Abstract
Wellbore instability is one of the major problems that arise in drilling shale formations. Drilling in these formations may lead to breakouts and induce fractures. In Pakistan, drilling companies face quiet a number of formations that are shaly in nature and are candidates for wellbore instability issues. To counter these issues, it is required to have good understanding about the composition of the shale and its chemical reactivity with drilling fluids. Shale samples of three different formations have been obtained. These samples belong to two different regions of Indus Basin of Pakistan and were subjected to cation exchange capacity test and X-rays diffraction analysis to determine the reactivity and mineralogy, respectively. The samples were then tested for swelling properties using linear dynamic swell meter. The testing was done in two different water-based drilling fluids. The increase in height and swelling percentage for each sample was then recorded against each type of drilling fluid. A comparative analysis was done as to what type of drilling fluid systems out of the two used in the swelling test would best inhibit the swelling nature of shale for each formation in these regions of Pakistan. Finally, analytical and numerical modeling was performed on each shale sample. It was observed that the swelling parameter A (total swelling) increases significantly for Middle Indus Basin Shale Formation, and on the contrary, the filtration term (C) becomes independent of time after certain period of testing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the major technical issues of the oil industry is the instability of the wellbore (Van Oort et al. 1996; Van Oort 2003; Muniz 2005; Stankovic 2010). The most common reason for this problem is considered to be the presence of shale formation (Chenevert 1998; Al-Bazali 2009). During drilling, over 75% of the formations encountered are shaly in nature and more than 90% of the times cause severe wellbore instability problems while drilling is being performed through them (Mody and Hale 1993; Lal 1999; Al-Bazali 2005). Instability of the wellbore can cause some major drilling issues including lost drilling time, increased cost of drilling and in the worse scenario the abandonment of the well (Van Oort et al. 1996). According to some estimates, more than $ 500 million/year is being lost by the oil industry pertaining to wellbore instability issues (Van Oort et al. 1996; Van Oort 2003; Al-Bazali 2009).
While there are a number of reasons for wellbore instability to be a problem in shale, the major and the most primary cause is the unfavorable interaction mechanism of shale minerals with water-based drilling fluids that results in sloughing shale (Chenevert 1998; Al-Bazali 2009). These mechanisms are complex in nature and include physical, chemical and hydraulic phenomena (Al-Bazali 2009). However, movement of water molecules as well as flow of ions into or out of the shale is the main factor behind all these phenomena (Al-Bazali 2005). Water molecules and ions invade the shale formation, raising the pore pressure and consequently increasing the overall stress around the wellbore which makes it instable as shown in Fig. 1. Additionally, the clay part of the shale formation reacts with water and starts developing swelling stress, which ultimately increases the overall wellbore instability of shale (Stankovic 2010). Shale is considered to be weak in nature and possesses a high amount of clay mineral composition residing in them. When water-based drilling fluids are used during drilling, the filtrate penetrates the shale formation, which results in increase in pore pressure, and due to the clay being a major part of shale, and the rock starts to swell. Both these factors ultimately result in wellbore failure (Stankovic 2010). During this process, different types of interactions occur between shale and the drilling fluid (Van Oort 1997). The first and the least of all these interactions between shale and drilling fluid is Darcy flow that occurred only when wellbore pressure is greater than shale pore pressure (Stankovic 2002). This convection flow takes place very slowly as the permeability of shale is small in magnitude. The second type of shale–fluid interaction is due to molecular diffusion (diffusion flow), governed by Knudsen flow model, that takes place as a result of osmotic forces and density gradient. This is the most vital flow which occurs due to the difference in concentration (concentration gradient). The relative activity of water in drilling fluid to the activity of shale is the major factor which determines whether the flow will occur into the shale or out of the shale (Stankovic 2010). Shale sloughing and the increase in shale pore pressure are because of the convection and diffusion of H+ ions from water molecules into the shale. This increase in pore pressure results in overall weakening of the rock and decrease in rock shear strength, which ultimately reduces the mean effective stress of the shale rock (Van Oort 2003). When pore water present inside the shale interacts with the clay minerals, swelling of shale takes place. It is because the clay minerals possess a negative charge and have a greater affection to adsorb and entice the water that is occupying the pores of shale (Lal 1999). Smectite clay mineral has the tendency to expand by up to 20 times its original volume by adsorbing water enclosed in their cells (Lal 1999). This mineral is tinier than the rest of the clay minerals, but depicts a high-volume change that conclusively leads to a higher degree of swelling in clay. The variation in smectite is because of the large surface area, which allows extra water molecules and various cations to adhere to its surface area as compared to the other clay minerals (Pettersen Skippervik 2014).
