Abstract
Produced water is the main residue from the petroleum extraction industry. Other critical factor in this sector is carbon dioxide emissions. This work presents a solution proposal for both problems throughout the development of an apparatus which allows the synthesis of salts dissolved in produced water with CO2 capture. The experimental unit developed in this work was based on the Solvay process, to convert sodium chloride (NaCl) into sodium bicarbonate (NaHCO3) from synthetic produced water and carbon dioxide (CO2). No previous work used the combination of produced water and CO2 aiming at the synthesis of new products. Four steps were made with different experimental setups. The best outcome for the reaction of bicarbonate attained a conversion of 44.5% of sodium chloride into sodium bicarbonate and capture of 250,000 tons of carbon dioxide per year. A preliminary financial analysis indicates an annual revenue of US$ 126,607,292.31 in sodium bicarbonate and ammonium chloride and US$ 2,862,897.23 in carbon credits per year. The studied methodology can be used as a starting point for new experimental works that have the purpose to obtain salts from produced water and can help for better understanding its potential as carbon capture agent and a source of valuable products, contributing to the reduction of the environmental impact and adding value to the production chain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oil is a fundamental energy source for many countries since it serves as input for countless industrial sectors (automotive, textile, agricultural, chemical, among others) (Aneel 2008). Due to its various derivatives, such as liquefied petroleum gas (LPG), gasoline, diesel, kerosene, naphtha, fuel and lubricant oils, marine fuel, and coke, including uses in the petrochemical industry (Almeida 2006; Fraser and Ellis 2009), it becomes difficult to replace, in the short term, in the energy matrix of any country (Almada and Parente 2013).
The importance that petroleum represents for the international economy and politics is unquestionable. However, the oil and gas sector is one of the activities that represent major impact on the environment (Magrini and Lins 2007; O’Rourke and Connolly 2003). In all stages of the petroleum production chain there are aspects that can lead to environmental pollution caused by liquid and solid wastes, or gaseous emissions (IFC 2007a).
The volume of produced water generated in the petroleum production activity, whether onshore or offshore, is one of the largest among all the streams generated by the oil and gas industry (IFC 2007b). In 2019, an estimated 21 billion barrels tons of produced water were discharged offshore. Average disposal costs range from US$ 4.00 to US$ 8.00 per barrel and can reach US$ 20.00 per barrel with transportation. However, produced water treatment and generation of co-products can also be an opportunity to add market value to a stream that has no economic value.
Produced water is a complex mixture of naturally occurring organic and inorganic chemical compounds that have been dissolved or dispersed in the form of particles from geological formations and migration routes, where this effluent has been dammed for thousands of years (Neff, J.; Lee, K.; DeBlois, 2011). Its physical and chemical properties can vary widely, according to the geological age, depth, geochemical characteristics and location of the rock formation, as well as the chemical composition of the oil and gas phases in the reservoir, and processes performed during production (Hosseini et al. 2012).
The oil/gas/water mixture may be processed through separation devices to separate the three phases from each other at the offshore platforms. But almost always some little amount of water is charged by the oil stream and reaches a tank in a crude oil terminal. Usually, the crude oil tanks at the terminal receive oil from different offshore platforms. After a residence time, water segregates from oil and needs to be treated before discharge. A sample of this mixed produced water was collected to be analyzed at the Research and Development Center (Cenpes) of Petrobras. The sample analysis of the mixed produced is similar to those presented by Filho (2020).
Analyzing these results and those presented by other works, it is possible to conclude that the composition of the produced water generated in Brazil is similar to that generated worldwide (Elkins, Vanner, Firebrace, 2005; Fakhru’l-Razi et al. 2009; Igunnu and Chen 2014; Isehunwa and Onovae 2011; Neff 2002). It was considered that these results are representative to portray the general chemical constitution of this effluent. It is important to highlight the amount of sodium chloride observed in the produced water.
