Abstract
Recently, nanoparticle additives have been used to improve stability and hence efficiency of chemicals during enhanced oil recovery. Herein, a comparative analysis of the application of nanoparticle-stabilized xanthan gum for oil recovery applications was investigated. The nanoparticles used as additives are silicon oxide (SiO2), metallic aluminium oxide (Al2O3), and titanium oxide (TiO2). Rheological measurements were carried out to examine the shear viscosity of the polymeric nanofluids under a range of salinity typical of reservoir conditions. Interfacial tension (IFT) experiment was conducted using Kruss tensiometer. Oil displacement studies were carried out to examine the incremental recovery factor of the polymeric nanofluids. The polymeric nanofluids exhibited better rheological behaviour compared to bare xanthan gum (XG) polymer. At 0.5 wt.% nanoparticle concentration, 0.5 wt.% polymer concentration, shearing rate of 10 s−1, and 3 wt.% NaCl concentration, rheology result shows that the shear viscosity of SiO2-XG, Al2O3-XG, and TiO2-XG is 423 mPa.s, 299 mPa.s, and 293 mPa.s, respectively. Moreover, the polymeric nanofluids lowered the IFT of the oil/brine interface due to adsorption at the nanoparticles at the interface. Finally, oil displacement result confirms that the incremental oil recovery after water flooding by Al2O3-XG, TiO2-XG, and SiO2-XG is 28.4%, 27.6%, and 25.2%, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
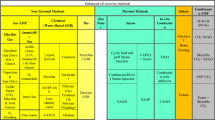
The contribution of oil to globalization and modern-day economy cannot be over emphasized (Afolabi et al. 2017). Meanwhile, literature suggests only one-third of the oil deposit is produced after primary and secondary recovery (Abbas et al. 2018; Yekeen et al. 2018). This implies that vast amount of oil is bypassed and left behind in the reservoir. Hence, the implementation of new technologies is desired to produce the remaining oil deposits. Recently, numerous enhanced oil recovery (EOR) methods have been proposed to recover bypassed and residual oil in the reservoir. These EOR methods are broadly classified into thermal and non-thermal EOR (Gbadamosi et al. 2019a, 2019b; Agi et al. 2020a).
Thermal EOR methods are unsuitable for reservoirs with huge depth and deep pay zones. Besides, this EOR method requires huge energy consumption and generates large CO2 emissions which are a major source of concern for the environment due to issues associated with global warming and climate change (Guo et al. 2016). Hence, non-thermal EOR methods such as gas EOR, microbial EOR, and chemical EOR have continued to receive prodigious attention to produce oil from hydrocarbon reservoirs.
Due to its ease of applicability, and ability to effectively recover oil of varying viscosities (light, intermediate and heavy oil), field application of chemical EOR has been reported (Saboorian-Jooybari et al. 2016). This method of oil recovery involves tuning the efficiency of injected water flood to alter the fluid/fluid and rock/fluid properties of reservoir rock, thereby aiding pore scale displacement and macroscopic sweep efficiencies (Olajire 2014). Conventional chemical EOR methods for oil recovery include alkaline flooding, surfactant flooding, foam flooding, and polymer flooding (Elhag et al. 2020; Kamal et al. 2015).
Alkaline and surfactant flooding lowers the IFT of the oil/brine interface and causes wettability alteration of the reservoir rock. On the other hand, polymer floods increase the viscosity of the injectant and cause disproportionate permeability reduction phenomenon (Kamal et al. 2015). Xanthan gum is a natural polysaccharide with good viscous property required to improve mobility ratio and improve conformance control in the reservoir (Olajire 2014). Despite its efficiency, conventional chemicals degrade in reservoir conditions which inhibits their optimal efficiency for flooding operations. The degradation of polymer molecules also occurs at high temperature and salinity conditions. The viscosity of the polymeric solution reduces with increase in reservoir temperature and salinity; hence, the efficiency of the polymer floods decreases (Bera et al. 2020; Agi et al. 2020b).
