Abstract
In order to improve the temperature and shear resistance of fracturing fluid, a kind of nano-zirconium-boron crosslinker, which is different from the traditional zicral-boron crosslinker, is prepared using 4wt% borax, 50 v/v% glycerol, 8 v/v% triethanolamine and 40 v/v % acetylacetone as raw materials, and its chemical structure is characterized of by infrared spectroscopy and its performance, such as viscoelasticity, temperature and shear resistance and gel breaking property, have also been evaluated. The results show that firstly the elastic modulus of the fracturing system is much larger than the viscous modulus at frequency of 0.1–10 Hz, indicating that the fluid is a typical structural fluid. Secondly the fracture fluid crosslinked by nano-zirconium-boron crosslinker is sheared at 180 °C, 170 s−1 for 2 h, and the viscosity is maintained above 60 mPa.s. Finally viscoelasticity, gel breaking property and damage evaluation also meet the requirements of national standard code for Chinese. Analysis of the temperature resistance mechanism of the HPG fracturing fluid crosslinked by nano-zirconium-boron crosslinker shows that its connecting lines are thicker and stronger to make the fracturing fluid have better temperature and shear resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the continuous development of oil and gas resource exploitation technology, the formation reconstruction technologies, such as waterflooding (Bryant et al. 1993), acidification (Evans et al. 2001) and fracture (Wang et al. 2013), have been paid more and more attention and widely used in the field of oil and gas fields, especially fracture plays an irreplaceable role. Fracturing fluid is mainly divided into water-based fracturing fluid (Geetanjali and Amit 2017; Zhang et al. 2020), oil-based fracturing fluid, foam fracturing fluid, slippery water fracturing fluid and so on, and among which water-based fracturing fluid is the most widely used in oil and gas resource exploitation (Pei et al. 2020). According to the type of thickener (Fu et al. 2017), water-based fracturing fluid can be divided into hydroxypropyl guar gum fracturing fluid, carboxymethyl guar gum fracturing fluid, hydroxypropyl-carboxymethyl guar gum fracturing fluid, cellulose fracturing fluid and xanthan gum fracturing fluid and hydroxypropyl guar gum (HPG), a high-performance organic polymer (Azzahari et al. 2018), is widely applied in oil and gas exploitation because of its environment-friendly (Wang et al. 2008; Singha et al. 2017), low price (Jain et al. 2005; Manjunath et al. 2016), abundant resources (Yang et al. 2018; Jayachandrabal and Thirugnanasambandam 2018) and excellent performance (Li and Liu 2008; Carvalho et al. 2018).
Due to the increasing depth of oil and gas resources for exploitation, temperature and shear resistance are important indicators for evaluating the excellent performance of fracturing fluids (Zhou et al. 2013; Zheng et al. 2019), and crosslinker is an important factor affecting the temperature and shear resistance of fracturing fluids, so it is of great significance to develop a crosslinker with good temperature resistance to improve the performance of fracturing fluid. At present, the types (Li 2011; Gedamu et al. 2014) of crosslinkers for HPG fracturing fluids include inorganic boron crosslinker, organic boron crosslinker, organic metal crosslinker and organic boron-metal crosslinker (Zhao et al. 2014; Bayramoğlu G Arıca MY. 2005), and they all have their own advantages and disadvantages. For example, organic boron crosslinker is cheap but has poor temperature resistance (Chen et al. 2017a; Chaib et al. 2021), and organometallic crosslinkers (Zhang et al. 2019, 2013) have good temperature resistance but poor shear resistance and hard-to-break. Generally speaking, the temperature resistance of fracturing fluid with hydroxypropyl guar gum as thickener is less than 140 °C, so in order to continue to use the low-cost thickener–hydroxypropyl guar gum, it is important to develop a new crosslinker with better temperature resistance and easily breaking to adapt to the exploitation of deeper oil and gas resources.