Down-hole forces acting on a single clay platelet (Van Oort 2003)
Using oil-based mud systems or synthetic-based mud systems can be a remedy for shale–fluid interaction problems as the interaction between oil/synthetic mud and shale is comparatively much simpler than that of water-based mud and shale (Ewy and Stankovich 2000). The use of oil-/synthetic-based mud may look pleasing to the eye keeping in mind its ability to inhibit wellbore instability issues; the use of oil-based mud has been experienced to be very expensive. Also the use of oil-/synthetic-based mud is legally restricted around due to their detrimental and dangerous environmental effects (Stankovic 2010). Hence, it is imperative for the oil industry to enhance the efficiency of water-based mud and make the use of water-based mud even more economical and environmentally friendly (Van Oort 2003; Al-Bazali 2005).
Scope of work
The main objective of this study is to monitor the effect of inhibition of water in different types of shales from across Pakistan. This involves going through the methods involved for identifying shale and the methods used for testing shale samples. The water inhibition in shale is measured using the shale’s swelling nature by introducing the shale sample in different drilling fluid systems. Finally, a comparison is made between the different shale formations across different basins of Pakistan on the basis of their reactivity and swelling nature. Swelling was also modeled for each samples; however, the tests performed are limited to atmospheric pressure and temperature conditions, with no confining pressure experienced by samples.
Methodology
Sample selection
Different shale samples from Talhar, Ranikot and Khadro Formations were obtained that belong to different regions of Pakistan. Talhar Formation belongs to the Early Cretaceous age and is classified as a source rock. This sample was obtained from the Lower Indus Basin of Pakistan. On the other hand, Ranikot Formation belongs to Paleocene age and this formation mainly consists of limestone, sandstone and shale. It is situated in the Middle Indus Basin of Pakistan. Lastly, Khadro Formation belongs to Early Paleocene group, which is made up of sandstone and shale and is normally found in Lower Indus Region of Pakistan. A Memmert UE 200 Moisture Extraction Oven with maximum output of 1100 W was used to extract all the moisture from each sample. The moisture was removed in order to determine the exact swelling of each formation. The list of samples and their respective depths are given in Table 1.
Sample preparation for XRD analysis
Each sample (except Sample 1) was initially air-dried in the open to evaporate the moisture present. Afterward, they were put in a hot oven to make sure all the moisture has been removed. After the samples have been completely dried, they were put in a motor grinder in order to have a powder form (grain size < 100 microns). It was important for the accurate QXRD analysis that a homogenous grain size is achieved for all samples. The shale samples were then run in a sieve shaker with a sieve of 0.07 microns to get a homogenous representative sample. XRD was performed in order to analyze the mineralogy of each shale sample. It can help in identifying what types of clay minerals are present in our shale sample, and consequently we can determine the reactivity of shale sample with drilling fluids. In this test, the sample is exposed to X-rays and rotated through different angles. The crystalline minerals inside the sample will diffract the X-rays to some extent. The amount deflected is unique for each mineral. The diffraction pattern is then compared with standard diffraction of pure crystals already fed into the software, which gives us the mineral composition of the sample. Each crystal has their own definitive peak and can be identified easily (Stephens et al. 2009). The XRD is a semiquantitative analysis. The most common nonreactive metals found are dolomite, calcite, pyrite, quartz, siderite, feldspars, hematite, while the reactive clays include illite, chlorite, kaolinite and smectite (Stephens et al. 2009). Table 2 shows the detail of the mineralogy that was obtained from XRD reports of each sample.