One of the available choices for the produced water segregated on oil terminal tanks is treating the water before discharge, to comply with environmental laws. The treatment is usually expensive and is not so easy. So, we have an effluent which has to be treated at lower costs and with high efficiency. On the other hand, we have another choice: to find a way to value the produced water as a raw material to a new process. Then, after some research, we chose sodium bicarbonate as one of the materials with more profit chances.
There are two main possibilities to produce carbonates and bicarbonates. The first one is the use of raw materials such as trona, which is an evaporite mineral, composed of hydrated sodium bicarbonate and carbonate (Bonaventura et al. 2017). In this case, the stream is first converted to sodium carbonate in a fluidized bed reactor operating at 180 °C–200 ºC and 1 bar. The heat required in the reactor can be supplied by renewable sources, such as solar energy or biomass. A fraction of the sodium is recirculated to capture carbon dioxide using the dry carbonate process. The remainder is converted into sodium bicarbonate in a carbonating tower by reaction with carbon dioxide and water. After the separation of sodium bicarbonate and other salts from water, the sodium bicarbonate produced is suitable for sale.
A widely used process with similar raw material is the Solvay process, also known as the ammonia-soda process, which was developed in 1881 by the Belgian chemist Ernest Solvay. The raw materials used in this process are salt and limestone, as well as coke or gas. Ammonia is a cyclic reagent, as it participates in reactions and can be recovered, with a small amount lost. Metallurgical coke is used to calcinate the limestone and, at the same time, provide the CO2 supplement. Sodium chloride participates in the reactions as saturated brine, usually from the rock salt.
Sodium bicarbonate is a chemical material fully requested by the industrial sector, being widely used in different kinds of processes. It is usually produced through the Solvay process and is a very important commodity for countries. However, the Brazilian production of sodium carbonate (and, consequently, sodium bicarbonate) is not enough to meet its demand (ABIQUIM 2019). This represents an opportunity for new production technologies and the salt recovery from produced water appears as a very attractive alternative, due to its potential availability and environmental appeal. It is in line with recent literature solutions.
For example, El-Naas et al. (2017) developed a study whose objective was to capture CO2 and reduce the salinity of a saline synthetic liquid sample. The authors indicated an uptake yield of around 99% of CO2 and the removal of 35% of the sodium present. The process was carried out without the presence of ammonia and the conclusions refer to the need for a pH greater than 10 and a temperature of 20 ºC.
Lee et al. (2021) propose the use of synthetic liquids for CO2 sequestration. The authors argue that conventional adsorbents are limited and unsafe for indoor environments. However, the authors do not comment on the costs of manufacturing these synthetic liquids.
And Inasaka et al. (2021) used an alkaline construction residue as a matrix for CO2 capture. According to the authors, the scavenging source must be alkaline because carbon dioxide lowers the pH of the medium, forming carbonate. Thus, a neutral residue becomes easier to treat, discard or reuse. In this case, the authors had as objective the formation of calcium carbonate for later separation.
No previous work, however, has evaluated the potential of produced water—a waste that demands significative treatment costs—as a carbon dioxide capture matrix and had sodium bicarbonate synthesis—with appropriate measurement—as objective.
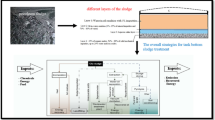
Therefore, based on the initial discussion, the objective of this work is the development of a methodology for obtaining sodium bicarbonate from a synthetic produced water. For this, an experimental apparatus was built, and the influence of some process parameters was analyzed. Figure 1 presents a general outline of the problem under study.
Experimental
From the literature analysis presented in the introduction section, we defined a synthetic solution of produced water to be used on the tests, with a chemical composition emulating a real produced water from Petrobras. A glass apparatus for simulating the Solvay process was built in accordance with Araújo et al. (1998). However, due to the lack of information from similar processes, experimental planning should seek to gather as much information as possible. Thus, for a preliminary assessment, the behavior of the gases and the apparatus that have been used were evaluated. Based on the bibliographic review and the samples’ compositions, the preliminary assessment was made only with CO2. This gas has less solubility in comparison with NH3 and does not offer any risks. The CO2 that was used in the essays was supplied by White Martins.