In this paper, a kind of nano-zirconium-boron crosslinker with temperature resistance up to 180 °C has been developed, which is different from the traditional organic zirconium-boron crosslinker, and it has the advantages of simple preparation, stable crosslinking reaction, high crosslinking strength and so on. Traditionally, the zirconium source of the organic zirconium-boron crosslinker is zirconium ions, and there are two main preparation methods. One is that zirconium ion reacts directly with tetrahydroxyborate ion through ligand to form organic zirconium-boron crosslinker; the other is that zirconium ion and tetrahydroxyborate ion react with organic ligand separately to obtain organic zirconium crosslinker and organic boron crosslinker (their respective ligands may be different), and organic zirconium crosslinker and organic boron crosslinker are compounded according to a certain proportion to obtain organic zirconium-boron crosslinker. However, the zirconium source of the nano-zirconium-boron crosslinker is zirconia, and zirconia has stable chemical properties and less impact on the environment than zirconium ion (Mozaffari et al. 2021; Yousefi and Ghasemi 2020; Saeedmozaffari and Muditdixit 2019). The preparation theory of nano-zirconium-boron crosslinker is that nano-zirconia reacts with tetra-hydroxy-boron ion to form nano-zirconium-borate ester compound, and nano-zirconium-borate ester compound further reacts with ligands to generate nano-zirconium-boron crosslinker. The nano-zirconium-boron crosslinker is characterized by infrared spectroscopy, and its viscoelasticity and temperature and shear resistance are evaluated by high-temperature rheometer. In addition, the gel breaking property, sand suspension property and core damage of the crosslinker are evaluated, and the mechanism of its preparation and temperature resistance is also analyzed.
Experimental section
Materials
Hydrochloric acid, glycerol, sodium hydroxide and borax (sodium tetraborate pentahydrate) are all AR Grade and are purchased from Chengdu Kelong Reagent Factory. Triethanolamine, acetylacetone and 0.5wt% nano-zirconium emulsion are produced by the laboratory, and the preparation method is shown in the corresponding references (Chen et al. 2017b).
Synthesis of Nano-zirconium-boron crosslinker
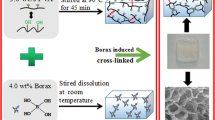
Take 50 mL of 0.5wt% zirconia emulsion into a three-necked flask, add 25 mL glycerol, stir to mix well, add 12 g borax, stir and dissolve at 40 ℃, then add an appropriate amount of sodium hydroxide to adjust the pH of the system to 10, and the reaction temperature is raised to 60 ℃ for 5 h, finally 4 mL triethanolamine and 12 mL acetylacetone are added, and the reaction is continued at 60 ℃ for 5 h to obtain a nano-zirconium-boron crosslinker (Fig. 1).
Configuration of the fracturing fluid
Take 1L deionized water, add 0.03wt% citric acid and 0.5% hydroxypropyl guar gum under stirring condition (300 rpm), continue stirring for one hour and free swelling for three hours to get 0.5wt% guar gum solution.
Take 100 mL guar gum solution, adjust the pH value of guar gum solution to 9 with 25wt% sodium carbonate solution and add nano-zirconium-boron crosslinker to prepare water-based fracturing fluid crosslinked by nano-zirconium-boron crosslinker.
Characterization of fracturing fluid crosslinked by the modified nano-ZrO2 crosslinking agent
The CVOR 200 rheometer (USA) is used to evaluate the viscoelasticity, temperature and shear resistance of the fracturing fluid.
The SSX-550 scanning electron microscope manipulating under an acceleration voltage of 2 kV is used to analyze its micromorphology and study its resistance temperature mechanism (Fig. 2).
Infrared spectra is recorded by a Nicolet Nexus 470 infrared spectrometer using the KBr method at room temperature (Fig. 3). In addition, sand suspension and core damage are also evaluated (Tables 1, 2).
Results and discussion
Synthesis mechanism of modified nano-ZrO2 crosslinking agent
As shown in Fig. 3, it is obtained that the peak at 3383 cm−1 is the stretching vibration peak of OH (Sagadevan et al. 2016), the peak at 2937 cm−1 and 2872 cm−1 are C-H absorption vibration peaks of methyl and methylene (Jin et al. 2006), the peak at 1653 cm−1 is the bending vibration peak of water molecules, and the absorption peak at 1562–1332 cm−1 is the stretching vibration absorption peak of C–O (Bayramoğlu G Arıca MY. 2005). The absorption peak at 1247 cm−1 is the vibrational absorption peak of the C-N single bond, the peak at 683 cm−1 is the Zr–O bond absorption peak of the tetragonal zirconia, the peak at 952 cm−1 is the B-O bond absorption peak (Jankowski and Risbud 1980), and the peak at 1084 cm−1 and 1037 cm−1 are the stretching vibration peak of Zr-O-B (Hirata et al. 1994), combined with the infrared characterization and temperature resistance experiments of modified nano-ZrO2 crosslinking agent, which shows that the modified nano-ZrO2 crosslinking agent is successfully prepared. These surface ligands in nano-ZrO2 play an important role in the crosslinking reaction with guar gum (Kookhaee et al. 2020).
