Abstract
Recently, interest in on-site heat generation has increased due to injection of thermochemical fluids as a complex effect on well productivity. The method of thermochemical treatment with H2O2 while restoring and increasing the filtration characteristics of the bottomhole formation zone is a relatively new and insufficiently studied technology. The article discusses the key factors affecting the exothermic decomposition of this fluid when this fluid is injected into the well. The heat effects, pressure growth and decomposition time of H2O2 were determined depending on the salinity of the water, the composition of terrigenous rock, and various concentrations of H2O2. Physical 1-D modeling of H2O2 injection was carried out on rock models with mobile and stationary oil, which demonstrated a sharp increase in temperature by 100–240 °C caused by the decomposition of H2O2 due to the catalyst and the presence of catalytic active sites in the rock. As a result of this thermochemical treatment, the rock was partially cleaned of immobile oil and heavy sediments. Injection of H2O2 with a catalyst has shown the effectiveness of displacement of mobile oil from the filled sand model. Thus, the results of this study can provide a preliminary assessment of the effectiveness of H2O2 thermochemical treatment in fields operated at a later stage of development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent analysis suggests many large global deposits are in the final stages of development. Thus, further development of producing oil deposits in mature fields requires intense processes for increasing recovery and well productivity. This involves the use of complex technologies for purposes such as cleaning the bottomhole formation zone and enhancing oil-saturated zones with deteriorated filtration-volumetric properties.
In wells producing from terrigenous reservoirs, the following types of impact on the bottomhole formation zone are most often used: thermogasochemical, electric heating, warming up with hot water and oil, treatment with solutions of surfactants and solvents, and hydraulic fracturing of the formation (Shah et al. 2010, Al-Bahlani et al. 2011, Hassan et al. 2019, Shafiai et al. 2020). Chemical methods are used to influence the bottomhole formation zone in carbonate reservoirs, where treatment with an aqueous solution of hydrochloric acid is most often carried out. For the treatment of terrigenous reservoirs with a carbonate content of less than 10%, clay-acid solutions are used, prepared from hydrochloric and hydrofluoric acids (AlMubarak et al. 2015; Dashti et al. 2007). However, the use of acids can lead to severe damage to the formation, which is associated with acid-oil emulsions, or the deposition of asphaltenes in some carbonate reservoirs with low permeability.
In practice, it is well known that the innovative method of thermochemical influence on the formation can help in such cases. This technology involves the injection of solutions, mainly inorganic salts of nitrates, chlorides, and nitrites, in addition to initiators of their decomposition, forming an energy-generating composition (Kuznetsov et al. 2017). Mahmud M. and colleagues are actively working in this direction, having studied in detail the kinetics and energy analyses of typical chemical compositions (NH4Cl and NaNO2) and preliminary evaluation of performance in recovery of heavy oil and gas condensate (Alade et al. 2019; Hassan et al. 2018). The dual salt process is the injection of two chemicals that react together in the downhole environment to generate heat and nitrogen, thereby significantly increasing pressure and temperature. Thermochemical stimulation for stimulation provides a number of advantages over the cyclic steam treatment technology, such as investment of lower capital costs, injection of a smaller volume of injection agent, reduction of well-operation time and reduction of the time to warm up the bottomhole zone to the required temperature.
A review of other chemical agents capable of generating increased energy and gas production during their injection into the formation allows us to consider the class of peroxide compounds for this purpose. Peroxides are characterized by a weak dissociation of the O–O bond (190–210 kJ/mol), which is less than half the strength of the C–C, C–H, and C–O and leads to easy breaking of the O–O bond (Bach et al. 1996). Therefore, a weak acid such as hydrogen peroxide (H2O2) can be considered as a suitable injection agent for thermochemical technology the reaction of its exothermic decomposition (∆Hd = −2884.5 kJ/kg), accompanied by a vigorous evolution of oxygen (Eq. 1) (Pedziwiatr et al. 2018, Plaucka et al. 2016). The use of H2O2 as an injection agent is attractive from the point of view of the availability of the reagent, as well as its compliance with environmental standards, having non-toxic decomposition products (water and oxygen):
One of the first studies to use H2O2 as a thermal enhanced oil recovery method was carried out by Bayless in 1989 (Bayless et al. 1989). This study determined that after H2O2 injection into the formation zone, there is a waiting period before the decomposition of H2O2, which leads to the release of the required amount of heat to increase the temperature of the formation. The peculiarity of using this reagent is that during its injection, not only a large amount of heat is released, but also active oxygen is released, which contributes to the exothermic process of oil oxidation, which contributes to additional heating of the formation. The decomposition of H2O2 has a few controllable parameters, such as temperature, concentration, pH and catalyst type, which according to Bayless make H2O2 a particularly useful agent in the production of high viscosity oils.