Recently, nanofluid flooding, the addition of nanoparticles to base fluid as injectant, has been investigated (Agi et al. 2020c). The presence of nanoparticles enhances the fluids property due to their small surface area and functionality. With ability to be transported in small pores, nanofluid flooding has demonstrated sufficient ability to increase oil production (Ngouangna et al. 2020). Nonetheless, the instability of the nanoparticles at reservoir conditions due to aggregation and agglomeration is the source of concern for pore throat blockage. More recently, the synergy of nanoparticles and conventional chemicals have been courted to overcome their limitations (Agi et al. 2020a, 2020b, 2020c, 2020d). The nanoparticles improve the efficiency of the conventional chemicals. On the other hand, the surfactant and polymer improve the stability of the nanoparticles.
Nanoparticle-assisted polymer flooding, also referred to as polymeric nanofluid, has received prodigious attention of researchers in the field of chemical EOR (Agi et al. 2020b). Maurya and Maurya and Mandal (2016) studied the rheological application of silica (SiO2) nanoparticles-assisted polymer flooding for high-temperature and high-salinity conditions. The polymeric nanofluid exhibited high viscosity and temperature tolerance compared to bare polymer. Haruna et al. (2017) observed improved rheological and thermal stability of HPAM with the addition of silica (SiO2) nanoparticles. Gbadamosi et al. (2019a, 2019b) also reported an improved rheological property of hydrolysed polyacrylamide with the addition of aluminium oxide nanoparticles.
Additionally, Haruna et al. (2019 formulated stable graphene oxide dispersions and investigated their impact on the dynamic and viscoelastic properties of HPAM. They noted that the addition of graphene oxide nanoparticles to the polymeric solution increased the elastic properties, high-temperature stability and viscosity of the polymer. Moreover, Aliabadian et al. (2020) investigated the oil displacement properties of graphene oxide nanosheets dispersed in HPAM. It was observed that the addition of functionalized graphene oxide improves the rheological properties of HPAM. Consequently, the oil displacement of the polymeric nanofluid improved by 7.8% over HPAM flooding.
Corredor et al. (2019) investigated nanoparticle-assisted xanthan gum rheological and flooding behaviour. They reported that the addition of nanoparticles to xanthan gum improved their viscosity properties at all conditions. Moreover, application of Fe(OH)3 and TiO2 nanopolymer sols caused 2% and 3% incremental oil recovery. Bera et al. (2020) reported improved rheological and oil displacement properties of guar gum polymer with the addition of nanoparticles. Besides, Keykhosravi et al. (20,121) examined the impact of TiO2 nanoparticles on xanthan gum polymer and its mechanism in oil-wet carbonate reservoir. The formulated polymeric nanofluid demonstrated an extra oil recovery of 25% original-oil-in-place (OOIP).
From the review of literature, the commonly used nanoparticles for improving rheological and oil displacement efficiency of polymers are the non-metallic and metallic nanoparticles. Despite the numerous research on nanoparticle application as additives in polymer flooding, a comparative study of the efficiency of metal oxide and non-metallic oxide nanoparticle on polymer flood remains obscure. Herein, a comparative analysis of the impact of metal oxide nanoparticles and non-metallic oxide nanoparticles on the rheological and oil displacement properties of xanthan gum was investigated. The metal oxide nanoparticles used are aluminium oxide (Al2O3) and titanium oxide (TiO2) nanoparticles. Meanwhile, silicon dioxide (SiO2) was utilized as the non-metallic oxide nanoparticle. The rheological property of the polymeric nanofluids was investigated in the presence of brine. Finally, oil displacement test was carried out to estimate incremental oil recovery.
