic groups’ removal. Responses at the interface between oil and water include decomposition and desorption of carboxylic acid functional groups and the reaction between carboxylic acid and cations (Al-Shalabi et al. 2015; Qiao et al. 2015). Aqueous reactions include all the reactions considered rapid reactions, and it is assumed that thermodynamic equilibrium takes place in the system by these reactions.
Spontaneous imbibition (SI) test
Three spontaneous imbibition tests were designed and conducted to examine and validate the results obtained from the contact angle measurement, which represents wettability alteration induced by smart water injection. The main goal of the two tests was to investigate the effects of ionic compounds in smart water, and in the other test, the effect of acidic compounds in oil was investigated. In these experiments, rock sample characteristics are mentioned in Table 3 with the same formation water saturation. The rock samples were first aged for 40 days depending on the purpose of the experiment with the desired crude oil and were exposed to the desired smart water.
The effect of sulfate ions
The core (2) with a porosity of about 16.61% and the liquid permeability of approximately 2.5 md is aged with crude oil (A). The oil saturation (So) in the core is about 76 percent. The results of CT scan and XRD and EDX tests confirm that the core has no micro-fracture and contains about 8% of anhydrite sediment. The rock slice was placed under a spontaneous imbibition test for 80 days. The results are displayed in Fig. 11. After the core plug was placed in the spontaneous imbibition cell, it was first exposed to formation water, and the oil recovery was recorded versus time. In this step, overall oil recovery was about 10%. This fluid production could be attributed to the water–oil displacement process, which is affected by a temperature of 90 °C. After that, the formation water was slowly replaced by seawater. So, sessile water droplets will remain on prepared rock surfaces. After 35 days from the beginning of the test, the observed oil recovery is approximately 25%. This demonstrates the impacts of seawater on the wettability alteration of the carbonate rock surface and the oil displacement from larger pores. Optimization of sulfate concentration in seawater has increased the oil recovery by 34%, confirming the positive effect of sulfate ions on oil recovery. Besides, doubling the concentration of sulfate ions exacerbates the process of wettability alteration and oil displacement from medium and smaller pores. One of the accepted mechanisms for this behavior is the adsorption and replacement of sulfate on a rock whose potential is reduced by magnesium and calcium cations. As a result, it leads to the separation of carboxylic groups. These reactions control the wettability and related change in the contact angle, which determines the capillary pressure, relative permeability, and the amount of saturated oil remaining in the rock.
The effect of calcium ion compared to the magnesium ion
The core (1) with a porosity of about 24.33% and liquid permeability of approximately 2 md is aged with oil (A). The oil saturation (So) in the core is about 85 percent. The results of CT scan and XRD and EDX tests confirm that the core has no micro-fracture and contains about 2% of anhydrite sediment. This rock slice was placed under a spontaneous imbibition test for 80 days. The results are displayed in Fig. 12. After the core plug was placed in the spontaneous imbibition cell, it was first exposed to formation water. In this step, the oil recovery was approximately 2%, attributed to the water–oil displacement process affected by a temperature of 90 °C. Then, formation water was slowly replaced by seawater. So, sessile water droplets will remain on prepared rock surfaces. After 33 days from the beginning of the test, the observed oil recovery is approximately 28%. This demonstrates the impacts of seawater on the wettability alteration of the carbonate rock surface and the oil displacement from larger pores. Doubling the concentration of calcium ions has led to an increase in oil recovery by 34%, confirming the positive impact of calcium ions. In fact, an increase in calcium ions concentration leads to a decrease in the dissolution rate of surface minerals. However, the increasing oil recovery trend clarifies that a higher concentration of calcium causes the congestion of positive cations on the surface, which in turn leads to the formation of a stable and ion-rich water film on the surface. Calcium cations are displaced and positioned between organic adsorbed components containing carboxylic acid and carbonate rock surface due to the ion-exchange process. This phenomenon will finally result in the separation of carboxylic groups. In this regard, better performance and effectiveness of magnesium ions could be noteworthy. Then, formulated smart water is replaced with seawater containing twice the concentration of magnesium cations. Almost 41% of incremental oil recovery was achieved in this process. In this regard, it was found that growth in the concentration of calcium and magnesium cations in the presence of sulfate ions aggravates the wettability alteration process and oil displacement from medium and smaller pores.