The process using CO2 aims to take advantage of the NH4OH available in the samples and to evaluate the formation of NaHCO3 through the reaction with NaCl, according to Eq. 1.
Then the parameters to be evaluated were defined. In the Solvay process, the brine contacts the gases in countercurrent flow in columns with several indentations (Araújo et al. 1998). In gas–liquid absorption processes, the most important column types used are: spray, bubble, packed and wet-wall (Revello 1998). The use of one or another type of column depends largely on the solubility of the components of interest—the gas phase in the liquid phase or vice versa—and the mass transfer resistance in the phases.
The chemical reaction increases the absorption rate and the mass transfer efficiency, due to the increase in solubility resulting from the reaction demand for the reagents. In addition, the manipulation of process parameters (temperature, pressure and flowrates) directly influences the reaction rates (Leite 2005). Therefore, in the present study, random packing was used to improve mass exchange without essentially modifying the design proposed by Araújo et al. (1998).
Based on what was discussed, the initial outline of the project has aimed to assess the formation of HCO3− from the produced water. For that, it was defined a set of parameters that influenced the anion’s formation.
Temperature is an important parameter, as it directly influences CO2 solubilization and reaction. CO2 has low solubility in water. Based on the review presented, the values of 10 and 30 °C were defined for this initial assessment.
To evaluate the influence of mass exchange on the process and the gas–liquid contact, two types of random packings were planned: Berl saddles and Raschig rings. The size (height) of the column was also evaluated. For this purpose, glass columns with sizes of 20 and 40 cm were tested. The experiments took place in a batch system that allows 250 ml of gas to be inserted each time. For the purpose of investigating the influence of reaction time, the times of 15 and 60 min were chosen to be evaluated.
To aid the solubilization and bicarbonate formation reaction, a molar excess of CO2 was used. Another parameter analyzed was pH, as it directly influences the chemical stability of bicarbonate. The planning considered two pH levels: the original pH of the sample (7.2) and a higher value (8.0) with the injection of NaOH.
The evaluation of the parameters was done with different bicarbonate concentrations. Based on this, a factorial experimental planning 23 was developed considering temperature (°C), flowrate (mL/min) and pH as parameters. A spreadsheet based on this planning was developed for each packing (Berl saddles and Raschig rings) and column height (20 and 40 cm). The CO2 gas pressure chosen was 2 bar, a value above ambient pressure to guarantee an atmosphere rich in the reference gas.
All measurements were taken in duplicate. In tests where duplicates showed deviations greater than 10%, a triplicate was performed, and the most distant result was neglected. The combined uncertainty was the propagated equipment deviations (σR). As the measurements were directly performed, the uncertainty, calculated through Eq. 2, was defined as the quadratic sum of the equipment deviation (Vuolo 1996).
where DE is the equipment deviation, x is the value of the measured property and N is the number of repetitions. The deviation calculated for each synthesis was related to the quadratic propagation of the error of each duplicate considered. Thus, the total number of tests reaches 72.
To better evaluate the outcomes, a synthetic produced water solution was used instead of the original produced water samples, because the complex compositions of the original samples could dissimulate the effects of the parameters. It makes all sense to evaluate that the performance of any experimental apparatus would be better when synthetic produced water is used instead of real samples, but it is not only difficult to simulate a general produced water, it is also important in this case.
Results and discussion
Assessment tests
The initial tests were carried out with the objective of evaluating the unit for the formation of bicarbonate. The experimental setup had the same design proposed by Araújo et al. (1998).
The assessment tests were developed to evaluate the influences of temperature (A), gas flow (B) and pH (C), besides the kind of packing and column height. The data were then submitted to a statistical evaluation in the Design-Expert 12 program, using the ANOVA method to measure the impact of each parameter on the process.
The results (Table 1) show that temperature presents the greatest influence between the three parameters, representing a maximum gain of 74.98% with its reduction. This is because higher temperatures imply lower CO2 solubility in the medium. Moreover, pH has exhibited the biggest positive gain with its increase, 19.86% in the best-case scenario, as HCO3− is stable in alkaline media, showing its importance in the process.