The possibility of using H2O2 solutions as a liquid oxidant in thermochemical methods for extracting high-viscosity oil was also studied by Khlebnikov et al. (2013). Their proposal was to introduce a catalyst (manganese oxide IV, ferrous oxides) in the form of an aqueous suspension to create a catalyst pad at the bottomhole, after which a 10–40 wt.% H2O2 solution is injected into the formation. According to their data, if the initial temperature of the injected solution is in the range of 10–20 °C, then as a result of the exothermic reaction, the temperature of the water–gas mixture can reach 86–286 °C when the concentration of hydrogen peroxide changes from 10 to 40 wt.%. They come to the conclusion that the heat released during the autooxidation reaction of residual oil will maintain high water temperatures and compensate for heat losses in the higher and lower horizons, as well as heating the reservoir rock and reservoir fluids.
The data discussed in the works cited above only indirectly answer questions about the possibility of thermochemical treatment of the formation with H2O2, without affecting detailed laboratory studies that play a significant role in the operation of this technology. And with the growing interest in using such technology in the field, data on thermodynamic and kinetic behavior are definitely important and are touched upon in the present work.
Successful initiation of thermal treatment with H2O2 in the formation zone can be caused by factors such as reaching the self-decomposition temperature (SADT) of H2O2, the presence of catalytic impurities (for example, most transition metals) or additional injection of a decomposition initiator (Takagi et al. 1985, Lousada et al. 2012, Xing et al. 2018). Catalysis of peroxide under standard reservoir conditions can take place both in the aqueous phase (homogeneous) and on a solid surface (heterogeneous). Regardless of the initial method of injection into the formation, upon contact with the surface of metal equipment and during filtration through the rock there will be a contribution from heterogeneous catalysis (Pham et al. 2012; Teel et al. 2007). The catalytic decomposition of H2O2 on the surface of metals or metal oxides has been investigated in recent years, including Fe, W, Cu, UO2, ZrO2, CuO, CuO2 (Yang et al. 2015, Bjorkbacka et al. 2015). The kinetics of decomposition of H2O2 upon contact with a different type of surface is determined by the first order rate constant, which includes the rate constant of decomposition in the aqueous phase (homogeneous) kh and at the wall surface ksur. Lin and colleagues investigated the kinetics of H2O2 decomposition on relatively inert materials including Teflon, glass, stainless steel and titanium (Lin et al. 1991). They found that the rate constant for H2O2 decomposition in a stainless-steel metal tube (k125 °C = 0.0016 s−1) is approximately 80 times higher than in a tube made of an inert material (k132 °C = 0.000019 s−1), and the activation energies varied from 68.2 to 106.3 kJ/mol. The observed rate of decomposition of H2O2 in volume on a solid catalytic surface can be determined as follows (Croiset et al. 1997; Liu et al. 2018):
where the value SA/V is the ratio of the overall surface area of a pure metal or solid catalytic particles to a unit volume of a liquid medium. In general, the catalytic effect of a solid catalyst is associated with the process of charge transfer with the participation of radicals which is determined according to the equation (Hiroki et al. 2005):
where I–intermediate compound (for example, OH. or O2−), obtained as a result of the reaction of H2O2 with the solid surface of the catalyst.
The main goal of the study is to understand the ability of H2O2 to act as a highly active thermochemical fluid in reservoir conditions. In the results and discussion section, the thermochemical and kinetic aspects of the decomposition of H2O2 depending on its different concentration, the influence of terrigenous rock, salt water and metal surface are analyzed, and also physical modeling of H2O2 injection into a model containing immobile heavy oil and saturated with oil to evaluate H2O2 as a possible bottom hole cleaning agent and its displacement capacity is discussed. Before the discussion, the methodological part of the conducted research is given.
