Abstract
As a surfactant solution system, microemulsion has attracted much attention due to its ultra-low interfacial tension, high solubilization and thermodynamic stability in the process of enhanced oil recovery. Different from water phase system of polymer flooding and ASP flooding, the microemulsion system shows a special phase state, and its existence system may be water phase, oil phase or microemulsion phase. The microemulsion phase can be divided into upper phase, middle phase and lower phase microemulsion according to the composition of the system. Different phase microemulsions have different oil displacement efficiency, and the middle phase microemulsion reaches ultra-low interfacial tension with oil/water, and the oil displacement efficiency is the highest. In order to ensure the middle-phase microemulsion flooding as far as possible during the oil displacement process, it is necessary to study the phase change process of microemulsion and the formation conditions of microemulsion in detail, and clarify the influence of surfactant concentration, additive concentration, salt content, water–oil ratio and temperature on the microemulsion phase transformation and the formation mechanism of microemulsion. The research results have some guiding significance for the formulation selection and slug design of microemulsion flooding system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microemulsion flooding is an oil displacement method which uses microemulsion (composed of water, oil, surfactant, additives and salts) as the driving medium. It is a concentrated surfactant flooding (Yang 2007). It has two basic types and one transition type, namely lower-phase microemulsion, upper-phase microemulsion and middle-phase microemulsion. The lower-phase microemulsion can be obtained by hydrophilic surfactants, and the upper-phase microemulsion can be obtained by lipophilic surfactants. When conditions are changed, the lower-phase microemulsion and the upper-phase microemulsion could be changed mutually, and all pass through the transition stage of middle-phase microemulsion. The middle-phase microemulsion has no interfaces with oil phase and water phase. Therefore, there’s no interface tension and capillary resistance, making the sweep coefficient often higher than that of ordinary water. Moreover, microemulsion has good displacement efficiency since it cannot be mixed with oil. The surfactant dosage needed in this method is relatively high. Compared with micelle flooding, the microemulsion flooding has the higher surfactant concentration and all molecules in the system exist as micelles. This not only manifested by all the mechanism of micelle flooding throughout the oil displacement, but also incurs the lower interface tension and stronger solubilizing power. In the process of microemulsion flooding, the system phase is instability due to the adsorption of rocks. There may occur many kinds of phase states, such as upper phase, middle phase and lower phase microemulsion. At present, some scholars have carried out microemulsion phase transformation in various fields (Akram et al. 2021; Ghasemi et al. 2021; Hamed et al. 2020; Ghasemi et al. 2020; Mozaffari et al. 2017; Nivedita et al. 2021). Michihiro Nagao 2001; Seto et al. 2000) have studied the effects of temperature and pressure on phase transitions in a microemulsion system. The results indicate that the mechanism of the phase transition induced by temperature is different from that by pressure. Han Seung Lee (2014) studied the formation process of oil-in-water nanoemulsions by means of cryogenic electron microscope. It was found that water continuous microemulsions produce initial nanoemulsions structures that are small and simple, most unilamellar vesicles, but microemulsion that are not water-continuous produce initial nanoemulsion structures that are larger and multilamellar. Wang Xin (2021) have taken the droplet as the macro research object, and the creep models of five dynamic states are given. Zhu Tongyu (Zhu et al. 2021; Wang et al. 2019; Wang et al. 2021) summarized microemulsion in EOR, and evaluated the characteristics of microemulsion system and phase transformation as a whole. Most of the above studies are from macroscopic perspective to study the effect of temperature and pressure on microemulsion phase change, and lack of micro angle microemulsion phase change mechanism. Therefore, based on the theory of microemulsion structural phase transformation, the factors affecting the microemulsion phase change and the mechanism of microcosmic formation are studied. It helps to ensure the displacement of middle-phase microemulsion in the process of oil displacement and improve the displacement efficiency of microemulsion. This study prepared a microemulsion system (Wang 2019) with 2.5% ionic surfactant, 2.0% n-butyl alcohol, 2.0% salt and water–oil mixture (1:1) to study the effects of surfactant concentration, additive concentration, salt content, water–oil ratio and temperature on the phase change of microemulsion. The research results have some guiding significance for the formulation selection and slug design of microemulsion flooding system.