Experimental
Materials
Xanthan gum (XG) polymer was purchased from Sigma-Aldrich, Malaysia, and used to prepare the polymer solutions. Non-porous aluminium oxide (Al2O3), titanium oxide (TiO2) nanoparticles and silicon dioxide (SiO2) with size range of 15–20 nm were purchased from Sky Spring Nanomaterial Inc., Houston, Texas, USA. The specific surface area (SSA) of Al2O3, TiO2, and SiO2 nanoparticles is 100 m2/g, 80 m2/g, and 160 m2/g, respectively. Sodium chloride (NaCl) was acquired from Merck Group to prepare the brine solution. Mineral oil with viscosity of 61 cP @ 27 °C was used as a representative of intermediate oil.
Sample preparation
The polymer solution was prepared by dissolving xanthan polymer powder in deionised water and stirred gently with the aid of a magnetic stirrer for 12 h. The polymer concentration used in this study is 0.5 wt.%. To prepare polymer in brine solution, 3 g of NaCl was added to obtain the desired salinity. Meanwhile, to prepare polymeric nanofluid suspension, the nanoparticle powder was first dissolved in water and subsequently ultrasonicated for 20 min in an ultrasonic bath (Crest Ultrasonics, USA) to form a stable homogenized dispersion. Thereafter, appropriate quantity of xanthan powder was added to make the nanopolymer sol.
Rheological measurement
Rheological measurement provides insight into the flow behaviour of injectant under stress and deformation. The rheology of the xanthan polymer solution and nanopolymer suspensions is measured using Brookfield rheometer. The rheometer is equipped with a water-bath to adjust the temperature of the system to desired temperature condition. Rheological measurements were taken to determine the steady shear between 1 and 1000 s−1. The experiment was carried out at high temperature condition of 80 °C to depict typical reservoir condition. To ensure and ascertain reproducibility, the experiment was repeated thrice and the average values reported.
IFT experiment
The IFT of oil/brine interface was measured using Kruss tensiometer. Besides, the behaviour of the xanthan gum polymer and polymeric nanofluids at the oleic interface was measured. The tensiometer is equipped with a water bath to adjust the temperature of the system to a desired range. The brine has a concentration of 3.0 wt.%, while the temperature used is 80 \(^\circ{\rm C}\). The measurement procedure involves the prior burning of the ring to remove impurities. Thereafter, the aqueous media is first placed in the glass up followed by appropriate quantity of the oleic phase without disturbing the surface of the dense phase (Fig. 1).
Oil displacement test
To estimate and compare the incremental oil recovery factor by various injectant, oil displacement test was carried out in high-pressure high-temperature core flooding equipment using a sandstone core. The experiment was carried out at 2500 psi and temperature of 80 °C. Four cores from the same outcrop were used to perform the experiment, and their properties are listed in Table 1. Prior to the experiment, each core sample is properly cleaned to remove impurities using toluene and acetone in a Soxhlet extractor. Thereafter, the cores are dried in an oven at 100 °C for 24 h. The procedure for the oil displacement test involves saturating the core with brine (3.0 wt.%) injected using the ISCO syringe pump. Thereafter, oil (61 cP @ 27 °C) is injected until connate water saturation is established. The oil saturated core is then aged for 24 h to ensure uniformity and establish equilibrium. Subsequently, water flooding was carried out at 0.5 ml/min until water breakthrough. Finally, xanthan gum solution and polymeric nanofluid flooding were injected to recover the bypassed oil and establish tertiary recovery. The same flow condition was used for water flooding and chemical flooding. Low flow rate was used to decrease the possibility of shearing and viscosity loss of the polymer/polymeric nanofluid encountered at high injection rates.
Results and discussion
Rheological measurement
Figure 2 shows the shear stress versus shear rate measurement of the xanthan gum polymer in deionised water. The polymer demonstrated a pseudoplastic behaviour as the shear stress increases with shear rate. Figure 3 depicts the polymer viscosity as a function of shear rate for each polymer concentration of 1000 ppm, 2000 ppm, and 5000 ppm. The polymer viscosity decreases with an increase in the shear rate, thereby exhibiting shear thinning behaviour. Low viscosity observed at high shear rate is due to the disentanglement and realignment of the xanthan polymer chains in the direction of flow (Jang et al. 2015). Although xanthan polymeric solution demonstrates shear thinning behaviour, they recover their initial viscosity when the shear rate is removed. Nonetheless, higher polymer concentration exhibits better rheological performance. For polymer flooding applications, the increase in viscosity minimises viscous fingering phenomenon by increasing the residual resistance factor and consequently improves the sweep efficiency.