Materials and methods
H2O2 with a concentration of 37 wt.% (Khimprom Novocheboksarsk, Russia) and sodium permanganate with a concentration of 40 wt.% (Hefei TNJ Chemical Industry, China) were used in this study. Crude oil, formation water and core samples were taken from one of the oil fields in Tatarstan Republic (Russia). Before experiments the provided full-size core from terrigenous reservoir was crushed. Extraction of the crushed core was extracted in a Soxhlet apparatus, the operation lasted a week at the condensation temperature of the solvent mixture. An ethanol–benzene mixture in a ratio of 1:2 with the addition of chloroform (equal to alcohol) was used as a solvent. At the end of the extraction, the rock was ventilated from the solvent in a thermostated laboratory oven until a constant weight of the sample was achieved. Table 1 lists the mineralogical composition of the terrigenous samples used. TG experiments were performed in the TG 209 F1 Libra thermobalance (Netzsch, Germany) to evaluate the content of total organic matters contained in rock samples after combustion tube experiments. A corundum crucible was employed for heating samples from 30 to 850 °C at the heating of 10 °C/min. About 50 mg sand sample was used in each experiment. Before performing experiments, calibration should be carried out.
Experimental procedure
Thermal Analysis An accelerating rate calorimeter ARC 254 manufactured by Netzsch (Germany) was used to study heat effect and kinetics of hydrogen peroxide decomposition. Calibration of a calorimeter is a process of heating an empty cell to the specified temperatures with a step of 50 °C (50, 100, 150…500 °C). Each of the obtained temperatures corresponds to a certain voltage. Calibration of the device is reduced to entering a coefficient and when multiplied by it, the reference temperature values for a given thermocouple are obtained. Experiments were carried out in heat-wait-search (HWS) mode according to our previous works (Zhao et al. 2019, Yuan et al. 2020). The sample was heated with a step of 5 °C. After each step, the waiting time for search of reaction was 30 min. The criterion for finding the reaction was the self-heating of the system by more than 0.02 °C/min. When a reaction was found, the ARC automatically switches to the adiabatic mode and follows the reaction. The closed spherical containers (nickel-molybdenum-chromium heat resistant alloy) with a 7 ml volume were used for the experiments. The volume of the reaction mixture for research was 1 ml. Temperature control is carried out by means of a thermocouple (Control TC), which is directly attached from the outside to the calorimetric cell.
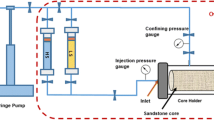
High-pressure reactor The study of the dynamics of pressure and temperature growth during the decomposition of H2O2 in a liquid state in a closed system was carried out in a sealed cylindrical high-pressure reactor (Fig. 1) made of steel grade AISI 304, equipped with an inlet for a pressure sensor. The experimental cell was filled by 37 ml H2O2 solutions of various concentrations from the total volume of the system in each measurement. For the safety of the process, this cell was in a special protected oven during the experiment.
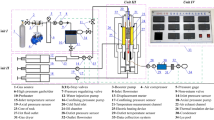
Filtration technique A self-designed experimental set-up for physical modeling of thermochemical methods for IOR studying the thermochemical effect of hydrogen peroxide injection in sand pack model under reservoir conditions (Fig. 2). The operation details of the unit were described in previous works (Minkhanov et al. 2021, 2020; Varfolomeev et al. 2021). The injection agent supply system consists of a precision high-pressure plunger pump (1), metering hydraulic fluid at a constant rate to the inlet to the piston containers (2), which are connected to a core holder (4) placed in the high-pressure chamber (3). The fluid collection system consists of a back pressure regulator (8), which maintains the reservoir pressure in the model and the fluid is discharged into the separation burette (9). A nitrogen cylinder is used to create pressure.
Scheme (left) and appearance (right) of self-designed experimental set-up for testing of thermochemical treatment on sand pack or core models: (1) high pressure plunger pump; (2) piston containers; (3) high pressure chamber; (4) core holder; (5) digital computer; (6) ceramic electric heaters; (7) thermocouples, (8) back pressure regulator; (9) separation burette
Core samples from the studied oilfield were crushed to a fraction of 0.1–1.0 mm. The resulting sample was pressed into a core holder with a diameter of 50 mm and a length of 300 mm. The permeability (K) of the disintegrated rock sample with immobile oil pressed into the core holder was 1056 mD. The permeability (K) of the model sand with mobile oil pressed into the core holder was 1243 mD. Reservoir conditions were created in the pressed-in model before fluid injection, and the initial pressure was 5 MPa. The injection of water and H2O2 was carried out at a constant rate of 1 ml/min, and the temperature changes along the entire length of the sand-pack model were measured using 4 thermocouples.
Results and discussion
Decomposition of H2O2 on various types of surfaces
For a quantitative interpretation of the processes in which the decomposition of hydrogen peroxide is possible both in the formation zone and during its injection, calorimetric studies were carried out in this work with the determination of thermodynamic parameters. Investigations of the process of decomposition of H2O2 in calorimetric experiments can be divided depending on the type of container surface and the effect of various chemical properties of individual substances (Table 2).