Modified nano-ZrO2 crosslinking agent is prepared by two-step method. The first step is to obtain nano-zirconia borate ester compound through the reaction of nano-zirconia and borax. The second step is to continue to react with high-temperature ligands triethanolamine and acetylacetone to obtain nano-zirconia-boron crosslinker. The chemical structure of nano-zirconia-boron crosslinker is shown in Fig. 4.
Morphology of crosslinked HPG fracturing fluid with the modified nano-ZrO2 crosslinking agent
When the crosslinking agent reacts with guar gum, the HPG fracturing fluid formed by different crosslinking agents has different temperature and shear resistance, but it is not possible to evaluate the superiority or inferiority based on eye observation. The microscopic appearance of fracturing fluid is observed by electron microscope, and the crosslinking strength of the crosslinker is evaluated according to the mesh density and mesh thickness, which reflect the temperature and shear resistance of the crosslinker and the crosslinking mechanism of different crosslinkers.
Quanta 450 environmental scanning electron microscope is used to investigate the micromorphology of the HPG fracturing fluid crosslinked with the modified nano-ZrO2 crosslinking agent and organoboron crosslinker. After sample preparation procedures such as nitrogen freeze-drying and gold spraying, the samples are placed in ESEM for observation. Different fracturing fluid formulations are: 0.6wt% HPG + 0.5% the modified nano-ZrO2 crosslinking agent and 0.6wt% HPG + 0.5% organic-boron crosslinker.
Figure 5-A-1 and Fig. 5-A-2 show the ESEM images of HPG fracturing fluid crosslinked by nan-zirconium-boron crosslinker and organic-boron crosslinker at a magnification of 500 times, and Fig. 5-B-1 and Fig. 5-B-2 show the ESEM images of HPG fracturing fluid at a magnification of 2000 times, respectively. It can be seen from Fig. 5- A-1 and Fig. 5-A-2 that at a magnification of 500, the morphology of the HPG fracturing fluid crosslinked by the modified nano-ZrO2 crosslinking agent and the organic boron crosslinker are relatively regular networks with a structure of a thin film between each node. However, comparing Fig. 5-A-1 and Fig. 5-A-2, it can be seen that the network structure of HPG fracturing fluid crosslinked by the modified nano-ZrO2 crosslinking agent is significantly stronger, and the network lines are convex and thick. As shown in Fig. 5-B-1 and Fig. 5-B-2, it can be seen more clearly that the network structure of the HPG fracturing fluid crosslinked by the modified nano-ZrO2 crosslinking agent is stronger and the lines are thicker at a magnification of 2000, indicating that the crosslinking strength of the zirconium-boron-crosslinked HPG fracturing fluid is stronger, which is also confirmed from the results of temperature and shear resistance evaluation of the fracturing fluid.
Viscoelasticity, temperature and shear resistance
Figure 6a shows the results of the HPG fracturing fluid's viscoelasticity evaluation. In the range of 0.1–10 Hz, the HPG fracturing fluid's elastic modulus (G ') is significantly larger than the viscous modulus (G "), indicating that the fracturing fluid is a typical structural solution (Bijesh et al. 2018). In addition, the change of G" of the nano-zirconium-boron-crosslinked HPG fracturing fluid is relatively stable, and G 'shows a steady upward trend, and its value has been maintained above 25. Moreover, there are two sharp peaks in Gʹ values around 5 Hz and 8 Hz, indicating that the fracturing fluid has greater elasticity at the corresponding frequency, which proves that it has stronger deformation resistance. Based on the above experiments, it is shown that the fluid is a highly elastic fluid, which is not easily deformed by external forces, has a strong crosslinking density and strength, and thus exhibits good shear resistance.