Effect of brine on polymer rheology
The impact of brine on the rheology of xanthan polymer is illustrated in Fig. 4. The brine concentrations investigated were 1 wt.%, 2 wt.% and 3 wt.% which depict typical reservoir salinity condition. At 0.5 wt.% xanthan polymer concentration, the viscosity of the polymer reduces as the brine concentration increases. At 10 s−1, the viscosity of the polymer suffers a 20%, 26.7%, and 41% reduction in 1 wt.%, 2 wt.%, and 3 wt.% brine concentrations, respectively. The addition of the brine to the polymer solution causes the cations of the brine to neutralize the ionic charge of the polymer macromolecular structure. Hence, the elongated molecules of xanthan gum are transformed into a helix molecular conformation which occupy smaller hydrodynamic volume (Zhong et al. 2013), resulting in viscosity loss. During field applications, this will impact negatively on the efficiency of the polymer flooding operation.
Effect of nanoparticle on xanthan gum (XG) rheological properties
Figure 5 depicts the shear stress versus shear rate relationship for the effect of nanoparticles of XG polymer in the presence of brine. The shear stress of the polymeric nanofluid increases in direct proportion with increase in shear rate. The effect of metallic (Al2O3 and TiO2) and non-metallic (SiO2) oxide nanoparticles on the viscous properties of xanthan polymer was studied and is presented in Fig. 6. The nanoparticles improved the viscosity of the polymer in the presence of brine. The SiO2-XG polymeric nanofluid had the highest impact on the viscous property of the polymer macromolecular structure compared to Al2O3-XG and TiO2-XG polymeric nanofluids. At 5000 ppm polymer concentration, 0.5 wt.% nanoparticle concentration, shearing rate of 10 s−1, and 3 wt.% NaCl concentration, rheology result shows that the shear viscosity of SiO2-XG, Al2O3-XG, TiO2-XG, and XG polymer are 423 mPa.s, 299 mPa.s 293 mPa.s, and 220.2 mPas, respectively. The viscosity increase in the polymeric nanofluids is due to the interlink bond formed between the nanoparticles and polymer moieties. Moreover, the nanoparticles shield and/or minimize the attack of the macromolecule from cations of the brine through it complex macromolecular structure, thereby strengthening the polymer (Keykhosravi et al. 2021). SiO2-XG polymeric nanofluid had high viscosities at all shear rate because the nanopolymer sol is well dispersed compared with Al2O3-XG and TiO2-XG polymeric nanofluid. Finally, the better rheological properties of SiO2-XG may also be due to lower adsorption of nanoparticles on the polymer compared to the metallic oxide nanoparticles that had higher adsorption.
Interfacial tension (IFT) result
The ability of injectant to lower IFT is one of the major pore scale displacement mechanisms. Meanwhile, oil recovery efficiency is a combination of pore scale and sweep efficiency. The IFT of the oleic-aqueous phase was investigated and is depicted in Fig. 7. The use of XG lowered the IFT infinitesimally from 19.8 mN/m to 17.2 mN/m. Meanwhile, SiO2-XG, TiO2-XG, and Al2O3-XG reduced the IFT to 12.5 mN/m, 11.6 mN/m, and 11.4 mN/m, respectively. The standard deviation of the experiment is \(\pm\) 0.2 mN/m. The use of polymeric nanofluids exhibited better IFT reduction due to the adsorption of the nanoparticles at the oil brine interface (Saha et al. 2018). With reduction in IFT, the work required to deform the oil in pores reduces; hence, trapped residual oil could be more easily recovered (Agi et al. 2019).
