Abstract
Carbon dioxide (CO2) sequestration through CO2 enhanced oil recovery (EOR) in oil reservoirs is one way to reduce this gas in the atmosphere. Undesirable chemical reactions that occur during these operations can affect the reservoir structure and characteristics. In this study, the effect of CO2-water-rock interaction on the rock permeability alteration and final oil recovery has been evaluated experimentally during CO2 injection into a carbonate rock. The effect of flow rate, displacement type and pressure were investigated during CO2 EOR injection. Different scenarios of miscible/immiscible displacement, secondary/tertiary recovery has been evaluated for different levels of connate water salinity and injection rate. The results show that the severity of damage is directly related to the injection rate, however change in displacement type from miscible to immiscible reduce the intensity of chemical reactions in porous medium. Moreover, in the tertiary CO2 injection, the chemical reactions become more severe due to the higher water saturations. Interestingly, this growth in the level of chemical reactions has a negligible impact on permeability reduction, since the major volume of possible reactions occurs in coarse and high permeable pores. Results reveal that damage is more intense in the case of more saline water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Greenhouse gas emissions have raised concerns about rising global temperatures. To reduce greenhouse gas emissions such as CO2, researchers have proposed solutions such as storage of this gas in underground formations. Sequestration of this gas will reduce its concentration in the Earth's atmosphere Bachu and Adams 2003. Also, after water flooding of oil reservoirs, CO2 injection can be considered as a proper candidate for tertiary EOR as it helps to decrease CO2 atmosphere (Aycaguer et al. 2001; Beckwith 2011; Han et al. 2016; Eliebid et al. 2017; Hamid et al. 2017). CO2 injection method has been studied in various light and heavy oil reservoirs (Mangalsingh and Jagai 1996; Nobakht et al. 2007; Ghedan 2009; Zekri et al. 2013; Ma et al. 2016; Seyyedsar et al. 2016). Effective parameters of this process based on the laboratory investigation, field application or simulation are reported by some researchers (Trivedi and Babadagli 2009; Ghani et al. 2015; Jaber and Awang 2017; Lashkarbolooki et al. 2017; Zarghami et al. 2017; Rostami et al. 2018; Bhatti et al. 2019; Biyanto et al. 2019; Fakher et al. 2019; Zivar and Pourafshary 2019).
Generally, in the process of CO2 injection into aquifer formations in order to sequester this gas, the reaction between the CO2 and the aquifer formation plus the salts present in the environment yields acids and soluble as well as insoluble compounds in the formation depending on the type of salt. This can affect the formation and change its petrophysical characteristics by activating the dissolution and sedimentation processes. During the secondary or tertiary recovery of oil by CO2, such reactions can also be repeated by the interaction between CO2 and formation water.
The injection of CO2 into the reservoirs alters the physical rock properties, including permeability and porosity resulting from the dissolution of rock minerals (Izgec et al. 2006, 2008). Changes that result from the interaction between CO2 and formation water in the aquifer formations along with its effect on the quality of the formation has a substantial study background. However, the interaction between CO2 and irreducible water in oil reservoirs due to the insignificant percentage of transportable water and its effect on the EOR process has not been studied so far.
The mechanism by which the sedimentation process reduces the permeability involves deposition on the pore walls. This way, some of the particles block the pore spaces and others pass through these spaces and are produced (Izgec et al. 2006, 2008; Pruess et al. 2001, 2003).
CO2-brine-rock interaction makes EOR and/or sequestration processes more complex. CO2 dissolution into the formation water causes to change in thermodynamic equilibrium of minerals that may result into further mineral dissolutions or depositions. The created acidic fluid can change mineral composition or reservoir properties such as porosity and permeability. Permeability reduction due to chemical reaction is been studied using simulation and experimental tools by many researchers (Izgec et al. 2006; Zhao et al. 2015; Sandeep et al. 2016). This reduction can reach to about 90% of the initial permeability as its intensity is a function of rock mineralogy and cementation, initial permeability, temperature, injection rate and injection duration (Moghadasi et al. 2004).
On the other hand, the dissolution of formation minerals during CO2 injection, can increase the rock permeability (Wang and Gu 2011). Effect of CO2 injection on permeability and porosity has been commonly studied (Zhao et al. 2015) but, rare study has been conducted for CO2-EOR projects due to the low percentage of immobile connate water saturation in the main body of the reservoir. A better understanding of CO2-brine-rock interactions and mechanisms in CO2-EOR can be obtained through laboratory studies.