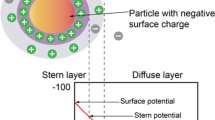
The electrostatic forces between two systems of carbonate rock—connate water and oil—connate water form a mono-layered and unstable water film that enhances the interaction between crude oil drops and carbonate rock polar surface. As a result, crude oil will be adsorbed to the rock surface. The phenomenon turns the surface wettability toward oil-wet condition and decreases the rate of crude oil recovery from carbonate reservoirs (Hirasaki and Zhang 2004). Injected smart water has different ionic compositions. This difference in ionic composition disturbs the main thermodynamic equilibrium in the reservoir. In this situation, geochemical reactions occur on the surface, which cause changes in the concentration of ions and finally lead to wettability alteration. Wettability can control capillary pressure and relative permeability. In turn, variation in these factors also leads to alteration of multiphase flow condition and crude oil recovery in the reservoir. Ions including \({\mathrm{H}}^{+}\) and \({\mathrm{OH}}^{-}\) can play an effective role in changing the surface charge of the rock and thus wettability alteration. Experimental results show that calcium, magnesium, and sulfate affect the surface potential, but calcium or magnesium cannot alter surface wettability without sulfate ions. Reactions (3)–(8) show that the sulfate concentration determines the solid surface potential. At the same time, magnesium and calcium are excreted with carboxylic acids from the interface between the liquid and solid, which leads to a certain change in wettability (Table 6).
These reactions describe the different roles of aqueous compounds. Reaction (5) shows the acid/base interface interaction between oil and water on a solid surface. Electrostatic attraction between oppositely charged phases leads to oil drops adsorption on a solid surface (Buckley et al. 1996). This reaction is similar to the competitive adsorption of carboxylic acid and sulfate on the interface between chalk and water. The competitive composition of Mg2+, Ca2+, and \({\mathrm{CaOH}}_{2}^{+}\) on the interface between oil and water is shown in reactions (4) and (5). Reactions (12) and (14) take place in an aqueous solution in carbonate reservoirs. These reactions are also important in PH measurement in aqueous medium. The results of experiments confirm this claim that any changes in electrostatic repulsion force between carbonate rock surface and brine/oil will affect the surface wettability and provide the conditions for the spontaneous imbibition process.
The effect of changes of oil composition
This section investigates the impact of acidic oil components on wettability alteration in carbonate rock, which is considered the initial wettability effect. Two carbonate cores with similar specifications have been selected according to table (3). Both cores are saturated with two different oil samples under the same conditions. Core (3) and core (4) are aged with oil sample A.s and crude oil sample A, respectively. The oil saturation in both core plugs is 70%. These core plugs have been placed under a spontaneous imbibition test for 70 days. The results are displayed in Fig. 13. The cores were first exposed to formation water in the spontaneous imbibition cell according to SI test procedures. The formation water was then replaced with seawater. The oil recovery was regularly recorded versus time. In all steps of this test, the ambient temperature was set at 90 °C. Core (3) showed a significant recovery factor of about 23% when it was in contact with formation water, while core (4) showed a recovery factor of about 6% under the same conditions. This fluid production could be attributed to the water–oil displacement process, which is affected by a temperature of 90 °C. Assuming that formation water is not able to change wettability, seawater will be used instead of that. Core (3) reacts with seawater after 10 days and starts to produce oil with a considerable delay. The final recovery in this sample is reported to be about 29%. However, under similar conditions for core (4), the final recovery has been reported to be about 37%. It is evident that these core surface minerals have reacted with seawater the faster the reaction proceeds. The results of this experiment conform to contact angle measurement findings. According to the results of the FTIR test in Fig. 2, it can see that at the frequency (1800 cm−1), the highest difference between crude oil A and oil A.S is related to group C=O or the same group carboxylic acid. Then, the C-H and C–OH bands are different peaks. Also, there are almost significant differences in the two categories of compounds C=N and C=S. Therefore, as a result, a decrease in polar content in crude oil, especially acidic functional groups, is not much effective and weakens the performance of the smart water injection process despite a lower initial wetting degree (neutral zone). The presence of acidic functional groups in crude oil is required to participate in the reactions between the three phases of oil, rock, and injected water, which provides suitable conditions for wettability alteration of carbonate surface and then facilitates the movement and absorption of oil from rock pores.