Oil displacement test
Oil displacement test was carried out to evaluate the efficiency of the various polymeric nanofluids as injectant for EOR. The use of bare XG yielded 55.4% OOIP. Figures 8, 9, and 10 depict the core flooding result of using SiO2-XG, TiO2-XG, and Al2O3-XG injectant, respectively. In the case of SiO2-XG flooding, secondary recovery by water flooding recovered 37.4% oil recovery. Meanwhile, SiO2-XG polymeric nanofluid recovered 25.2% of OOIP after water flooding and cumulatively recovered 62.6% OOIP. The residual oil saturation after the EOR process is 21.5. On the other hand, TiO2-XG suspension has an oil recovery percentage of 27.6% of OOIP after water flooding and cumulatively recovered 64.3% OOIP and residual oil saturation of 19.1%. Finally, Al2O3-XG yielded 28.4% OOIP and cumulative recovery of 66.6% OOIP, and residual oil saturation is 18.5%.
The tertiary recovery stage using SiO2-XG, TiO2-XG, and Al2O3-XG is depicted in Fig. 11. SiO2-XG exhibited the lowest oil recovery percentage amongst the polymeric nanofluids. Meanwhile, the use of metal oxide polymer nanofluids (TiO2-XG, and Al2O3-XG) showed high recoveries. This can be adduced to the inherent properties of the metal oxide nanoparticles. In addition to improving rheological properties, metal oxide nanoparticles can reduce oil viscosity in situ via aquathermolysis process (Iskandar et al. 2016; Lakhova et al. 2017). This makes easier the conformance control and ability of the injectant to sweep oil toward the production well. Finally, considering the metal oxide polymeric nanofluids, the higher specific surface area of Al2O3 nanoparticles may have accounted for its increased activity over TiO2 nanoparticles.
Conclusion
This study investigates the impact of metallic and non-metal oxide nanoparticles on rheological, IFT, and oil displacement properties of xanthan gum polymer for enhanced oil recovery applications. The synergic application of nanoparticles improved the steady shear behaviour of the polymer. SiO2-XG exemplified the best steady shear performance. The shielding effects of the nanoparticles reduced the effect of brine on the viscous property of the polymer. Core flooding results showed that the polymeric nanofluids of Al2O3-XG, TiO2-XG, and SiO2-XG had incremental oil recovery of 28.4%, 27.6%, and 25.2% OOIP after water flooding, respectively. The higher recovery performance of metal oxide nanoparticles was adduced to their ability to lower viscosity of the crude oil during flooding operations. Overall, polymeric nanofluids shows positive and promising potential for field applications of EOR.
Abbreviations
- SiO2 :
-
Silicon oxide
- Al2O3 :
-
Aluminium oxide
- TiO2 :
-
Titanium oxide
- IFT:
-
Interfacial tension
- XG:
-
Xanthan gum
- SiO2-XG:
-
Silica xanthan gum suspension
- Al2O3-XG:
-
Alumina xanthan gum suspension
- TiO2-XG:
-
Titanium xanthan gum suspension
- EOR:
-
Enhanced oil recovery
- CO2 :
-
Carbon dioxide
- Fe(OH)3 :
-
Iron hydroxide
- NaCl:
-
Sodium chloride
- cP:
-
Centipoise
- OOIP:
-
Original oil in place
References
Abbas AH, Sulaiman WRW, Jaafar MZ, Gbadamosi AO, Ebrahimi SS, Elrufai A (2018) Numerical study for continuous surfactant flooding considering adsorption in heterogeneous reservoir. J King Saud Univ Eng Sci. https://doi.org/10.1016/j.jksues.2018.06.001
Afolabi F, Ojo T, Udeagbara S, Gbadamosi A (2017) Bitumen extraction from tar sands using solvent techniques. Int J Sci Eng Res 8:783–790