In this paper, the influence of CO2-EOR injection on the carbonate rock’s physical properties has been studied experimentally. Specifically, the permeability alteration of the rock has been investigated. The initial permeability (absolute permeability) and permeability after flooding tests were compared. Indeed, this study intends to answer the question of whether the interaction of CO2 and irreducible water is significant during flooding experiment, and what its effects are on the reservoir characteristics and how these changes affect the final recovery. High pressure-high temperature experiments of CO2 injection into carbonate cores are conducted to investigate how the injection rate, miscibility region, type of injection (secondary/tertiary) and value of connate salinity may affect the permeability and the oil recovery factor.
Materials and methods
Fluid properties
Oleic phase used in the experiments (kerosene) and injected gas phase (carbon dioxide) are introduced in Table 1. The reason for using Kerosene is that the aim of the present study is to investigate the rate of chemical interaction between rock and CO2 in the presence of water. The use of crude oil in the reservoir adds many uncertain factors to the experiment making it impossible to examine various parameters.
NaCl (Merck Company) was used to make synthetic brine with concentration of 40,000 ppm.
Rock properties
Different rock samples, were extracted from reservoir carbonate rocks. The cores dimensions were 8.8 cm in length and 3.8 cm in diameter as their porosity and absolute permeability varied in range of 22–27% and 1–5 md, respectively. X-ray powder diffraction (XRD) analysis and core CT-scan was conducted to recognize rock’s mineralogy and possible heterogeneity.
The result of XRD test (presented in Table 2) indicated that the rock sample is a carbonated one mainly consisted of calcite.
Rock porosity and permeability can also be estimated using indirect methods such as CT-scan imaging (Hounsfield 1975; Hunt et al. 1988) when direct measurements are impossible, for instance; during conduction of an experiment. In carbonated rocks porosity can be introduced as a function of CT number (output of CT-scan setup that is a measure of X-ray absorption) using following correlation (Saadat et al. 2011):
As an example, variation of porosity at different cross sections of one of the cores has been illustrated in Fig. 1. As is shown, rock is almost homogeny. Other studies have used similar methods, such as a linear X-ray machine, to investigate the homogeneity of the sample and to obtain porosity profile (Hassani et al. 2016).
Experimental setup and procedure
Tests’ temperature was fixed at 63 °C as a typical value of oil reservoirs. The MMP of kerosene was considered about 1500–1800 psi. Therefore, to reduce the uncertainty of experiments, miscible and immiscible experiments have been designed to be conducted at test pressure of 2700 psi and 1000 psi, respectively. To study the effect of injection rate on the changes of permeability and oil recovery, three tests were introduced at different injection rates of 10, 20 and 30 cc/h. The changes in permeability were measured after 25 pore volumes injection into the core. Salinity variation was also considered in two different levels of 40,000 PPM and 130,000 PPM. Table 3 summarizes the specifications of the conducted experiments.
The core-flood experiments have modeled CO2 injection into the saturated rock samples to study the effect of CO2/water chemical interaction on permeability evolution and final oil recovery. The design followed the basic principles of conventional core-flood experiments for multi-phase flow in porous media.
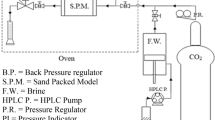
Figure 2 demonstrates the schematic of our experimental setup. The flooding setup consisted of a core holder, two hydraulic transfer vessels for gas and oil injection, a positive displacement pump, and an air bath. A back pressure regulator was used to guarantee the stability of test pressure. The collected data was sent to a computer for monitoring and further controlling of major parts of the systems. During the experiments, the core holder was adjusted to the vertical position and CO2 was injected into the oil saturated core samples in presence of connate water from the upper input port. Total oil recovery was calculated based on the amount of produced oil, correcting the oil volume variation from reservoir condition to standard condition. Rock permeability has been calculated before and after each test in order to monitor the evolution. Experimentally, the initial permeability is absolute permeability. Using Darcy’s equation, absolute permeability was measured at different flow rates. In the following figures, the permeability measured after flooding is defined based on initial permeability for easier comparison.
Results and discussion
Several experiments were designed and summarized in Table 3 in order to cover all major aspects of CO2 chemical and physical impacts on the core permeability and oil production.
The effect of injection rate (tests 1, 2 and 3)
Test 1, test 2 and test 3 have been initiating and operating at the same condition (miscible condition) the only difference was their injection rate. Figure 3 depicts the trend and the amount of oil production versus CO2 pore volume injection.