Figure 6b is a temperature and shear resistant curve of HPG fracturing fluid crosslinked by the modified nano-ZrO2 crosslinking agent under a shear condition of 180 °C and 170 s−1 for 2 h. It can be seen from Fig. 3b that the viscosity of the HPG fracturing fluid gradually decreases as the temperature continues to increase. However, the viscosity increases as the temperature continues to increase in the first 10 min, and this phenomenon is because while the crosslinking structure of fracturing fluid is destroyed, the chemical structure of guar gum is still relatively complete, and the crosslinking agent reacts with the ortho cis hydroxyl of guar gum again, so as to partially restore the viscosity of guar gum fracturing fluid. During this shear process, secondary crosslinking occurs especially the first 40 min. When the temperature reaches 180 °C, the viscosity is basically stable. Above 50 mPa.s, after continuous shearing for 2 h, the viscosity of the HPG fracturing fluid finally maintained above 60 mPa.s, indicating that the fracturing fluid has good high-temperature shear resistance and can fully adapt to 180 °C formation reform.
Gel breaking property
Potassium persulfate is used as the breaker, and the experimental temperature is 180 °C. Under the experimental conditions of different amounts of breaker, the trend of viscosity change of 0.6wt% HPG fracturing fluid is observed over time. The results are shown in Table 1.
In general, the viscosity of the breaker fluid shows a declining trend with the increase of the amount of breaker. When the amount of potassium persulfate is 0.07wt%, although only 30 min later, the viscosity of the breaker fluid has been greatly reduced to 15 mPa.s, but after 2 h, the viscosity change is not obvious, the viscosity value is 10 mPa.s, which is greater than the requirement of viscosity less than 5 mPa.s in the evaluation standard of water-based fracturing fluid SY/T5107-2005. When the amount of potassium persulfate is increased to 0.1wt%, the viscosity of the breaker fluid is almost impossible to measure after 2 h, and the viscosity value is clearly below 5 mPa.s, and the breaker fluid could be effectively returned, and the corresponding residue content is 105 mg/L, which meets the standard of SY/T 6376–2008 fracturing fluid general technical conditions for the residue not more than 600 mg/L.
Suspended sand property
Sand suspension is one of the important properties of fracturing fluids. It is an important factor that determines whether the fracturing fluid can bring proppant to the fractures in the destination layer, and the suspension properties of fracturing fluid are evaluated by experiments.
Pour a certain amount of fracturing fluid into a 100-mL graduated cylinder, accurately measure the height of the liquid surface(h), gently place the preferred 10 uniformly sized ceramsites on the liquid surface and press the timer at the same time to record the time when the first ceramic particle reaches the bottom of the graduated cylinder(t). Finally, the fall rate is calculated. It is generally believed that the fall rate is in the range of 0.08–0.18 mm/s (0.48–1.08 cm/min) (Huang et al. 2009), which indicates that the sand suspension performance of the fracturing fluid system is good and it can well meet the construction requirements in practical applications.
It can be known from the experiment that the sedimentation rate of ceramsite in the fracturing fluid is very slow, and the sedimentation rate is 0.037 cm/min, which is much smaller than the reference value and meets the requirement of the application.
Core damage
After the fracturing fluid enters the formation, it will cause certain damage to the formation, making the permeability of the formation worse, resulting in poor oil and gas circulation, which is not conducive to the recovery of oil and gas resources. So the damage of nano-zirconium-boron crosslinked HPG gel breaker is evaluated by artificial core.
First test the permeability K1 of the dry core directly, then test the permeability K2 after saturating the core with the breaker fluid for 1 h, and perform two parallel operations, and calculate K1 and K2 according to formula (1), calculate damage rate ηd according to formula (2).
where K is permeability, Q is flow rate(mL/s), μ is air viscosity (μ = 17.9 × 10–3 MPa·s), L is core axial length (cm), Δ P is core inlet and outlet pressure difference (Mpa), A is core cross-sectional area (cm2).
ηd is the damage rate of core permeability, K1 is the matrix permeability of dry core, and K2 is the matrix permeability after being saturated by the breaker fluid. The core axial length is 3.6 cm and the core diameter is 2.5 cm, and the damage rate of core permeability is shown in Table 2.
According to Table 2, the permeability damage rate of the fracturing fluid crosslinked by modified nano-ZrO2 crosslinking agent is 28.58%, indicating that there is still some damage to the fracturing fluid, but it meets the requirements of the standard general technical conditions for fracturing fluid (SY/T 6376–2008) that the damage rate is less than 30%. Some researchers have statistics that the damage of polymer fracturing fluid is generally 35%, while the damage of guar fracturing fluid is generally 43.7% (Fu and Qin 2006), so the result shows that the fracturing fluid crosslinked by modified nano-ZrO2 crosslinking agent is suitable for the exploitation of low permeability oil and gas resources.