Phase transition theory of microemulsion system
Microemulsion is generally formed spontaneously by surfactant, additives, oil, water or saline water under appropriate mixing ratio. It can be divided into three types according to structure, namely water in oil type (W/O) oil in water type (O/W) and bi-successive type (B.C) (Fig. 1). According to the quasi-equal model of microemulsion, ① the W/O-type microemulsion is composed of oil continuous phase, water nucleus and interface membrane (Fig. 1a). There are few additives in the water nucleus and some additives and water in the oil continuous phase. The interface membrane is composed of surfactant and additives. Moreover, surfactant in the microemulsion system only exists on the interface membrane. Polar groups of surfactant and additives on the interface membrane orient to the water nucleus, with fraction ratio of 1:2. ② The O/W microemulsion is composed water continuous phase, oil nucleus and interface membrane (Fig. 1b). Polar groups of surfactant and additives on interface membrane orient to the oil nucleus. ③ In the B.C-type microemulsion, oil and water both are continuous phase and it has comprehensive characteristics of W/O type and O/W type. However, water droplets and oil droplets are not spherical, but become networks formed by water pipes in oil media (Fig. 1c).
Microscopically, balance of micelles and association phase changes in the microemulsion system are very complicated. In the process of phase change, there exist intermediate phases, which are determined by structure of surfactant, temperature and existence of other additives. Currently, theories of microemulsion structures mainly include dual-membrane theory, geometric arrangement theory and R-ratio theory.
In 1955, Schulman and Bowcott (Wang 2008) proposed the concept that adsorption monolayer of surfactant and additive is the third phase or intermediate phase, thus developing the dual-membrane theory. As the third phase, the mixed membrane has two surfaces that contact with water phase and oil phase, respectively. The relative strengths (interface tension) of interaction between these two surfaces and water and oil determine the bending and direction of interfaces, thus determining whether the microemulsion system is the W/O type or O/W type. When the water-side interface pressure is larger than the oil-side interface pressure, the interface membrane expansion bends toward the oil phase, thus forming the O/W microemulsion. If the water-side interface pressure is smaller than the oil-side interface pressure, the interface membrane expansion bends toward the water phase, forming the W/O microemulsion. If the water-side interface pressure is equal to the oil-side interface pressure, the interface membrane bears equal stresses at two sides and it is not bended, forming dual continuous microemulsion. Such penetration of oil and water into the interface membrane indeed reflect the hydrophilic and oleophylic effects of the interface membrane. Generally, the lower alcohol/surfactant needed to form the O/W-type microemulsion is relatively low, while the lower alcohol/surfactant needed to form the W/O-type microemulsion is relatively high. Obviously, the lower alcohol serves for the hydrophilic-lipophilic balance of the interface membrane, thus influencing the spontaneous bending direction of the membrane.
Robbins (Li 1995) et al. proposed the geometric arrangement theory based on the dual-membrane theory, which interpreted the prior bending of interface membrane and structural problem of microemulsion. This theory believes that polar hydrophilic groups and nonpolar alkyl chain of surfactant form separately uniform interfaces with water and oil, respectively. On the water-side interface, polar head is hydrated into the hydration layer. On the oil-side interface, oil molecules penetrate into alkyl chains. With considerations to geometric filling of surfactant on interface, the filling coefficient was defined: P = V/a0lc, where V is the volume of alkyl chains in surfactant molecules, a0 is the minimum cross section of polar groups of each surfactant on the flat interface, and lc is the length of alkyl chain (80% ~ 90% of full extension length). The prior bending of interface is determined by this filling coefficient which is influenced by swelling of polar heads and alkyl chain in water and oil. If the filling coefficient (P) is 1, the interface is flat and it will not bend toward any direction firstly, forming the dual-continuous microemulsion. If P > 1, the interface bends to the oil phase firstly, forming the opposite-phase micelle (W/O-type microemulsion). If P < 1, the interface bends toward the water phase firstly. However, the O/W-type microemulsion can be formed only when 1/3 < P < 1. When P < 1/3, positive micelles are formed. The geometric filling model theory interprets properties of additives, electrolytes and oil as well as influences of temperature on interface curvature and thereby on type or structure of microemulsion successfully.