CEC analysis
Cation exchange capacity (CEC) test was done using the CEC testing equipment to analyze the reactivity of each sample. After the CEC analysis was done on all the samples, it was important to select those samples for linear swell meter test that shows the highest reactivity. Positively charged ions (cations) are trapped between the layers of clay. The amount of these ions that can be exchanged is determined by CEC test. CEC is a direct measure of the reactivity of the shale as only the exchangeable ions contribute to the reactivity.
The cations mostly found in illite, smectite, montmorillonite and bentonite are sodium, calcium, iron, potassium and magnesium (Institute 1997). Methylene blue test (MBT) is the most common method of measuring the CEC. The CEC is measured is mill equivalents per 100 g of clay (meq/100 g) (Institute 1997). A small sample of 1 g of dried shale (ground and powdered) is usually required for each test. The sample is placed in a flask and then immersed in 50 mL of water (deionized). The suspension is then boiled gently and then brought to about room temperature again. The solution is then titrated using a methylene blue solution. The methylene blue indicator is added to the solution in increments of 0.5 mL. At each increment, a rod stirrer is dipped and then a drop from the stirrer is placed on the filter paper. If the drop has a blue halo, then the endpoint has been achieved (Institute 1997). Figure 2 and Eq. 1 show the testing kit and the relationship that was used to obtain the CEC for each sample
Sample preparation for swelling analysis
Fifteen grams of each sample was taken and put in the compactor for 30 min at a pressure of 6000 psi. The process was done twice for each shale sample as two bullets were required for each sample for testing with two separate mud systems; 350 ml that is equivalent to 1 barrel was prepared for two different water-based mud systems naming Salt Polymer Glycol and Silicate which were prepared for the swelling test. The composition and rheology of each mud system are given in Table 3 and Table 4. The additives such as poly-anionic cellulose and xanthan gum are added in order to have the desired rheological properties as well as better filtration control. Potassium chloride is added with the purpose of getting strong inhibition characteristics. Partially hydrolyzed polyacrylamide (PHPA) with a molecular weight of 1.9 × 106 is used as a viscosifier in water-based drilling mud. Polyethylene glycol is also used as a shale inhibitor in salt polymer mud system.
Swelling test is performed by using dynamic linear swell meter. This test effectively defines the degree of interaction between the shale sample and the fluid. It measures the swelling tendency of the rock sample in that particular drilling fluid. It is helpful in determining the changes in fluid/shale interaction for short periods of time (0–5 min) as well as for longer periods (up to days).The rock sample is set between two metal plates and then exposed to the fluid. The fluid then hydrates the sample and results in the consequential swelling (Stephens et al. 2009). When the sample swells, the distance between the two plates increases and is recorded with respect to time. The linear variable differential transducer is used to measure this expansion. A graph is plotted between the percentage swell and time. If the results show a higher percentage of swell with respect to time, it can be inferred that the drilling fluid used has poor inhibitive qualities (Stephens et al. 2009). Figure 3 shows the typical setup of an OFITE linear dynamic swell meter.
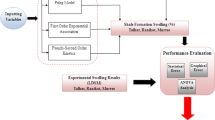
A complete flow path schematic for the study is presented in Fig. 4.