Conclusions
Using 4wt% borax, 50 v/v% glycerol, 8 v/v% triethanolamine and 40 v/v % acetylacetone as raw materials, 5 h reaction time, 60℃ reaction temperature, the modified nano-ZrO2 crosslinking agent is successfully prepared, and its chemical structure is characterized of by infrared spectroscopy. Analysis of the temperature resistance mechanism of the HPG fracturing fluid crosslinked by the modified nano-ZrO2 crosslinking agent shows that its connecting lines are thicker and stronger, and the fluid is a typical structural fluid, and the stronger crosslinking network gives the fracturing fluid stronger temperature and shear resistance, which makes it have the excellent performance of easy gel breaking and low damage, and has a wide range of research and application value.
References
Azzahari AD, Mutalib N, Rizwan M et al (2018) Improved ionic conductivity in guar gum succinate–based polymer electrolyte membrane [J]. High Perform Polym 30(8):993–1001
Balachandramohan J, Sivasankar T et al (2018) Ultrasound assisted synthesis of guar gum-zero valent iron nanocomposites as a novel catalyst for the treatment of pollutants [J]. Carbohyd Polym 199(11):41–50
Bayramoğlu G, Arıca MY (2005) Ethylenediamine grafted poly(glycidylmethacrylate-co-methylmethacrylate) adsorbent for removal of chromate anions [J]. Sep Purif Technol 45(3):192–199
Bijesh K, Paul G, Hamed K et al (2018) Robust charge transfer plasmons in metallic particle-film systems [J]. ACS Photonics 10(5):4022–4029
Bryant RS, Stepp AK, Bertus KM et al (1993) Microbial-enhanced waterflooding field pilots [J]. Dev Pet Sci 39(2):289–306
Carvalho DV, Loeffler N, Hekmatfar M et al (2018) Evaluation of guar gum-based biopolymers as binders for lithium-ion batteries electrodes [J]. Electrochim Acta 265(3):89–97
Chaib S, Benali N, Arhab R et al (2021) Preparation of thymus vulgaris essential oil microcapsules by complex coacervation and direct emulsion: synthesis, characterization and controlled release properties [J]. Arabian J Sci Eng 46(6):5429–46
Chauhan G, Verma A et al (2017) Rheological, structural and morphological studies of Gum Tragacanth and its inorganic SiO2 nanocomposite for fracturing fluid application [J]. J Taiwan Inst Chem Eng 80(11):978–998
Chen F, Yang Y, Tao BU et al (2017a) Preparation of boron cross linking agent for fracturing fluid using waste fly ash [J]. Bull Chin Ceram Soc 3(12):287–292
Chen F, Yang Y, He J et al (2017b) The gelation of hydroxypropyl guar gum by nano-ZrO2 [J]. Polym Adv Technol 29(12):564–568
Evans CD, Cullen JM, Alewell C et al (2001) Recovery from acidification in European surface waters [J]. Hydrol Earth Syst Sci 5(3):283–298
Fu C, Qin L (2006) A study of formation damage of fracturing fluid [J]. Nat Gas Ind 26(1):109–111
Fu C, Yang Y, Jie H et al (2017) The gelation of hydroxypropyl guar gum by nano-ZrO 2 [J]. Polym Adv Technol 29(12):123–127
Gedamu D, Lupan O, Mishra YK et al (2014) Integration of metal and metal oxide nanowires directly on chip by top-down technology and their electrical characteristics [J]. J Nanoelectron Optoelectron 9(2):239–246
Hirata T, Asari E, Kitajima M (1994) Infrared and raman spectroscopic studies of ZrO2 polymorphs doped with Y2O3 or CeO2 [J]. J Solid State Chem 110(2):201–207
Huang L, Chen F, Chen H et al (2009) Laboratory research on application of VES fracturing fluid in coal seams [J]. Inner Mongolia Petrochem Ind 35(2):4–5
Jain R, Anjaiah V, Babbar SB (2005) Guar gum: A cheap substitute for agar in microbial culture media [J]. Lett Appl Microbiol 41(4):345–349
Jankowski PE, Risbud SH (1980) Synthesis and characterization of an Si-Na-B-O-N Glass [J]. J Am Ceram Soc 63(5–6):350–352
Jin D, Xu L, Zou G et al (2006) Solvothermal synthesis of single crystalline ZnTe nanorod bundles in a mixed solvent of ethylenediamine and hydrazine hydrate [J]. J Cryst Growth 291(1):183–186