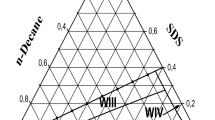
In the process of microemulsion flooding, phase changes can be expressed by the ternary phase diagram of water, oil and surfactant, because phase, components, time and spaces change as a result to migrations of water, oil, surfactant and additives. Three fixed points of the phase diagram represent saline water, surfactant + additive and oil. Moreover, this phase diagram is the function of salt content. With changes of salt content in the microemulsion system, the phase diagram shows continuous changes. Winsor I, Winsor II and Winsor III phase states change accordingly. The phase diagram of ternary system is the basis for composition simulation of microemulsion flooding. Surfactant is soluble with water phase and oil phase at any ratio to describe phase-state change process of microemulsion accurately, it has to analyze migration of surfactant, salt and other components in microemulsion phase, water phase and oil phase. The main influencing factors for a crude oil-saline water-surfactant-additive system to have phase-state changes including surfactant concentration, additive concentration, salt content, water–oil ratio and temperature. The surfactant system develops changes from I → III → II by increasing the total surfactant concentration, concentration of additive alcohol (C4, C5 and C6), salt content and water–oil ratio, and by decreasing the temperature (ionic surfactant)/when the phase state of microemulsion changes from I-type to III-type, the soluble oil content and water content are equal optimal salt content. The microemulsion phase is equal to interface tension between excessive oil phase and excessive water phase, which is the optimal condition for displacement of reservoir oil.
Preparation of microemulsion system
The preparation of microemulsion is a spontaneous emulsifying process, in which no external work is needed. It mainly depends on matching of different components in the microemulsion system, but it will be influenced by oil phase, temperature, salt content and surfactant. There are conventional methods to prepare microemulsion: ① Schulman method: oil, water, surfactant and inorganic salts are mixed uniformly to form microemulsion. Later, a certain amount of additives are dropped into the microemulsion, and the system becomes transparent suddenly. In this case, the prepared solution is the microemulsion. Changes of types and concentration of components in the system trigger changes in structure and type of microemulsion accordingly. ② Shah method: oil, additive, surfactant and inorganic salts mixed, in which a certain amount of water is added. Later, the system becomes transparent instantly. Under this circumstance, the prepared solution is the W/O-type microemulsion. A certain amount of water is added in continuously and the discrete phase changes in the form of sphere → cylinder-laminar structure or in the form of dual continuous structure → cylinder → sphere. The microemulsion system is changed from the W/O-type → dual-successive type → O/W type, as is shown in Fig. 2.
Microemulsion system is composed of surfactants, additives, salt, water and oil. Microemulsion as an oil displacement medium, on the one hand, contacts with the oil phase and water phase in the reservoir, changing the composition of oil and water components in the microemulsion system, and on the other hand, it is adsorbed by the reservoir rocks, reducing the concentration of surfactants and additives in the microemulsion system. The concentration of each component in the system changes, which directly affects the phase state of the microemulsion. Therefore, the level selection of surfactants, cosurfactant, salt and water/oil ratio parameters should cover all microemulsion phase states (upper phase, middle phase and lower phase microemulsion) as far as possible. Experimental oil was prepared by the crude oil and kerosene from the 10th Oil Extraction Plant in Daqing Oil Field according to certain proportion. Viscosity of the experimental oil was 10 mPa•s. in crude oil from Daqing Oil Field, the alkane content is higher than 50% and it has high paraffin content (26% ~ 30%), high condensation point (about 30 ℃), low sulfur content (0.10%), and low n-heptane and asphaltene content (< 2.5%). It belongs to a typical low-sulfur paraffin-based crude oil. The degree of mineralization of experimental water was about 5758.4 mg/L, which was consistent with mineral type and salinity of groundwater in Daqing Oil Field. In the experiment, microemulsion was composed of 2.5% ionic surfactant, 2.0% n-butyl alcohol, 2.0% salt and water–oil mixture (1:1). The experimental temperature was set 45 ℃. The single control variable method was applied in the experiment to investigate influences of surfactant concentration, additive concentration, salt content, water–oil ratio and temperature on phase state of microemulsion. Given the fixed other conditions, phase changes of microemulsion are observed by increasing any one influencing factor gradually. Masses of different phases at stability and effects of influencing factors on phase state of microemulsion were recorded. The experimental schemes are listed in Table 1.
The experimental procedures are as follows: according to the ratio of mass fraction described in Table 1, mix the surfactant, additive and salt, and add oil and water to the total mass 100 g of the solution at a given temperature according to the water–oil ratio. The solution was stirred evenly, and then obtain the microemulsion with obvious interfacial phase after separation, and separate the phases of the microemulsion to determine the quality of each phase solution. The mass fraction of microemulsion was calculated, and the mass fraction of microemulsion was microemulsion content.
Microscopic formation mechanism of phase transition of microemulsion
Mass fraction of phases in the prepared microemulsion systems was determined through experiments. Phase types of microemulsion were judged and the microscopic formation mechanism of microemulsion was interpreted from the perspective of molecular structure.