Numerical and analytical modeling of shale
The equation describe below is used to model the swelling response of shale in water-based mud system. Also the validation of this equation was verified doing the course of modeling:
where A is in %, B is in % and C is estimated to be between 0 and 1
Equation 2 is derived by combining the continuous growth rate equation and the instantaneous filtration loss term. Parameter A shows the total swelling that a sample experiences during the course of testing, parameter B represents the swelling at a particular instant of time and parameter C defines the filtration loss rate parameter. These parameters are obtained by fitting the defined equation on the experimental data obtained from linear swell meter. The line of best fit was investigated by calculating the root-mean-square error (RMSE) value for a given set of these parameters. Equation 3 is used to find the analytical solution for swelling of shale sample. Initial height of the sample was determined using the Vernier Caliper; on the other hand, the height at time ‘t’ was obtained using the data from linear swell meter at different time intervals.
Results and discussion
QXRD analysis and cation exchange capacity (CEC) of each sample
Three different samples of shale comprised of Talhar, Ranikot and Khadro Formations were acquired from different regions of Pakistan, and their compatibility with water-based mud (WBM) was investigated. Initially, from the results of XRD reports, minerals were grouped based on their chemical compositions as clay, silicates, carbonates, salt and iron ores. Results for each formation are shown from Fig. 5 till Fig. 7. It was observed that the percentage of reactive clay minerals in Ranikot Formation was greatest among all the three formations. Further analysis showed that the quantity of smectite shared the major portion in Ranikot Formation. Quantitative investigation is shown in Table 2. Apart from the clay mineral, all the three formations consist of a greater percentage of silicates, especially quartz, which is almost 50% of the total bulk sample. It was further examined that Talhar and Khadro consist of a 25% and 20% of carbonates, respectively, which is one-fourth and one-fifth of total formation composition. All the samples were collected at the depth of less than 4000 m, respectively. The percentage of iron ore in all the samples is not that substantial as seen from the figures below. All the three samples were gathered from two different basins of Pakistan that include Middle Indus Basin and Lower Indus Basin. From the study, it was perceived that these three formations had the characteristics of a cap rock and they all belong to different geological timescales (Figs. 6, 7).
Once the QXRD reports were generated, each sample was then tested for its cation exchange capacity (CEC) as shown in Table 5. It was experimentally determined that Ranikot Formation had the greatest cation exchange capacity of all as the weight percentage of clay minerals, specifically smectite, is very high. This group of clay mineral is comprised of a hydratable clay mineral known as montmorillonite, which has the largest surface area that ranges from 700 to 800 m2/g. Calcium ion (Ca2+) and sodium ion (NA+) are the typical exchangeable ions on its surface. Apart from montmorillonite, this clay mineral has also got a high weight percent of illite. This mineral has a surface are ranging from 100 to 200 m2/g and an intermediate CEC in comparison with other clay minerals. On the other hand, Talhar Formation shows the least CEC, as the sum of weight percentages of clay minerals is very low.
Mud systems A and B linear dynamic swell meter testing
The samples were then immersed in two different mud systems of varying composition as shown in Tables 3 and 4. The idea behind the study was to check the effectiveness of these mud systems in controlling the swelling of shale when they come in contact with these formations during drilling phase. The standard testing procedure is to record the swelling until 18 h of immersion. The swelling percentage of each sample was then plotted against time to determine swelling of each sample. Each of the three samples was originally brought in contact with freshwater with the purpose of determining the maximum swelling percentages. As shown in Table 6, the swelling percentage of Ranikot Formation that is 27.79% is largest among all the formations when coming in contact with freshwater. Figure 8 till Fig. 10 show the experimental analysis, numerical modeling and analytical modeling of each sample when they are in contact with the mud system A. It was observed during experiment that the sample from Ranikot Formation shows 1.97 mm increase in height that is equal to 17.62%. This result corresponds to the cation exchange capacity as shown in Table 5. In this case, expansion of the sample results because of the presence of smectite which is 15% by weight. This type of clay mineral shows most of the swelling characteristics as the interlayered spacing between this clay mineral is largest among all the clay types. The mud system A was unable to provide a better isolation in this sample, which results in water molecules, especially H+, migrating into the nanointerlayer spacing and that ultimately causes swelling. It was further observed that the Mud A got 18 grams of KCl associated with it. This KCl system is used to develop a better barrier around the shale that tends to provide stability and minimized sloughing shale problems. From the investigation, it was observed that KCl decreases significantly the swelling percentage of Ranikot Formation. Figure 14 and Table 7 show a comparative analysis of height for each formation during the 18 h of testing. It was perceived that Talhar Formation shows the least change in height of all the samples with 0.49 mm change, while Khadro shows 1.15 mm of change in its original height. The swelling characteristic of each sample was modeled using Eqs. 2 and 3. It was observed that parameter A is a strong function of CEC (Shadaab Maghrabi et al. 2013) and is maximum in Ranikot Formation as shown in Table 8 as its CEC was maximum of all the shale samples. It was further observed that the value of filtration loss parameter (C) lies between 0 and 0.2 for the samples of these basins and it only contributes to maximum of 8 h; after that, the swelling term became independent as shown from the curves (Figs. 9, 10).