Thermal decomposition of H2O2 by adiabatic calorimetry
Factors such as concentration, temperature and pressure are important considerations when H2O2 is injected into the wellbore. The influence of each factor is an important condition for assessing the total exo-effect, the reaction start time, as well as the process safety associated with these characteristics. This is due to the possible explosive boiling of an aqueous solution during the decomposition of H2O2 (Nikitin et al. 1992). The most serious H2O2 accidents are usually associated with one of three types of decomposition processes: organic pollution; inorganic pollution; and decomposition caused by alkali (Hart et al. 2011).
In this work a series of thermochemical studies were conducted to determine the heat power of the decomposition of H2O2 by the method of adiabatic calorimetry (ARC)33,34, to analyze the criteria selectable when using H2O2 as a thermochemical agent in the formation and treatment of the bottomhole zone (West 1963; Duh et al. 1996).
The first thing that was studied at the ARC was the effect of concentration on the exo-effect of the reaction of decomposition of H2O2 with formation water involvement (Fig. 3). The ∆T of a dilute H2O2 solution from 7 to 30 wt.% (initial form) varies in the range from 9,0 to 78,6 °C and is described by a linear equation (R2 = 0.99) depending on the concentration (Table 3). Using this correlation, it is possible to predict the amount of heat released for various concentrations of hydrogen peroxide on a surface of the type B (Table 2). Therefore, the selection of the correct concentration of H2O2 and/or addition of an inhibitor can lead to reliable control of the exothermic decomposition reaction of H2O2, and therefore a given heat release.
To select an agent for pushing H2O2 through tubing into the formation (distilled or formation water), it is necessary to investigate the impact of the water salinity on the time for initiation of H2O2 decomposition. It is also important to consider the effect of water salinity on the wellhead equipment and oil-well tubing, that do not have an inert surface and can therefore affect the process of H2O2 decomposition. Salt water can damage not only low-alloy steels, but also stainless steel, copper, aluminum, nickel alloys (Fink 1963). The composition of water can affect the decomposition of H2O2, since an increase in water salinity can lead to both a zero effect with the content of ferrous sulfate and chloride in formation water, and a possible increase in the reaction rate with the content of sodium and magnesium chlorides.
Experiments utilizing ARC have shown that an increase in the salinity of an H2O2 solution upon dilution with water characterized by a high salinity in the range of 250 g/l leads to a reduction in the time for the onset of the decomposition reaction (Fig. 4). While the importance of water salinity on the decomposition of H2O2 is recognized, the exact mechanism of the effect of salts in solution in combination with the effect of the ARC cell surface (type B) is not known. This study shows that in the region where there is a reduction in the initiation time of the reaction, there is a region of linear dependence, which is proportional to the degree of diluted formation water. With a decrease in the salt solution concentration (> 50%), the effect of H2O2 decomposition is less intense and the reaction time increases (Fig. 5).
The bottomhole formation zone contains a large quantity of solid particles (minerals, mixtures of oxides, etc.), which are effective catalysts for the decomposition of H2O2. The removal of iron-containing material from the oil-well tubing into the bottomhole zone can provide an environment for the decomposition of H2O2. At the same time, the rock can exhibit varying reactivity in relation to H2O2, depending on the area and properties of the metal surface. It is known that the coordination between the surface of mixed phases of Fe, Al, and Si oxides (for example, iron-containing aluminosilicate minerals) is more efficient in the decomposition of H2O2 than a surface of pure oxides. Thus, to predict the decomposition during injection of H2O2 in the bottomhole formation zone, it is necessary to assess the effect of given catalysts within the given reservoir.
In this work, two types of rocks were analyzed in the ARC experiments: non-extracted rock containing immobile oil and fully extracted rock. In a calorimetric cell, mixtures were prepared in two different ratios of 1 g of rock/1 g of H2O2 solution, as a model of the behavior of the system, and 5 g of rock/1 g of H2O2 solution, as a model of the behavior of the system close to the reservoir. The ratio 1:5 is considered in relation to the porosity of the rock equal to 20%. H2O2 solutions with a concentration of 20 wt.% and 30 wt.% were prepared in distilled water to eliminate the contribution of the metal cell wall. H2O2 with a concentration of 37 wt.% was not used in this experiment, so as not to create a stronger thermobaric effect in the cell. Figure 6 shows curves of temperature variation with time of decomposition of H2O2 of various concentrations under the influence of each of the rocks.