Surfactant concentration
With the increase of total surfactant concentration, the microemulsion system develops phase transition from I → III → II, as is shown in Fig. 3. When the total surfactant concentration is lower than 1.0%, the surfactant mainly exists in water and the whole system is Winsor I type, with about 60% of lower-phase microemulsion (O/W type) coexisting with excessive oil phases. As the surfactant concentration increases, the whole system begins to change toward the Winsor III type, forming middle-phase microemulsion. In this case, the excessive oil phase decreases, while the excessive water phase increases. When the surfactant concentration is increased to 2.5%, there are equal masses between excessive water phase and excessive oil phase in the middle-phase microemulsion system, which is the optimal system. In the optimal middle-phase microemulsion system, the microemulsion content is 44.84%. When the surfactant concentration further increases to 4.0%, the excessive oil phase disappears and the total system becomes the Winsor II type, with about 60% of upper-phase microemulsion (W/O type) coexisting with excessive water phases.
The influencing mechanism of surfactant concentration on microemulsion phases was studied from the perspective of system structure. It can be seen from Fig. 4 that surfactant concentration can promote formation of micelle molecular structure and increase solubility of oil phases. Hence, increasing the total surfactant concentration can trigger I → III → II transition of the microemulsion system.
When there’s no surfactant in the whole system, water, oil, additives and salts coexist, with a distinctive water–oil interface (Fig. 4a). With the increase of surfactant dosage, the added surfactant monomers concentrate on the interface. When surfactant concentrations reach the critical micelle concentration (CMC), surfactant molecules in water phase form small-sized micelles with additives (Fig. 4b and c). As the surfactant concentration is increasing continuously, the formed small-sized micelles begin to solubilize oil phases, thus increasing grain size of micelle molecules and decreasing mass fraction of oil phase. As a result, the whole system becomes a Winsor I type (Fig. 4d). Subsequently, more and more O/W-type microemulsion is formed and the solubilizing ability is enhanced with the further increase of surfactant concentration, which causes a density difference between O/W microemulsion and water phases. O/W microemulsion is thereby separated from water phases and gathers on the water–oil interface. Subsequently, grain size of microemulsion increases and the interface membrane become instable. The O/W microemulsion formed gradually became a W/O/W based microemulsion. Under this circumstance, the whole system is Winsor III. The excessive oil phase decreases, while excessive water phase increases. The optimal middle-phase microemulsion is achieved when the masses of excessive water phase and excessive oil phase are equal (Fig. 4c and f). As the surfactant concentration continues to increase, the residual surfactant molecules form micelle molecules and these micelles are able to solubilizing intra-phase fluids infinitely. In other words, excessive oil phases disappear and W/O microemulsion is formed. The whole system is changed to Winsor II (Fig. 4g).
Additive concentration
With the increase of additive concentration, microemulsion system develops phase transition from I → III → II, as is shown in Fig. 5. When the additive concentration is lower than 0.83%, Winsor I-type microemulsion system is formed, with about 55% of lower-phase microemulsion (O/W type) coexisting with excessive oil phase. As the additive concentration increases, the whole system begins to change toward Winsor III, forming the middle-phase microemulsion. At this moment, excessive oil phase decreases, whereas the excessive water phase increases. The masses of excessive water phase and excessive oil phase in the middle-phase microemulsion system are equal when the additive concentration is 2.0%. This middle-phase microemulsion system is determined as the optimal middle-phase microemulsion system, when the microemulsion content is 44.84%. As the additive concentration further increases to 3.16%, the excessive oil phase disappears and the whole system becomes Winsor II. The upper-phase microemulsion (W/O) accounts for about 60% and it coexist with excessive water phases.
Influencing mechanism of additive concentration on microemulsion phases was discussed from system structure. It can be seen from Fig. 6 that increasing the additive concentration can trigger I → III → II transition of the microemulsion system by weakening the charge repulsion among surfactant molecules and improve bending flexibility of interface membrane.