The best values for packing and height are Berl saddles and 40 cm (Table 2). However, the analysis showed that the efficiency of the procedure was not high enough to precipitate NaHCO3.
Apparatus improvement
Another essay were made by modifying the initial experimental setup proposed by Araujo et al. (1998) to improve the solubilization of the gas. Thus, the gas injection was modified by inserting a hose with 1-mm holes and a porous stone at the end, where the gas was bubbled into the system. From the assessment tests, the conditions of 10 °C were repeated with Berl saddles, 40 cm columns and pressure of 2 bar. In addition, a peristaltic pump was adapted to the system, which allowed the sample to be recirculated, aiming to increase the gas/liquid contact in the system.
In order to understand the influence of the increase in pH, solutions with pH 10 and 11 were prepared, adding NaOH to the samples. The tests were also carried out with and without recycle, to assess its influence on the process. The best final average concentration, 1300 mg/L of bicarbonate ion (Table 3), revealed an improvement compared to the previous runnings, representing a gain of 113.72% using recycle, with a temperature of 10 ºC and 60 min of testing.
The results showed that the apparatus is technically feasible. In addition, it was possible to conclude that Berl saddles provide better results in comparison with Raschig rings. For the evaluated system, the saddles allowed a better solubilization of the gas in the liquid phase. However, NaHCO3 precipitation still did not occur.
Process improvement with sample doping
A third attempt was made by changing the characteristics of the synthetic produced water, since the improvement tests did not point out the precipitation of NaHCO3. The following issues were considered:
-
(a)
Low Na+ in solution—The produced water samples contain sodium in the range of 17 g/L. The brine used in the Solvay process contains 300 g/L and does not contain other constituents. A possible solution is to dope the produced water with NaOH to increase the sodium concentration in the medium and consequently the pH, which increases the formation of NaHCO3. NaOH was added until pH 10 and 11.
-
(b)
Need for NH4+—In the Solvay process, NH3 is used to form NH4HCO3, which, in turn, reacts with NaCl to form NaHCO3. The produced water contains NH3 in its composition; however, this amount was not enough to precipitate the salt of interest, so its increase and influence in the medium must be evaluated. A possible solution is to dope the produced water with NH4OH, which will increase the concentration of NH3 in the medium and increase pH, without the need for using it in the gas form (which is dangerous). NH4OH was added up to 0.10 and 0.45 g/L.
-
(c)
Low formation of NaHCO3—The amount of HCO3− in the medium is below the required for precipitation of ammonium bicarbonate and its subsequent conversion to sodium bicarbonate in the presence of a sufficient NaCl concentration. It could be solved with the insertion of NaCl, as both tend to reduce the solubility of bicarbonate in aqueous media (Ellingboe and Runnels 1965; Carneiro 2013). The amount of NaCl added was 15 g/L, the same value observed in the produced water sample, and the double (30 g/L), which allowed to measure the real effect of the initial amount of sodium in the process.
New synthetics samples were made, mainly by adding NH4OH and NaOH (Table 4 and Fig. 2). The results obtained were better than those for second run. For example, pH equal to 10.9 and 0.10 mg/L of NH4OH resulted in the best outcome, at 3271.4 mg/L of NaHCO3. A statistical analysis (Table 5) showed that NaCl mass concentration is more dominant than NH4OH and NaOH concentrations, which can be explained by its influence on the amount of initial reagent (Na+) and its importance in reducing the solubility of NaHCO3 in the solution.
Finally, another step was made to evaluate the following parameters: NaCl concentration, time and temperature. The conditions of the tests (Table 6 and Fig. 3) showed that, among eight runs, the best result was 3337.4 mg/L of NaHCO3, with 30 g/L of NaCl, 60 min of reaction and 10 °C.