We show that when the ratio of H2O2 to the rock is 1:5, there is no rise in temperature due to the low diffusion rate during the propagation of heat through the dense membrane of the rock (Fig. 6, purple curve). This also proves that the rate of H2O2 decomposition in porous media depends not only on the surface area of solid catalytically active particles, but also on the pore size in the system. When calculating the parameters of H2O2 decomposition on the surface of solid mineral systems, it is desirable to additionally intro-duce an average pore size parameter, which embraces all the catalytic centers of the rock. When the ratio of H2O2 solution to rock is 1:1, an increase in heat release is observed. The ∆T of H2O2 diluted with distilled water in the case of a 30% solution of H2O2 was 55,0 °C on the oil saturated and 49,7 °C on the extracted rock, in the case of a 20% solution of H2O2, 34,2 °C and 27,4 °C, respectively (Table 4). A slightly greater exo-effect on non-extracted rock may indicate the influence of metal complexes in the composition of immobile oil.
Decomposition of H2O2 in the oil phase
When studying the effect of the oil phase on the decomposition of H2O2, an inert surface (type A) was used. For reliable comprehension of phase behavior during the decomposition of H2O2 in the oil phase, the oil-H2O2 solution (in a 1:1 wt. ratio) with a catalyst was used. For this purpose, pure oil and an H2O2 solution were placed in a glass cylinder connected to a gas burette. To accelerate the decomposition of H2O2, an iron oxide-based catalyst was initially added to the solution. The initial temperature of the experiment was 25 °C. As soon as we carried out the catalytic decomposition of H2O2, migration of released oxygen from the aqueous phase to the oil phase was observed, as a result of which oil foamed and caused a gradual increase in the volume of the oil phase (Fig. 7). In this process, there was no gas (oxygen) breakthrough from under the oil cap.
The dynamics of the increase in the volume of oil ΔV/V0 are shown in Fig. 8. The rate of increase in the volume of the oil phase with a decrease in concentration from H2O2 37 wt.% solution to 10 wt.% was expected to drop. Liquid oil in the presence of H2O2 does not undergo serious heat treatment and itself is not capable of increasing the exo-effect, as a result of which such a fluid cannot lead to a reduction in the time of H2O2 decomposition in the oil formation. The experiment carried out indicated that the continuous increase (displacement) of the oil phase caused by the liberated oxygen from the aqueous phase may be a favorable factor for oil displacement in a porous medium.
Pressure growth dynamics during the decomposition of H2O2
One of the important factors that should be considered when injecting H2O2 into a well is the pressure increase associated with an increase in the volume of oxygen during the decomposition of H2O2. If the process is very violent, it can provoke the destruction of underground equipment, so it is desirable that this process has strict control.
Thus, a study was undertaken to evaluate the effects of the catalytic environment, including the combination of metal surface (type C) and water salinity, which collectively affect the decomposition of hydrogen peroxide. A pressure growth analysis was carried out in a sealed system, which allows online recording of the pressure rise. In the experiments, H2O2 solutions were used, diluted with formation and distilled water to concentrations of 15, 20, 30 wt.%.
A study on a solid metal surface with the participation of formation water showed that the decomposition of hydrogen peroxide proceeds much more intensively than with a liquid that does not contain salt ions. For solutions of lower concentrations of 15–20 wt.%, the pressure rise slowed down and is comparable in time. The rate of pressure increases in the static mode for a H2O2 30 wt.% solution diluted with formation water reaches 1 MPa/h, for 15 and 20% H2O2 solutions—0.6 MPa/h (Fig. 9). The decomposition time of H2O2 solutions mixed with distilled water of 10–20 wt.% water in the cell is reduced by 60–100 times (Fig. 10). Thus, the experiment carried out showed that injection of H2O2 with a concentration of 15–30 wt.% is possible, however, an increased content of saline medium should be avoided to reduce the catalytic effect of the pumping and compressor equipment. The presence of a spacer fluid can also allow H2O2 injection to be controlled to the required parameters for safe injection, but coiled tubing technology is also recommended. The well must be equipped with blowout preventers to exclude water and gas manifestations during the thermochemical treatment.
Filtration test of H2O2 injection in a model with different states of oil
Studies carried out at ARC and in a closed metal cell have shown that the decomposition of H2O2 is capable of generating different amounts of heat and contributing to a rapid increase in pressure in systems, which depends on the catalytic capacity of the surface, the salinity of the liquid, the mineralogy of the rock and the concentration of hydrogen peroxide itself. Therefore, this fluid can be considered a high-potential energy and gas generating agent for injection into the formation zone. To assess the effect of the decomposition of H2O2 on a real rock in reservoir conditions, two filtration tests were carried out with the injection of H2O2 in a commercial form of 37 wt.% into a disintegrated rock containing immobile heavy oil fractions. To assess the displacement ability of hydrogen peroxide, a filtration test was carried out through the injection of oil-saturated sand in order to determine the cumulative oil recovery. Previously, in both experiments, a 5 wt.% sodium permanganate solution was injected for a sharp initiating effect of the reaction. It can be noted here that, with terrigenous rocks, the initiation of the hydrogen peroxide decomposition reaction occurs regardless of the presence of a catalyst, as noted in Sect. 3.1, but has a delay time.