Kookhaee H, Tesema TE, Habteyes TG (2020) Switching a plasmon-driven reaction mechanism from charge transfer to adsorbate electronic excitation using surface ligands [J]. J Phys Chem C 124(41):22711–22720
Li LU (2011) Research on synthesis technology of organic boron crosslinker CY-2 and evaluation of fracturing fluid [J]. Drill Fluid Complet Fluid 1(3):23–27
Li S, Liu X (2008) Synthesis, characterization and evaluation of semi-IPN hydrogels consisted of poly(methacrylic acid) and guar gum for colon-specific drug delivery [J]. Polym Adv Technol 19(5):371–376
Manjunath M, Anjali Gowda D V et al (2016) Guar Gum and Its Pharmaceutical and Biomedical Applications [J]. Adv Sci 7(25):589–602
Mozaffari S, Li W et al (2019) The role of nanoparticle size and ligand coverage in size focusing of colloidal metal nanoparticles [J]. Nanoscale Adv 1(10):4052–4066
Mozaffari S, Ghasemi H, Tchoukov P et al (2021) Lab-on-a-chip systems in asphaltene characterization: a review of recent advances [J]. Energy Fuels 35(11):9080–9101
Pei Y, Zhang N, Zhou H et al (2020) Simulation of multiphase flow pattern, effective distance and filling ratio in hydraulic fracture [J]. J Petrol Explor Product Technol 10:933–42
Sagadevan S, Podder J, Das I (2016) Hydrothermal synthesis of zirconium oxide nanoparticles and its characterization [J]. J Mater Sci: Mater Electron 27(6):5622–5627
Singha NR, Mahapatra M, Karmakar M et al (2017) Synthesis of sustainable guar gum-g-(Acrylic Acid-co-Acrylamide-co-3-Acrylamido Propanoic Acid) interpenetrating polymer network via in situ attachment of 3-acrylamido propanoic acid for analyzing superadsorption mechanism of Pb(II)/Cd(II)/Cu(II) and dyes [J]. Polym Chem 2(44):34–38
Wang W, Zheng Y, Wang A (2008) Syntheses and properties of superabsorbent composites based on natural guar gum and attapulgite [J]. Polym Adv Technol 19(12):1852–1859
Wang M, He J, Zhang W (2013) Performance research of oil-based gelled fracture fluid based on the phosphoric acid ester and ferric iron [J]. J Southwest Petrol Univ 1(4):23–27
Yang Y, Chen F, Chen Q et al (2018) Synthesis and Characterization of grafting polystyrene from guar gum using atom transfer radical addition [J]. Carbohyd Polym 176(15):266–272
Yousefi S, Ghasemi B (2020) Precipitator concentration-dependent opto-structural properties of MgO nanoparticles fabricated using natural brine [J]. SN Appl Sci 2(5):1
Zhang J, Pu G, Dubay MR et al (2013) Repositionable pressure-sensitive adhesive possessing thermal-stimuli switchable transparency [J]. J Mater Chem C 1(6):1080–1086
Zhang Z, Mao J, Zhang Y et al (2019) Improved fracturing fluid using: organic amino boron composite crosslinker with BN covalent bond [J]. J Appl Polym Sci 11(2):23–26
Zhang K, Zhao Z, Tang M et al (2020) A new type of experimentally proposed in situ heat/gas clean foam fracturing fluid system [J]. J Petrol Explor Product Technol 10(8):3419–3436
Zhao G, Dai C, Wang S et al (2014) Synthesis and application of nonionic polyacrylamide with controlled molecular weight for fracturing in low permeability oil reservoirs [J]. J Appl Polym Sci 132(11):23–26
Zheng Y, Tang S, Wang J et al (2019) Effect of micelle structure on the viscosity of sulfonate gemini surfactant solution [J]. Arab J Sci Eng 44(1):259–267
Zhou L, Lin C, Gou S et al (2013) Synthesis and properties of the poly(acrylamide-acrylic acid-4-allylmorpholine) for enhanced oil recovery [J]. Shiyou Huagong/petrochem Technol 42(11):1256–1261
Funding
The study did not get any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, Y., Qi, C., Chao, Z. et al. Study on gelation, temperature resistance and micromorphology of modified nano-ZrO2 crosslinked hydroxypropyl guar gum. J Petrol Explor Prod Technol 12, 451–459 (2022). https://doi.org/10.1007/s13202-021-01378-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-021-01378-w