When no additive is in the whole system, water, oil, surfactant and salt coexist. Surfactants mainly exist in water. They concentrate on the water–oil interface as monomers and exist in water phase as micelles (Fig. 6a). With the increase of additives, the additive concentration in water climbs up. Additives can be embedded between two adjacent surfactant molecules due to their small molecular size, thus weakening charge repulsion among surfactant molecules and thereby decreasing curvature of interface membrane. Small micelles are formed due to the collaborative action of surfactant molecules and additive molecules (Fig. 6b and c). With the continuous increase of additive concentration, more and more small-sized micelles are formed and the solubilizing capacity of oil phase is increased, thus increasing grain size of micelle molecules and decreasing the mass fraction of oil phase. The whole system is Winsor I (Fig. 6d). As the additive concentration continues to increase, the content of O/W-type microemulsion for form the small-curvature interface membrane increases. Besides, microemulsion droplets agglomerate to increase solubilizing capacity and volume of oil phases. Therefore, density differences between O/W microemulsion and water phases are developed, so that O/W microemulsion can be separated from water phase and concentrate on the water–oil interface. As a result, grain size of microemulsion expands and the interface membrane become instable. The formed O/W microemulsion is changed to W/O/W or O/W/O microemulsion gradually. Under this circumstance, the whole system is Winsor III. Subsequently, the excessive oil phase decreases, while the excessive water phase increase with the increase of additive concentration. The optimal middle-phase microemulsion system is achieved at the equal masses between excessive water phase and excessive oil phase (Fig. 6e). The interface membrane begin to bulge toward the oil phase as a result to the membrane pressure if the additive concentration continues to increase, thus forming W/O microemulsion. Accordingly, the dual successive microemulsion disappears and the whole system becomes Winsor II (Fig. 6f). Later, residential additives by continuous increasing additive concentration mainly exist in excessive water phase. The phase types and microemulsion content remain basically unchanged (Fig. 6g).
Salt content
The surfactant system develops phase transition from I → III → II with the increase of salt content, as is shown in Fig. 7. The whole system is Winsor I when salt content is lower than 0.8%, in which lower-phase microemulsion (O/W type) content accounts for about 60% and coexists with excessive oil phase. As salt content increases, the whole system begins to change toward Winsor III and middle-phase microemulsion is formed. Excessive oil phase decreases, while excessive water phase increases, reaching the balance at 2.0% of salt content. At this moment, the system is determined the optimal middle-phase microemulsion system, which consists of 44.84% microemulsion. When salt content increases to 3.2%, excessive oil phase disappears and the whole system becomes Winsor II. Upper-phase microemulsion (W/O) accounts for 60% and exists with excessive water phases.
The influencing mechanism of salt content on microemulsion phases was discussed from system structure. It can be seen from Fig. 8 that increasing salt content can trigger the phase transition of microemulsion system from I → III → II by compressing the diffusion double-electronic layer of charged particles in water phases and changing the ionization balance state of ionic surfactant.
When salt content in the microemulsion system is 0, water, oil, surfactant and additive coexist and the formed microemulsion interface membrane is composed of surfactant and additives. This interface membrane shows stronger hydrophilicity than lipophilicity. According to the dual membrane theory, the interface membrane bulges toward the water phase to form O/W microemulsion, which is known as Winsor I (Fig. 8a). After salt is added in, surfactant and oil experience salt precipitation. On the one hand, salt precipitation compresses double-electronic layer and decrease repulsion of microemulsion droplets. Therefore, microemulsion droplets are easy to approach and agglomerate mutually, which further weaken charge repulsion among surfactant molecules. Consequently, active molecules of surfactants form more tightened arrangement. On the other hand, salt precipitation lowers the critical micelle concentration (CMC) of surfactant. As a result, micelle clusters increase, which further increase solubilizing capacity. Mass fraction of oil phase decreases and the system is changed to Winsor I (Fig. 8b). As the salt content continues to increase, inorganic salts enter into the interface membrane and charges in the interface membrane to adjust hydrophile-lipophile balance (HLB) value of surfactant, destroy stability of O/W microemulsion system. After salt content reaches a certain value, some micelles are separated from the original spheres, showing hemispheres or ovals. The interface membrane begins to elongate and pave gradually, accompanied with a growth in lipophilicity. Hence, oil phases in the O/W microemulsion system are exposed. These exposed oil phases agglomerate together and form successive oil phases. HLB value of surfactant reaches the relatively equilibrium and the membrane pressure is not enough to make the interface bend toward any phase. As a result, a W/O/W based microemulsion is formed. Under this circumstance, the whole system is Winsor III (Fig. 8c). With the continuous increase of salt content, excessive oil phase decreases, while excessive water phase increases and the optimal middle-phase microemulsion system is formed at equal masses between excessive water phases and excessive oil phase (Fig. 8d). With the continuous increase of salt content, the system is transformed into a O/W/O based microemulsion (Fig. 8e). Later, “salt precipitation” of surfactant and oil is intensified by increasing salt content continuously. More surfactants and additives enter into oil phases. At this moment, microemulsion shows stronger lipophilicity than hydrophilicity. Excessive oil phases all enter into middle-phase microemulsion. Dual successive structures of W/O/W and O/W/O microemulsion systems are broken, forming successive oil phases and discrete water phases. Hence, the interface membrane bulges toward the oil phase and W/O microemulsion is formed. The whole system becomes Winsor II (Fig. 8f). With the continuous increase of salt content, the double-electronic layer is compressed to the maximum extent. Phase types and microemulsion content become stable (Fig. 8g).