Conclusions
Experimental results show that smart water has an excellent potential to change the surface wettability to improve oil recovery from carbonate reservoirs. However, this method is a complex technique with multiple uncertainties that depend on various variables such as reservoir conditions and reactions in the rock–brine–oil system interface. A small change in any of the effective parameters can cause a change in the functionality of the mechanism. Therefore, to achieve the proper formulation of smart water, a mechanistic understanding of how wettability changes in a three-component system of rock–brine–oil are essential. In addition to paying attention to these parameters, the physical condition of the reservoir must also be considered. This leads to the design of injection water for each specific reservoir according to its effective parameters. The results also indicate that the type of mechanism involved in the rock–brine–oil interaction is very effective on the wettability followed by the distribution of phases in the reservoir and the resulting recovery enhancement. Therefore, conditions must be provided such that they lead to the formation of a stable film layer on the surface, rich in positive ions. An increase in disjoining pressure leads to further desorption and deallocation of crude oil components. Therefore, in this research, an attempt has been made to investigate the experiments in more realistic conditions and close to the reservoir conditions. Also, in this study, we have tried to investigate the effect of active ions separately on the interaction of rock, injected fluid, and oil. In general, it can be concluded that:
-
The results confirm that the existence of three basic conditions of polar compounds of oil, the presence of active ionic compounds in injected water, and the stable physical conditions of the reservoir is essential for performing a flooding process with smart water to increase oil recovery efficiency.
-
According to the spontaneous imbibition test results, since both cores were low permeability, they could have good efficiency in injecting smart water.
-
According to the comparison of the results of the SI test, the efficiency in the core (1) was about 7% higher compared to the core (2). In this regard, considering that the physical properties of both cores were similar, the increase in divalent calcium and magnesium cations in the presence of the minimum optimal sulfate ion can be seen (in this study, the concentration of sulfate in seawater). It can have a positive performance.
-
Initial wettability is an effective factor in predicting reservoir behavior in the smart water injection process.
-
Given the experimental results, the presence of acidic compounds or the same active compounds in the crude oil is obligatory to participate in the reactions between the three phases of oil, rock, and injecting water. Moreover, the interaction between oil active compounds and brine ones can control the rate of wettability change in a smart water injection process.
-
The predominant mechanism in this study of smart water injection is the ion-exchange mechanism.
References
Al-Shalabi EW, Sepehrnoori K, Pope G (2015) Geochemical interpretation of low-salinity-water injection in carbonate oil reservoirs. SPE J 20:1212–1226
Austad T, Shariatpanahi S, Strand S, Black C, Webb K (2012) Conditions for a low-salinity enhanced oil recovery (EOR) effect in carbonate oil reservoirs. Energy Fuels 26:569–575
Austad T, Strand S, Høgnesen E, Zhang P (2005) Seawater as IOR fluid in fractured chalk. In: SPE international symposium on oilfield chemistry. Society of Petroleum Engineers
Awolayo A, Sarma H, AlSumaiti AM (2014) A laboratory study of ionic effect of smart water for enhancing oil recovery in carbonate reservoirs. In: SPE EOR conference at oil and gas West Asia. Society of Petroleum Engineers
Bazhanova M, Pourafshary P (2020) Impact of SO42−, Ca2+, and Mg2+ ions in Caspian Sea ion-engineered water on the rate of wettability alteration in carbonates. J Pet Explor Prod Technol 10:3281–3293
Brady PV, Krumhansl JL (2012) A surface complexation model of oil–brine–sandstone interfaces at 100 C: low salinity waterflooding. J Petrol Sci Eng 81:171–176
B Brady PV, Krumhansl JL, Mariner PE (2012) Surface complexation modeling for improved oil recovery. In: SPE improved oil recovery symposium. OnePetro
Buckley JS, Bousseau C, Liu Y (1996) Wetting alteration by brine and crude oil: from contact angles to cores. SPE J 1:341–350
Denekas M, Mattax C, Davis G (1959) Effects of crude oil components on rock wettability
Drummond C, Israelachvili J (2004) Fundamental studies of crude oil–surface water interactions and its relationship to reservoir wettability. J Petrol Sci Eng 45:61–81
Fathi SJ, Austad T, Strand S (2010a) “Smart water” as a wettability modifier in chalk: the effect of salinity and ionic composition. Energy Fuels 24:2514–2519