Agi A, Junin R, Abbas A, Gbadamosi A, Azli NB (2019) Effect of dynamic spreading and the disperse phase of crystalline starch nanoparticles in enhancing oil recovery at reservoir condition of a typical sarawak oil field. Appl Nanosci. https://doi.org/10.1007/s13204-019-01102-5
Agi A, Junin R, Gbadamosi A, Manan M, Jaafar MZ, Abdullah MO, Arsad A, Azli NB, Abdurrahman M, Yakasai F (2020) Comparing natural and synthetic polymeric nanofluids in a mid-permeability sandstone reservoir condition. J Mol Liq 317:113947. https://doi.org/10.1016/j.molliq.2020.113947
Agi A, Junin R, Abbas A, Gbadamosi A, Azli NB (2020b) Influence of ultrasonic on the flow behaviour and disperse phase of cellulose nanoparticles ar fluid-fluid interface. Nat Resour Res 29:1427–1446
Agi A, Junin R, Jaafar MZ, Mohsin R, Arsad A, Gbadamosi A, Fungm CK, Gbonhinbor J (2020c) Synthesis and application of rice husk silica nanoparticles for chemical enhanced oil recovery. J Market Res 9(6):13054–13066
Agi A, Junin R, Gbadamosi A (2020d) Tailloring of nanoparticles for chemical enhanced oil recovery. Int J Nanomanuf 18(2):107–147
Aliabadian E, Sadeghi S, Moghaddam AR, Maini B, Chen Z (2020) Application of graphene oxide nanosheets and HPAM aqueous dispersion for improving heavy oil recovery: effect of localized functionalization. Fuel 265:1–11
Bera A, Shah S, Shah M, Agarwal J, Vij RK (2020) Mechanistic study on silica nanoparticles-assisted guar gum polymer flooding for enhanced oil recovery in sandstone reservoirs. Colloids Surf A Physicochem Eng Asp 598:124833. https://doi.org/10.1016/j.colsurfa.2020.124833
Corredor LM, Husein MM, Maini BB (2019) Effect of hydrophobic and hydrophilic metal oxide nanoparticles on the performance of xanthan gum solutions for heavy oil recovery. Nanomaterials 9:1–13. https://doi.org/10.3390/nano9010094
Elhag HH, Abbas AH, Gbadamosi A, Agi A, Oseh J, Gbonhinbor J (2020) Evaluation of Continuous Surfactant Flooding in North East Africa: Case Study of Bentiu Reservoir, in: Nigeria Annual International Conference and Exhibition Originally Scheduled to Be Held in Victoria Island, Lagos, Nigeria,11–13 August 2020. pp 1–11. https://doi.org/10.2118/203702-MS
Gbadamosi A, Radzuan J, Manan M, Agi A, Oseh J (2019) Nanotechnology application in chemical enhanced oil recovery: current opinion and recent advances. IntechOpen, London, pp 26–50
Gbadamosi AO, Junin R, Manan MA, Agi A, Oseh JO, Usman J (2019) Effect of aluminum oxide nanoparticles on oilfield polyacrylamide: rheology, interfacial tension, wettability and oil displacement studies. J Mol Liq 296:111863. https://doi.org/10.1016/j.molliq.2019.111863
Guo K, Li H, Yu Z (2016) In-situ heavy and extra-heavy oil recovery: a review. Fuel 185:886–902. https://doi.org/10.1016/j.fuel.2016.08.047
Haruna MA, Pervaiz S, Hu Z, Nourfkan E, Wen D (2019) Improved rheology and high-temperature stability of hydrolyzed polyacrylamide using graphene oxide nanosheet. J Appl Polym Sci 136(22):47582
Hu Z, Haruna M, Gao H, Nourafkan E, Wen D (2017) Rheological properties of partially hydrolyzed polyacrylamide seeded with nanoparticles. Ind Eng Chem Res 56(12):3456–3463
Iskandar F, Dwinanto E, Abdullah M, Khairurrijal OM (2016) Viscosity reduction of heavy oil using nanocatalyst in aquathermolysis reaction. Kona Powder Part J 33:3–16