Water–oil ratio
The microemulsion system develops phase transitions from I → III → II with the increase of water–oil ratio, as is shown in Fig. 9. It is a Winsor I-type system when the water–oil ratio is lower than 0.33, where content of lower-phase microemulsion (O/W type) ranges between 0 and 55% and it coexists with excessive oil phases. As water–oil ratio increases, microemulsion changes toward Winsor III and middle-phase microemulsion is formed. The excessive oil phase decreases, but the excessive water phase increases, reaching the balance when water–oil ratio is 1. The corresponding middle-phase microemulsion system is the optimal one, which consists of 44.84% of middle-phase microemulsion. Excessive oil phase disappears when water–oil ratio is higher than 3 and the whole system is Winsor II. The content of upper-phase microemulsion (W/O type) ranges between 0 and 55% and it coexists with excessive water phases.
The influencing mechanism of water–oil ratio on microemulsion phases was discussed from system structure. It can be seen from Fig. 10 that increasing water–oil ratio can trigger phase transition of I → III → II in the microemulsion system by promoting micelle molecular structure formation and increasing solubility of surfactant molecules in water.
When water content in the whole system is close to 0, oil, surfactant, additive and salts coexist. There’s an distinctive interface between lipophilic phases and hydrophilic phases (Fig. 10a). When water–oil ratio increases gradually, hydrophilic phases are dissolved in water phases if their mass is lower than that of oil phases and small micelles are formed in water phases. These small micelles begin to solubilizing oil phases to further expand micelle molecules and decrease mass fraction of oil phases gradually. The whole system is Winsor I (Fig. 10b). As the water–oil ratio continues to increase, more and more O/W microemulsion is formed, which increase solubilizing ability and capacity of oil phases. As a result, density difference between O/W microemulsion and water phases is formed. O/W microemulsion is separated from water phase gradually and concentrates on the water–oil interface, thus expanding microemulsion grains and making interface membrane instable. A W/O/W based microemulsion or a O/W/O based microemulsion is formed gradually. Under this circumstance, the whole system is Winsor III (Fig. 10c). With the increase of water–oil ratio continuously, excessive oil phase decreases, while excessive water phase increase. The optimal middle-phase microemulsion is achieved when the masses between excessive water phase and excessive oil phase (Fig. 10d). With the continuous increase of salt content, the system is transformed into a O/W/O based microemulsion (Fig. 10e). When water–oil ratio continues to increase, the dual successive microemulsion is destroyed and the excessive oil phase disappears. According to dual membrane theory, the interface membrane bulge toward the oil phase under the membrane pressure, forming W/O microemulsion. The whole system is Winsor II (Fig. 10f and g).
Temperature
When temperature of the ionic surfactant system rises from 0 ℃ to 100 ℃, the microemulsion system experiences the phase transition from II → III → I and microemulsion content increases gradually, as is shown in Fig. 11. According to comparison of ternary phase diagram under 45 ℃ and 60 ℃, partial two-phase microemulsion is changed into single-phase microemulsion, thus expanding the area of single-phase microemulsion area. Given the same surfactant concentration, microemulsion content is the higher, which is conducive to increase efficiency of microemulsion flooding. When temperature is 0 ℃, the whole system is Winsor II, in which upper-phase microemulsion (W/O type) accounts for 57.44% and exists with excessive water phases. When the temperature ranges between 25 ℃ and 80 ℃, the total system begins to be evolving toward Winsor III and middle-phase microemulsion is formed. The excessive water phase declines, while excessive oil phase and middle-phase microemulsion increase. The masses of excessive water phase and excessive oil phase are equal at 45 ℃, when the optimal middle-phase microemulsion system is achieved. The optimal system contains 44.84% of middle-phase microemulsion. As temperature continues to increase to 100 ℃, excessive water phase disappears and the whole system is changed to Winsor I. In Winsor I, the lower-phase microemulsion (O/W) accounts for 62.78% of total content and it coexists with excessive oil phases.