The statistical analysis (Table 7) revealed that the highest temperature (30 °C) has not led to improvements in the solubilization of CO2 and NH4+. That is, the temperature of 10 °C seems ideal. The longer time (60 min) showed better results, while more NaCl was also better, but the gain was low. In this step, the highest NaHCO3 concentration obtained was 3337.4 mg/L, which is still below the solubility limit at these conditions. However, this value represents a conversion of 44.5% NaCl to NaHCO3, what can be considered a very good result, as can be observed in Fig. 4.
The ammonium hydroxide formed in Eq. 1 will generate ammonium carbonate and bicarbonate according to Eqs. 3 and 4.
Ammonium bicarbonate forms, with sodium chloride, ammonium chloride and sodium bicarbonate, according to Eq. 5:
The equations show the adsorption of CO2 in two moments of the process. Considering the experimental efficiency obtained (44.5%), the mass balance and a factory operating according to the data and prices in Table 8, a preliminary financial analysis indicates an annual revenue of US$ 126,607,292.31 in sodium bicarbonate and ammonium chloride, and US$ 2,862,897.23 in carbon credits per year. These results are for a scenario that process a produced water with 30 g of NaCl per liter, at a flowrate of 50,000 L/min. The revenue in products and in carbon credits is shown in Fig. 5 for different flowrates and NaCl concentrations.
Summary and conclusions
In the present study, we propose a methodology for CO2 scavenging using petroleum produced water, synthesizing sodium bicarbonate. After the assessment tests, it was possible to conclude that the following conditions are the best for the proposed methodology: low temperature and high pH. It is important to keep the water at low temperature to avoid the reduction of bicarbonate. This occurs because at higher temperatures the CO2 solubility is reduced, but the gas is in equilibrium with the bicarbonate. This conclusion was obtained throughout the tests at 30 °C, which showed a lower concentration of bicarbonate.
The result was the technical feasibility of the apparatus to recover sodium bicarbonate from synthetic produced water. Unfortunately, the precipitation of NaHCO3 was not possible in the tested conditions and it requires more essays.
The main contributions of this work are:
-
The development of an experimental unit for bicarbonate recovering from produced water;
-
The presentation of new data and knowledge about the evaluated process;
-
The best conditions obtained with the development of the unit were: 10 ºC, 2 bar, recycling, 60 min of residence time, NH4OH doping, pH 11.
Although some progress has been made using our different experimental setups, these approaches provide only a partial answer to the problem. The disadvantage of the proposed methodology is the use of ammonium hydroxide, which requires stricter security protocols. A limitation of the present work was the fact that it was not possible to carry out tests at higher pressures, since the apparatus was developed in glass. Besides that, further studies are needed to determine new reaction parameters to precipitate bicarbonate salts, in substitution or complementary to those used so far.
References
ABIQUIM (2019) Brazilian chemistry association, Yearbook, p 120
Almada LP, Parente V (2013) Oil & Gas industry in Brazil: a brief history and legal framework. Panor Brazilian Law 1(1):223–252
Almeida J (2006) Introdução a industria do petroleo. FURG–CTI. Rio Grande. PETROBRAS– Petróleo Brasileiro S.A, p 76
Aneel (2008) Agência nacional de Energia Elétrica, Atlas de Energia Elétrica do Brasil, Brasília, 3a edição. p 263
Bonaventura D, Chacartegui R, Valverde JM, Becerra JA, Verda V (2017) Carbon capture and utilization for sodium bicarbonate production assisted by solar thermal power. Energy Convers Manag 149:860–874. https://doi.org/10.1016/j.enconman.2017.03.042
Carneiro A R (2013) Study physical‐chemical gives precipitation in bicarbonate in sodium in brine (NaCl (aq)). Dissertation, masters degree, UFRJ, Rio de Janeiro
de Araújo AL, Neves CA, Ferreira AMC, Koiti A (1998) Simulação do processo solvay no laboratório didático. Quim Nova 21(1):114–116. https://doi.org/10.1590/s0100-40421998000100018
Elkins P, Vanner R, Firebrace J (2005) Management of produced water on offshore oil installations: a comparative assessment using flow analysis. Working Paper 1–89
John L Ellingboe, John H Runnels (1965) Purification of trimesic acid USA patent US3504023A. avaliable in: US3504023A-Purification of trimesic acid-Google Patents
El-Naas MH, Mohammad AF, Suleiman MI, Al Musharfy M, Al-Marzouqi AH (2017) A new process for the capture of CO2 and reduction of water salinity. Desalination 411:69–75. https://doi.org/10.1016/j.desal.2017.02.005
Fakhru’l-Razi A, Pendashteh A, Abdullah LC, Biak DRA, Madaeni SS, Abidin ZZ (2009) Review of technologies for oil and gas produced water treatment. J Hazard Mater 170(2–3):530–551
de Filho LEAB (2020) Caracterização da água produzida associada ao petróleo na indústria petrolífera Do Rio Grande Do Norte. Dissertation, Course completation, UFERSA, RN.