All the three samples were then again tested for swelling at ambient condition in mud system B. This system is comprised of 7% by volume of sodium silicate in the initial base fluid. Table 9 shows the change in height that was observed in each of the samples. Figure 11 till Fig. 13 show the swelling percentages of each of the samples while in contact with silicate mud for 18 h along with numerical and analytical modeling. Table 10 shows the details of the modeling parameters that are obtained from Eq. 2 along with the RMSE values for mud system B. It was observed that the parameter A that defines the saturation swelling volume of shale increases in all the formations. It was further investigated that the RMSE value for Talhar was maximum, which indicates that the numerical model for this formation is not completely in correspondence with the experimental analysis. It was observed that Ranikot Formation again shows the maximum swelling of all the three samples; however, in comparison with Mud A, it was reduced by 5%. This reduction was observed based on using the silicate volume in the mud system. It was investigated that the inhibition characteristics of shale were improved. This will ultimately enhance the time-dependent failure, as silicate-based mud develops an ideal membrane around the shale that will increase its overall stability (Elkatatny et al. 2019). Another reason for the reduction in swelling can be because of the generation of oligomers as a result of polymerizes of monosilicate. These then penetrate into the shale formation by the mechanism of diffusion and form a three-dimensional gel network (Nediljka Gaurina 2004). The development of the gel isolates the filtrate invasion from the mud and creates a barrier around the sample, and it is also a mean of providing stability to the sample (Nediljka Gaurina 2004). However, the sodium ion was unable to block the pore throat in this sample; as a result, 16.98% swelling of the sample was still observed during testing. For the other two samples, the percentage of the swelling increases that shows that silicate mud was not an ideal mud in this case. For Talhar Formation, the swelling increases from 4.4 to 4.8%, whereas for Khadro, it increases from 5.7% to 6.39%, respectively. This gives the idea that in these formations the development of the membrane is ineffective and that results in transportation of the water molecules, especially the hydrogen ion, inside the layering of the formation (Figs. 12, 13, 14, 15).
Conclusion
It was concluded from the study that
-
Formations that are comprised of a greater percentage of clay minerals can be tackled using silicate mud that is comprised of Na+ and K+ ions.
-
All the formations found in Middle and Lower Indus Basin are mainly composed of large weight percentage of silicates.
-
Talhar shale showed very little reactive nature in the CEC and least swelling of all the samples in swell meter test. However, its swelling was increased using silicate mud. This concludes that in Middle Indus Basin of Pakistan Salt, polymer mud is a good source of drilling fluid in order to drill shale formations.
-
It is recommended that for Ranikot Formations that are located in the Middle Indus Basin of Pakistan, silicate mud can be used to avoid shale swelling process and issues related to wellbore instability.
-
Formations that are to be found in the Lower Indus Basin of Pakistan can be drilled using salt polymer mud system.
-
Swelling saturation for the Middle Indus Basin shale shows a drastic increase in silicate muds.