The influencing mechanism of temperature on microemulsion phases is discussed from the perspective of system structure. It can be seen from Fig. 12 that temperature rise is in favor of phase transition of the microemulsion system from II → III → I by increasing area of surfactants on the interface and solubility of surfactant molecules in water.
When the whole system is in a low temperature, water, oil, surfactant, additive and salt coexist and the formed interface membrane is mainly composed of surfactant and additives. The microemulsion presents the low molecular activity and stronger lipophilicity than hydrophilicity. According to the dual membrane theory, the interface membrane bulges toward oil phases under membrane pressure to form W/O microemulsion, which is known as Winsor II (Fig. 12a). With the increase of temperature, molecular activity is enhanced and electronic repulsion among surfactant molecules is weakened, accompanied with growths in oil solubilizing capacity and micelle grain size as well as a reduction in mass fraction of oil phases (Fig. 12b). With the further increase of temperature, micelle grain size increases continuously. When the acting forces among molecules on the interface membrane are lower than the acting forces among spherical microemulsion molecules, the interface membrane starts to elongate and spread around. The hydrophilicity is increased to expose water phases from the W/O microemulsion system. These exposed water molecules cluster together and form successive water phases. HLB of surfactant is achieved and the membrane pressure is not enough to make the interface bend toward any phase. Finally, a O/W/O based microemulsion is formed. At this moment, the whole system is Winsor III (Fig. 12c). With the continuous increase of temperature, hydrophilicity is increased, which leads to reductions in excessive water phases and increases in excessive oil phases and middle-phase microemulsion content. When masses of excessive water phase and excessive oil phase are equal, the optimal middle-phase microemulsion system is realized (Fig. 12d and e). When temperature continues to increase, solubility of surfactant molecules in water further increases and more surfactants and water enter into middle-phase microemulsion, thus decreasing density difference between the middle-phase microemulsion and water phase. Excessive water phases all enter into the middle-phase microemulsion to destroy dual successive structures in W/O/W or O/W/O microemulsion systems. Water becomes successive phases and oil becomes diffusive phases. Surfactant bends toward water phases due to influences of membrane pressure, forming O/W microemulsion. The whole system is Winsor I (Fig. 12f). With the further increase of temperature, the interface membrane becomes increasingly instable and area of the surfactant molecules on the interface increases. As a result, the adsorbing capacity is decreased, thus decreasing oil solubilizing capacity directly and increasing mass fraction of oil phases. Phase type and microemulsion content become stable (Fig. 12g).
To sum up, microemulsion phase states can be influenced by surfactant concentration, additive concentration, salt content, water–oil ratio and temperature. Increasing the total surfactant concentration can promote micelle molecular structure formation and increase solubilizing capacity of oil phases. Increasing additive concentration can lower the charge repulsion among surfactant molecules and increase bending flexibility of interface membrane. Increasing salt content can compress diffusive double-electronic layer of charged particles in water phases and change ionization equilibrium state of ionic surfactant. Increasing water–oil ratio can promote micelle molecular structure formation and increasing solubility of surfactant molecules in water. Consequently, the microemulsion system develops phase transitions from I → III → II. Temperature rise is conducive to expand area of surfactant molecules on interface and increase solubility of surfactant molecules in water, thus triggering phase transition of the microemulsion system from II → III → I.
Conclusion
-
1.
Given the fixed types of surfactant, additive and salt, influencing factors of phase changes in the microemulsion system mainly include surfactant concentration, additive concentration, salt content, water–oil ratio and temperature. Moreover, the influencing mechanisms of these factors on different types of microemulsion systems are disclosed from the microscopic perspective.
-
2.
Increasing total surfactant concentration can promote micelle molecular structure formation and increase solubilizing capacity of oil phases. Increasing additive concentration can weaken charge repulsion among surfactant molecules and increase bending flexibility of interface membrane.
-
3.
Increasing salt content can compress the diffusive double-electronic layer of charged particles in water phase and change the ionization balance state of ionic surfactant. Similarly, increasing water–oil ratio can promote micelle molecular structure formation and increase solubility of surfactant molecules in water. The microemulsion system develops phase transition from I → III → II. Increasing temperature can expand area of surfactant molecules on the interface and increase solubility of surfactant molecules in water. Microemulsion system develops the phase transition from II → III → I.