Fraser GS, Ellis J (2009) The Canada-Newfoundland Atlantic accord implementation act: transparency of the environmental management of the offshore oil and gas industry. Mar Policy 33:312–316
Hosseini A, Brown JE, Gwynn JP, Dowdall M (2012) Review of research on impacts to biota of discharges of naturally occurring radionuclides in produced water to the marine environment. Sci Total Environ 438(September):325–333. https://doi.org/10.1016/j.scitotenv.2012.08.047
IFC (2007a) Environmental, Health, and Safety Guidelines. Offshore oil and gas development. International Finance Corporation, p 39
IFC (2007b) Environmental, Health, and Safety Guidelines. Offshore oil and gas development. International Finance Corporation, p 52
Igunnu ET, Chen GZ (2014) Produced water treatment technologies. Int J Low-Carbon Technol 9(3):157–177. https://doi.org/10.1093/ijlct/cts049
Inasaka K, Trung ND, Kimitoshi Hayano HY (2021) Evaluation of CO2 captured in alkaline construction sludge associated with pH neutralization. Soils Found 61(6):1699–1707
Isehunwa SO, Onovae S (2011) Evaluation of produced water discharge in the Niger-Delta. J Eng Appl Sci 6(8):66–72
Lee JW, Kim M, Jung HS, Xu R, Kim S, Kang YT (2021) Liquid-like adsorbent assembled by CNTs: serving as renewable CO2 capture materials for indoor air. J Energy Chem. https://doi.org/10.1016/j.jechem.2021.08.027
Leite ABLLBABC (2005) Absorção química de dióxido de nitrogênio (NO2). Engenharia Sanitaria e Ambiental 10(1):1–12
Magrini A, Lins LS (2007) Integration between environmental management and strategic planning in the oil and gas sector. Energy Policy 35:4869–4878
Neff JM (2002) Effect of contaminants from oil well produced water, 1a edn. Elsevier, Amsterdam
Neff J, Lee K, DeBlois E (2011) Produced water: overview of composition, fates and effects. In: Science S (ed) Produced water environmental risks and advances in mitigation technologies. Springer, London, p 608
O’Rourke D, Connolly S (2003) The distribution of environmental and social impacts of oil production and consumption. Annu Rev Environ Resour 28:567–617
Revello J H P (1998) Mass transfer in absorption columns: a numerical and experimental approach. 141 Dissertation, masters degree. Florianopolis, SC
Vuolo JH (1996) Fundamentos da Teoria de Erros (Edgar Blucher LTDA, 2ª. São Paulo, SP
Acknowledgements
The authors thank financial support from Cenpes/Petrobras.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have participated in (a) conception and design, or analysis and interpretation of the data; (b) drafting the article or revising it critically for important intellectual content; and (c) approval of the final version.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Villardi, H.G.D., Yokoyama, L., Young, A. et al. Experimental methodology for CO2 capture and sodium bicarbonate synthesis with producedwater from oil industry. J Petrol Explor Prod Technol 12, 2577–2585 (2022). https://doi.org/10.1007/s13202-022-01479-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-022-01479-0