Physical bottomhole zone treatment 1-D modeling
When simulating the effect of H2O2 on crushed rock with immobile oil, the model rock was saturated with a catalyst pressed into the core holder, with H2O2 subsequently injected. In this case, the rock from the terrigenous reservoir was taken in a milled state to assess the greater coverage of catalytic centers in the rock for the decomposition of H2O2 in order to observe the maximum exo-effect. After doing so, the temperature in the model rose from 25 to 240 °C (Fig. 11). The sharp increase in the initiation of H2O2 decomposition is influenced by a combination of factors: the participation of the introduced catalyst, the content of solid metallic material in the rock, and the presence of active metal centers in the composition of the residual oil. After pressing out from the core holder, a change in the oil saturation of the rock is visually observed (Fig. 12). From the first to the last zone of the model, a gradual increase in the immobile part of oil is observed. Removal of immobile oil from this rock in the initial zones leads to its intense discoloration. The organic matter content was determined to be 8.9% before H2O2 was injected into the model. After the experiment in each of the zones, the content of organic matter was: 0.78% (zone 1); 1.4% (zone 2); 3.1% (zone 3); 14.1% (zone 4).
Physical 1-D modeling mobile oil recovery
When simulating H2O2 treatment on a model with oil-saturated (mobile oil) sand, the temperature rise above 100 °C was observed on the model of the compressed oil-saturated sand (Fig. 13), after which the oil inflow was observed on the line of the fluid inflow. The most intense oil displacement occurred in the range from 0.5 up to 1.5 pore volume units (Fig. 14). The cumulative oil recovery factor was 61%. In the absence of a solid catalytic surface in the rock and active sites in the residual oil for the decomposition of H2O2, the exo-effect is accompanied only by the participation of the catalyst, which becomes sufficient for the advancement of the fluid flow. After processing the H2O2 model and stopping the temperature rise, formation water was pumped, but no more oil dis-placement from the model was observed.
Summary and conclusions
On the example of a terrigenous field (RF), the possibility of using the H2O2 injection technology as a method for treating the bottom-hole formation zone has been explored.
-
Based on the performed filtration tests of physical 1-D modeling on disintegrated rock with mobile and immobile oil, a sharp increase in temperatures of 100–240 ºC was demonstrated, caused by the decomposition of H2O2 with a powerful exo-effect, as a result of which either the rock was cleaned from immobile oil, or the displacement of the liquid phase of oil was observed with determination of the recovery factor.
-
It was shown that the key factor that contributes to the decomposition of H2O2 in the formation zone is the catalytic ability of the mineralogical composition of the terrigenous rock to activate the decomposition of H2O2.
-
It is also shown in this study that an important factor for assessing the risks when injecting H2O2 into a well is the catalytic feature of the metal surface and water salinity, which together affect the decomposition of hydrogen peroxide and lead to a pressure increase.
Based on the data obtained, in the future, attention should be focused on mathematical modeling to justify the conduct of thermochemical treatment on a real reservoir in order to take into account how to increase the productivity of the well, as well as to assess the risks associated primarily with the presence of gaseous oxygen and water vapor, and the resulting increase in pressure.