References
Chengzhi Y (2007) Improving oil recovery by chemical flooding. Petroleum Industry Press, Beijing, pp 236–287
Ebrahimi A, Tamnanloo J, Mousavi SH, Miandoab ES, Hosseini E, Ghasemi H, Mozaffari S (2021) Discrete-continuous genetic algorithm for designing a mixed refrigerant cryogenic process. Ind Eng Chem Res 60(20):7700–7713
Ganzuo Li (1995) Microemulsion theory and its application. Petroleum Industry Press, Beijing
Gautam N, Kesavan K (2021) Phase transition microemulsion of brimonidine tartrate for glaucoma therapy: preparation, characterization and pharmacodynamic study. Curr Eye Res 46(12):1844–1852
Ghasemi H, Darjani S, Mazloomi H, Mozaffari S (2020) Preparation of stable multiple emulsions using food-grade emulsifiers: evaluating the effects of emulsifier concentration, W/O phase ratio, and emulsification process. SN Appl Sci 2(12):1–9
Ghasemi H, Mozaffari S, Mousavi SH, Aghabarari B, Abu-Zahra N (2021) Decolorization of wastewater by heterogeneous Fenton reaction using MnO2-Fe3O4/CuO hybrid catalysts. J Environ Chem Eng 9(2):105091
Jian W (2008) Theory and application of physicochemical percolation in chemical flooding. Petroleum Industry Press, Beijing
Kookhaee H, Tesema TE, Habteyes TG (2020) Switching a plasmon-driven reaction mechanism from charge transfer to adsorbate electronic excitation using surface ligands. J Phys Chem C 124(41):22711–22720
Lee HS, Morrison ED, Frethem CD, Zasadzinski JA, McCormick AV (2014) Cryogenic electron microscopy study of nanoemulsion formation from microemulsions. Langmuir 30(36):10826–10833
Mozaffari S, Tchoukov P, Mozaffari A, Atias J, Czarnecki J, Nazemifard N (2017) Capillary driven flow in nanochannels–application to heavy oil rheology studies. Colloids Surf A Phys Eng Asp 513:178–187
Nagao M, Seto H, Takeda T, Kawabata Y (2001) Effects of temperature and pressure on phase transitions in a ternary microemulsion system. J Chem Phys 115(21):10036
Seto H, Okuhara D, Kawabata Y, Takeda T, Nagao M, Suzuki J, Kamikubo H, Amemiya Y (2000) Pressure and temperature effects on the phase transition from a dense droplet to a lamellar structure in a ternary microemulsion. J Chem Phys 112(23):10608–10614
Wang D, Yin D, Gong X (2019) Numerical simulation of microemulsion flooding in low permeability reservoir. J Chem. https://doi.org/10.1155/2019/5021473
Wang D, Yin D, Wang J, Zhou Y, Zhang C (2021) Prediction and programming of microemulsion phase behavior simulation. Pet Sci. https://doi.org/10.1016/j.petsci.2021.10.024
Wang X, Zhang R, Mozaffari A, de Pablo JJ, Abbott NL (2021) Active motion of multiphase oil droplets: emergent dynamics of squirmers with evolving internal structure. Soft Matter 17(10):2985–2993
Wang D (2019) Numerical simulation theoretical research on microemulsion flooding in ultra-low permeability reservoirs. Northeast Petroleum University
Zhu T, Kang W, Yang H, Li Z, Zhou B, He Y, Wang J, Aidarova S, Sarsenbekuly B (2021) Advances of microemulsion and its applications for improved oil recovery. Adv Colloid Interface Sci 299:102527
Funding
This work is supported by Northeast Petroleum University Talent Introduction Scientific Research Start-Up Foundation Project "dynamic characterization and prediction method of phase behavior of microemulsion flooding" and National Natural Science Foundation of China under grant No. 12002083 and Excellent Scientific Research Talent Cultivation Fund Project of Northeast Petroleum University No. LJYCHB201901 and Postdoctoral Research Initiation Fund of Heilongjiang Province No. LBH-Q20075 and Nature Science Foundation of Heilongjiang Province (LH2020E013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the co-authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dongqi, W., Daiyin, Y., Junda, W. et al. Influencing factors and microscopic formation mechanism of phase transitions of microemulsion system. J Petrol Explor Prod Technol 12, 2735–2746 (2022). https://doi.org/10.1007/s13202-022-01475-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-022-01475-4