Jang HY, Zhang K, Chon BH, Choi HJ (2015) Enhanced oil recovery performance and viscosity characteristics of polysaccharide xanthan gum solution. J Ind Eng Chem 21:741–745. https://doi.org/10.1016/j.jiec.2014.04.005
Kamal MS, Sultan AS, Al-Mubaiyedh UA, Hussein IA (2015) Review on polymer flooding: rheology, adsorption, stability, and field applications of various polymer systems. Polym Rev 55:491–530. https://doi.org/10.1080/15583724.2014.982821
Keykhosravi A, Vanani MB, Aghayari C (2021) TiO2 nanoparticle-induced Xanthan Gum polymer for EOR: assessing the underlying mechanisms in oil-wet carbonates. J Pet Sci Eng 204:108756. https://doi.org/10.1016/j.petrol.2021.108756
Lakhova A, Petrov S, Ibragimova D, Kayukova G, Safiulina A, Shinkarev A, Okekwe R (2017) Aquathermolysis of heavy oil using nano oxides of metals. J Pet Sci Eng 153:385–390. https://doi.org/10.1016/j.petrol.2017.02.015
Maurya NK, Mandal A (2016) Studies on behavior of suspension of silica nanoparticle in aqueous polyacrylamide solution for application in enhanced oil recovery. Pet Sci Technol 34:429–436. https://doi.org/10.1080/10916466.2016.1145693
Ngouangna EN, Manan MA, Oseh JO, Norddin MNAM, Agi A, Gbadamosi AO (2020) Influence of (3–Aminopropyl) triethoxysilane on silica nanoparticle for enhanced oil recovery. J Mol Liq 315:113740. https://doi.org/10.1016/j.molliq.2020.113740
Olajire AA (2014) Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: prospects and challenges. Energy 77:963–982. https://doi.org/10.1016/j.energy.2014.09.005
Saboorian-Jooybari H, Dejam M, Chen Z (2016) Heavy oil polymer flooding from laboratory core floods to pilot tests and field applications: half-century studies. J Pet Sci Eng 142:85–100. https://doi.org/10.1016/j.petrol.2016.01.023
Saha R, Uppaluri RVS, Tiwari P (2018) Silica nanoparticle assisted polymer flooding of heavy crude oil: emulsification, rheology, and wettability alteration characteristics. Ind Eng Chem Res 57:6364–6376. https://doi.org/10.1021/acs.iecr.8b00540
Yekeen N, Manan MA, Idris AK, Padmanabhan E, Junin R, Samin AM, Gbadamosi AO, Oguamah I (2018) A comprehensive review of experimental studies of nanoparticles-stabilized foam for enhanced oil recovery. J Pet Sci Eng 164:43–74. https://doi.org/10.1016/j.petrol.2018.01.035
Zhong L, Oostrom M, Truex MJ, Vermeul VR, Szecsody JE (2013) Rheological behavior of xanthan gum solution related to shear thinning fluid delivery for subsurface remediation. J Hazard Mater 244–245:160–170. https://doi.org/10.1016/j.jhazmat.2012.11.028
Acknowledgements
Special thanks to Ministry of Higher Education (MOHE) Malaysia, and Universiti Teknologi Malaysia, Johor Bahru for providing materials and equipment for this research.
Funding
The manuscript was submitted on 24th July 2021. The introduction of APC commenced on October 1, 2021. Our submission precede the introduction of APC. Moreover, we have been notified of been covered by a BMC/SpringerOpen membership Account number JS-JPPT-PEPT-0921. Hence, we write to notify you and request for the production of the without APC charges.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gbadamosi, A., Yusuff, A., Agi, A. et al. Mechanistic study of nanoparticles-assisted xanthan gum polymer flooding for enhanced oil recovery: a comparative study. J Petrol Explor Prod Technol 12, 207–213 (2022). https://doi.org/10.1007/s13202-021-01334-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-021-01334-8