Fathi SJ, Austad T, Strand S, Puntervold T (2010b) Wettability alteration in carbonates: the effect of water-soluble carboxylic acids in crude oil. Energy Fuels 24:2974–2979
Fathi SJ, Austad T, Strand S, Puntervold TJE (2010c) Wettability alteration in carbonates: the effect of water-soluble carboxylic acids in crude oil. Energy Fuels 24:2974–2979
Fathi SJ, Austad T, Strand SJE (2011) Water-based enhanced oil recovery (EOR) by “smart water”: optimal ionic composition for EOR in carbonates. Energy 25:5173–5179
Hiorth A, Cathles L, Madland M (2010) The impact of pore water chemistry on carbonate surface charge and oil wettability. Transp Porous Media 85:1–21
Hirasaki G, Zhang DL (2004) Surface chemistry of oil recovery from fractured, oil-wet, carbonate formations. Spe J 9:151–162
Lager A, Webb KJ, Black CJJ, Singleton M, Sorbie KS (2008) Low salinity oil recovery-an experimental investigation1. Petrophys- SPWLA J Form Eval Reserv Descr 49(1)
Ligthelm DJ, Gronsveld J, Hofman J, Brussee N, Marcelis F, van der Linde H (2009) Novel waterflooding strategy by manipulation of injection brine composition. In: EUROPEC/EAGE conference and exhibition. Society of Petroleum Engineers
Montazeri M, Fazelabdolabadi B, Shahrabadi A, Nouralishahi A, HallajiSani A, Moosavian SMA (2020) An experimental investigation of smart-water wettability alteration in carbonate rocks–oil recovery and temperature effects. Energy Sour, Part A: Recov, Util, Environ Effects 1–13
Myint PC, Firoozabadi A (2015) Thin liquid films in improved oil recovery from low-salinity brine. Curr Opin Colloid Interface Sci 20:105–114
Pokrovsky O, Schott J (2002) Surface chemistry and dissolution kinetics of divalent metal carbonates. Environ Sci Technol 36:426–432
Puntervold T, Strand S, Austad TJE (2009) Coinjection of seawater and produced water to improve oil recovery from fractured North Sea chalk oil reservoirs. Energy 23:2527–2536
Purswani P, Tawfik MS, Karpyn ZT (2017) Factors and mechanisms governing wettability alteration by chemically tuned waterflooding: a review. Energy Fuels 31:7734–7745
Qiao C, Li L, Johns RT, Xu J (2015) A mechanistic model for wettability alteration by chemically tuned waterflooding in carbonate reservoirs. SPE J 20:767–783
Rashid S, Mousapour MS, Ayatollahi S, Vossoughi M, Beigy AH (2015) Wettability alteration in carbonates during “Smart Waterflood”: underlying mechanisms and the effect of individual ions. Colloids Surf, A 487:142–153
Seccombe JC, Lager A, Webb KJ, Jerauld G, Fueg E (2008) Improving wateflood recovery: LoSalTM EOR field evaluation. In: SPE symposium on improved oil recovery. Society of Petroleum Engineers
Snosy MF, Abu El Ela M, El-Banbi A, Sayyouh H (2022) Comprehensive investigation of low salinity waterflooding in carbonate reservoirs. J Pet Explor Prod Technol 12:701–724
Song J, Rezaee S, Guo W, Hernandez B, Puerto M, Vargas FM, Hirasaki GJ, Biswal SL (2020) Evaluating physicochemical properties of crude oil as indicators of low-salinity–induced wettability alteration in carbonate minerals. Sci Rep 10:1–16
Webb KJ, Black CJJ, Tjetland G (2005) A laboratory study investigating methods for improving oil recovery in carbonates. In: International petroleum technology conference. International Petroleum Technology Conference
Winoto W, Loahardjo N, Xie SX, Yin P, Morrow NR (2012) Secondary and tertiary recovery of crude oil from outcrop and reservoir rocks by low salinity waterflooding. In: SPE improved oil recovery symposium. Society of Petroleum Engineers
Wolery TJ (1992) EQ3/6, a software package for geochemical modeling of aqueous systems: package overview and installation guide (version 7.0) (No. UCRL-MA-110662-PT. 1). Lawrence Livermore National Lab
Yousef AA, Al-Saleh S, Al-Kaabi AU, Al-Jawfi MS (2010) Laboratory investigation of novel oil recovery method for carbonate reservoirs. In: Canadian unconventional resources and international petroleum conference. Society of Petroleum Engineers
Yousef AA, Al-Saleh S, Al-Kaabi A, Al-Jawfi M (2011) Laboratory investigation of the impact of injection-water salinity and ionic content on oil recovery from carbonate reservoirs. SPE Reserv Eval Eng 14:578–593
Zahid A, Shapiro AA, Skauge A (2012) Experimental studies of low salinity water flooding carbonate: a new promising approach. In: SPE EOR conference at oil and gas West Asia. Society of Petroleum Engineers
Funding
This study was funded by the Department of Chemical Engineering at the Iran University of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bovard, S., Sadeghi, M.T., Kazemzadeh, E. et al. Experimental study of effective compounds in a smart water injection process. J Petrol Explor Prod Technol 13, 471–485 (2023). https://doi.org/10.1007/s13202-022-01561-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-022-01561-7