-
After a certain period of swelling, the filtration loss parameter C becomes independent of swelling.
Abbreviations
- A:
-
Total swelling
- B:
-
Swelling at a particular instant of time
- C:
-
Filtration term
- t:
-
Time
- CEC:
-
Cation exchange capacity
- MBT:
-
Methylene blue test
- XRD:
-
X-rays diffraction
- YP:
-
Yield point
- PV:
-
Plastic viscosity
- PHAP:
-
Partially hydrolyzed polyacrylamide
- RMS:
-
Root-mean-square
References
Al-Bazali TM (2005) Experimental study of the membrane behavior of shale during interaction with water-based and oil-based muds. Petroleum and Geosystems Engineering, The University of Texas at Austin. PhD
Al-Bazali TM et al. (2009) An experimental investigation on the impact of capillary pressure, diffusion osmosis, and chemical osmosis on the stability and reservoir hydrocarbon capacity of shales. In: offshore Europe. SPE offshore Europe oil and gas conference and exhibition. Aberdeen, UK
American Petroleum Institute (1997) Standard procedure for testing drilling fluids
Chenevert ME, Pernot V (1998) Control of shale swelling pressures using inhibitive waterbase muds. In: SPE annual technical conference and exhibition
Elkatatny S, Jafarov T, Al-Majed A, Mahmoud M (2019) Formation damage avoidance by reducing invasion with sodium silicate-modified water-based drilling fluid. Energies 12:1485
Ewy RT, Stankovich RJ (2000) Pore pressure change due to shale-fluid interactions: measurements under simulated wellbore conditions. In: 4th North American rock mechanics symposium. A. R. M. Association
Gaurina-Medimurec N, Simon K (2004) Laboratory Investigation of silicate mud contamination with calcium. In: 3rd South East Europe conference on sustainable development of energy, water and environment systems. Serbia
Lal M (1999) Shale stability: drilling fluid interaction and shale strength. In: SPE Asia pacific oil and gas conference and exhibition. S. O. P. Engineers
Mody FK, Hale AH (1993) Borehole-stability model to couple the mechanics and chemistry of drilling-fluid/shale interactions. J Petroleum Technol 45(11):9
Muniz ES, Fontoura SA, Lomba RFT (2005). Rock-drilling fluid interaction studies on the diffusion cell. In: SPE latin American and Caribbean petroleum engineering conference. S. O. P. Engineers
Pettersen Skippervik C (2014) Study on the swelling potential of some selected rocks, Norwegian University of Science and Technology MSc
Shadaab Maghrabi DK, Teke K, et. al (2013) Modeling of shale swelling behavoir in aqueous drilling fluid. In: SPE middle east oil and gas show and conference SPE. Manama, Bahrain
Stankovic RE (2002) Shale swelling, osmosis and acoustic changes measured under simulated download conditions. SPE Drilling Completion 25:177–186
Stankovic RJ, Ewy RT (2010) Shale swelling, osmosis, and acoustic changes measured under simulated downhole conditions. SPE Drilling Completion 25(02):177–186
Stephens M, Gomez S, Churan M (2009). Laboratory methods to assess shale reactivity with drilling fluids
Van Oort E (1997) Physico-chemical stabilization of shales. In: International symposium on oilfield chemistry. S. O. P. Engineers
Van Oort E (2003) On the physical and chemical stability of shales. J Petroleum Sci Eng 38(3):213–235
Van Oort E et al (1996) Transport in shales and the design of improved water-based shale drilling fluids. SPE Drilling Completion 11(03):137–146
Funding
No external funding received for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khan, M.A., Haneef, J., Lalji, S.M. et al. Experimental study and modeling of water-based fluid imbibition process in Middle and Lower Indus Basin Formations of Pakistan. J Petrol Explor Prod Technol 11, 425–438 (2021). https://doi.org/10.1007/s13202-020-01022-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-020-01022-z