References
Alade OS, Mahmoud M, Hassan A, Al-Shehri D, Al-Nakhli A, Bataweel M (2019) Evaluation of kinetics and energetics of thermochemical fluids for enhanced recovery of heavy oil and liquid condensate. Energy Fuels 33(6):5538–5543. https://doi.org/10.1021/acs.energyfuels.9b00681
AlBahlani AM, Babadagli T (2011) Steam-over-solvent injection in fractured reservoirs (SOS-FR) technique as a new approach for heavy-oil and bitumen recovery: an overview of the method. Energy Fuels 25(10):4528–4539. https://doi.org/10.1021/ef200809z
AlMubarak T, AlKhaldi M, AlMubarak M, Rafie M, Al-Ibrahim H, AlBokhari N (2015) Investigation of acid-induced emulsion and asphaltene precipitation in low permeability carbonate reservoirs. SPE Saudi Arabia section annual technical symposium and exhibition. https://doi.org/10.2118/178034-MS
Bach RD, Ayala PY, Schlegel HB (1996) A reassessment of the bond dissociation energies of peroxides. An ab initio study. J Am Chem Soc 118(50):12758–12765. https://doi.org/10.1021/ja961838i
Bayless JH, Williams RE (1989) Recovery of viscous oil from geological reservoirs using hydrogen peroxide. Patent US № 4,867,238, Appl. № 195,764
Björkbacka A, Yang M, Gasparrini C, Leygraf C, Jonsson M (2015) Kinetics and mechanisms of reactions between H2O2 and copper and copper oxides. Dalton Trans 44:16045–16051. https://doi.org/10.1039/C5DT02024G
Croiset E, Rice SF, Hanush RG (1997) Hydrogen peroxide decomposition in supercritical water. AIChE J 43(9):2343–2352. https://doi.org/10.1002/aic.690430919
Dashti QM, Rezaul Kabir MM, Raj VU, Al-Ruhaimani FA, Liu H (2007) An integrated evaluation of successful acid fracturing treatment in a deep carbonate reservoir having high asphaltene content in Burgan Field, Kuwait. International petroleum technology conference. https://doi.org/10.2523/IPTC-11347-MS
Duh YS, Hsu CC, Kao CS, Yu SW (1996) Applications of reaction calorimetry in reaction kinetics and thermal hazard evaluation. Thermochim Acta 285:67–79. https://doi.org/10.1016/0040-6031(96)02899-7
Fink FW (1963) Saline-water development poses problems in metal corrosion. Groundwater 1(4):21–25. https://doi.org/10.1111/j.1745-6584.1963.tb01929.x
Hart PW, Rudie AW (2011) Modeling an explosion: the devil is in the details. Educ Chem Eng 45(1):15–20
Hassan AM, Mahmoud MA, Al-Majed AA, Al-Nakhli AR, Bataweel MA (2019) Water blockage removal and productivity index enhancement by injecting thermochemical fluids in tight sandstone formations. J Pet Sci Eng 182:106298. https://doi.org/10.1016/j.petrol.2019.106298
Hassan AM, Mahmoud MA, Al-Majed AA, Elkatatny S, Al-Nakhli AR, Bataweel MA (2018) A novel technique to eliminate gas condensation in gas condensate reservoirs using thermochemical fluids. Energy Fuels 32(12):12843–12850. https://doi.org/10.1021/acs.energyfuels.8b03604
Hiroki A, LaVerne JA (2005) Decomposition of hydrogen peroxide at water ceramic oxide interfaces. J Phys Chem B 109:3364–3370. https://doi.org/10.1021/jp046405d
Khlebnikov VN, Vinokurov VA, Zobov PM, Gushchina YF, Mishin AS, Antonov SV, Bardin ME (2013) Viscous oil production method. Patent RF № 2534870C2
Kuznetsov NM, Aleksandrov EN (2017) Safety in applying binary mixtures for oil production stimulation. Georesursy 19(4):379–382
Lin CC, Smith FR, Ichikawa N, Baba T, Itow M (1991) Decomposition of hydrogen peroxide in aqueous solutions at elevated temperatures. Int J Chem Kin 23:971–987. https://doi.org/10.1002/kin.550231103
Liu Z, Shen Q, Zhou C, Fang L, Yang M, Xia T (2018) Kinetic and mechanistic study on catalytic decomposition of hydrogen peroxide on carbon-nanodots/graphitic carbon nitride composite. Catalysts 8(10):445. https://doi.org/10.3390/catal8100445
Lousada CM, Johansson AJ, Brinck T, Jonsson M (2012) Decomposition of hydrogen peroxide upon exposure to transition metal particles. J Phys Chem C 116(17):9533–9543. https://doi.org/10.1021/jp300255h
Minkhanov IF, Bolotov AV, Al-Muntaser AA, Mukhamatdinov II, Vakhin AV, Varfolomeev MA, Slavkina OV, Shchekoldin KA, Darishchev VI (2021) Experimental study on the improving the efficiency of oil displacement by co-using of the steam-solvent catalyst. Oil Ind 2021(6):54–57. https://doi.org/10.24887/0028-2448-2021-6-54-57
Minkhanov IF, Marvanov MM, Bolotov AV (2020) Improvement of heavy oil displacement efficiency by using aromatic hydrocarbon solvent. International multidisciplinary scientific geoconference surveying geology and mining ecology management, SGEM 2020:711-718
Nikitin ED, Pavlov PA, Popov AP (1992) Spontaneous boiling and critical parameters of aqueous solutions of hydrogen peroxide. TPS 30(3):508–512
Pędziwiatr P, Mikołajczyk F, Zawadzki D, Mikołajczyk K, Bedka A (2018) Decomposition of hydrogen peroxide—kinetics and review of chosen catalysts. F Acta Innovations 26:45–52
Pham ALT, Doyle FM, Sedlak DL (2012) Kinetics and efficiency of H2O2 activation by iron-containing minerals and aquifer materials. Water Res 46:6454–6462. https://doi.org/10.1016/j.watres.2012.09.020
Plaucka A, Stanglandb EE, Dumesic JA, Mavrikakisa M (2016) Active sites and mechanisms for H2O2 decomposition over Pd catalysts. PNAS 113(14):E1973–E1982. https://doi.org/10.1073/pnas.1602172113
Shafiai SH, Gohari A (2020) Conventional and electrical EOR review: the development trend of ultrasonic application in EOR. J Petroleum Explor Prod Technol 10:2923–2945. https://doi.org/10.1007/s13202-020-00929-x
Shah A, Fishwick R, Wood J, Leeke G, Rigby S, Greaves M (2010) A review of novel techniques for heavy oil and bitumen extraction and upgrading. Energy Environ Sci 3:700–714. https://doi.org/10.1039/B918960B
Takagi J, Ishigure K (1985) Thermal decomposition of hydrogen peroxide and its effect on reactor water monitoring of boiling water reactors. Nucl Sci Eng 89:177–186. https://doi.org/10.13182/NSE85-A18191
Teel AL, Finn DD, Schmidt JT, Cutler LM, Watts RJ (2007) Rates of trace mineral-catalyzed decomposition of hydrogen peroxide. J Environ Eng 133:853–858. https://doi.org/10.1061/(ASCE)0733-9372(2007)133:8(853)
Varfolomeev MA, Yuan C, Bolotov AV, Minkhanov IF, Mehrabi-Kalajahi S, Saifullin ER, Marvanov MM, Baygildin ER, Sabiryanov RM, Rojas A, Emelianov DA, Al-Muntaser AA, Ganiev BG, Zaripov AT, Beregovoi AN, Shaihutdinov DK (2021) Effect of copper stearate as catalysts on the performance of in-situ combustion process for heavy oil recovery and upgrading. J Pet Sci Eng 207:109125. https://doi.org/10.1016/j.petrol.2021.109125
West ED (1963) Heat exchange in adiabatic calorimeters. J Res Natl Bur Stand A Phys Chem 67A(4):331–341. https://doi.org/10.6028/jres.067A.035
Xing M, Xu W, Dong C, Bai Y, Zeng J, Zhou Y, Zhang J, Yin Y (2018) Metal sulfides as excellent co-catalysts for H2O2 decomposition in advanced oxidation processes. Chem 4(6):1359–1372. https://doi.org/10.1016/j.chempr.2018.03.002
Yang M, Zhang X, Grosjean A, Soroka I, Jonsson M (2015) Kinetics and mechanism of the reaction between h2O2 and tungsten powder in water. J Phys Chem C 119:22560–22569. https://doi.org/10.1021/acs.jpcc.5b07012
Yuan C, Varfolomeev MA, Khachatrian AA (2020) Interaction between aromatics and n-alkane for in-situ combustion process. J Pet Sci Eng 187:106770. https://doi.org/10.1016/j.petrol.2019.106770
Zhao S, Pu W, Varfolomeev MA, Yuan C, Qin S, Wang L, Emelianov DA, Khachatrian AA (2019) Thermal behavior and kinetics of heavy crude oil during combustion by high pressure differential scanning calorimetry and accelerating rate calorimetry. J Pet Sci Eng 181:106225. https://doi.org/10.1016/j.petrol.2019.106225
Funding
This research was supported by the Ministry of Science and Higher Education of the Russian Federation under agreement № 075-15-2022-299 within the framework of the development program for a world-class Research Center “Efficient development of the global liquid hydrocarbon reserves”. We express our gratitude for the work done by A.A. Khachatryan, an employee of the Department of Physical Chemistry of Kazan Federal University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the co-authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anikin, O.V., Bolotov, A.V., Minkhanov, I.F. et al. Factors influencing hydrogen peroxide decomposition dynamics for thermochemical treatment of bottomhole zone. J Petrol Explor Prod Technol 12, 2587–2598 (2022). https://doi.org/10.1007/s13202-022-01507-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-022-01507-z