As is expected, there is no significant difference in oil recovery of these tests because of the miscibility regime. The permeability alternation is strongly dependent on the injection rate (Fig. 4). As the injection rate increases, ratio of permeability after flooding to initial permeability reduces and permeability alteration is turned into core damage.
Vertical flow injection could result in rock’s mineral dissolution in the upper part of the core and precipitation in lower part. In a vertical process, if dissolution occurs in the system, particles inside the core are displaced and move to the bottom of the core. So, particle removal from the environment will lead to increased permeability, where if the particles cannot be removed from the core and accumulate at the end of the core, this can lead to reduced permeability. Verticality and horizontality of the flow and its effects on the process have been studied by other researchers (Izgec et al. 2006, 2008).
In addition, it may result into fine migration that could lead to either permeability improvement of rock or permanent damage to the core plug depending on the rock’s mineralogy and/or fine’s diameter. Increase of the injection rate could accelerate the fine grain displacement process. As the connate water is placed in the fine pores of the core plugs, the chemical reaction mainly occurs within fine pores.
In test 1, the permeability does not depict a significant change. We could conclude that in test 1, there was a balance between the dissolution and precipitation in porous media. Test 2 has a lower connate water saturation which result less chemical reaction, and in consequence slight permeability damage was observed. Test 3 has a highest impact on the permeability evolution of the carbonate rock. It expresses clearly how exceeding from the optimum injection rate during a CO2 injection process could result in severe reduction in permeability. It was predicted that if dissolution and precipitation processes occur within the core, with increasing flow rate, the permeability would diminish. Because in this case, the extent of particle displacement to the bottom of the core increases and since the particle outflow capacity is limited, this would decrease the permeability. Note that permeability was reassessed after cleaning the core by chemical solvents.
Miscible/immiscible displacement (test 3 and test 4)
From the fourth experiment until the end of the experimental process, the flow rate was considered constant to allow the possibility of examining the influence of other parameters on the process. Pressure was assumed to be 1000 psi in the fourth experiment where the experiment was in an immiscible mode. Figure 5 compares the variation of oil recovery factor versus injected pore volume of CO2 for an immiscible and miscible vertical flood. As is shown, there is a great difference in the final results. In an immiscible displacement, low flexibility of fluid’s interface causes a large amount of oil saturation to remain against fine pore thoughts or to be bypassed in fine dead-end-pores. Therefore, lower ultimate oil recovery factor can be gained during immiscible CO2 injection. In addition, in this regime of injection, minimum chance of chemical reactions does exist due to disability of CO2 to enter the fine pores where connate water is allocated. Thus, permeability does not change significantly during immiscible injection (Fig. 6). While during miscible injection rock permeability has been improved, considerably. In immiscible CO2 flooding test, the injection gas is not able to penetrate the micro pores of the core. Thus, in addition to the fact that part of the fluid volume within the core is almost not recoverable anymore, the effect of CO2 injection on the irreducible formation water, generally present in the micro pores, is reduced. Eventually, this will lead to a decline in acid production and consequently a reduction in chemical reactions as well as permeability changes. In contrast, the miscible state allows CO2 to penetrate the micro pores of the core more easily, and enhance the recovery of the fluids. Then, the miscible state allows CO2 to more easily access and interact with irreducible formation water, resulting in more acid production and ultimately more dramatic changes in the physical properties of the rock.
Tertiary recovery (test 5 and test 6)
Test 5 (tertiary miscible injection) and test 6 (tertiary immiscible injection) have been performed to be compared with the secondary injection tests in tertiary recovery, strong chemical reaction is expected due to the presence of large amount of water saturation through the coarse and high permeable pores.
In these two tests, water flooding of the core was first performed with brine to remove all Kerosene that could be removed by water from the system. This stage of the experiment was carried out under ambient temperature and pressure, so that it would have the minimum effect on the physical conditions of the formation during the injection. CO2 injection was then performed at a flow rate of 30 cc/h. The reason for the presence of water was to create a mobile aqueous phase along with the mobile oil phase inside the sample and to augment the water saturation percentage in the system. This would allow observing what the rate of reaction is and examining its impact on the rock with the presence of a higher percentage of water in the system, which is mobile unlike previous tests.
Before starting the CO2 injection, three phase can be detectable within the porous media, saturation of mobile water, oil phase and immobile connate water. The fraction of each fluid is mentioned in Table 4.
Figure 7 shows the oil recovery factor versus injected pore volume of CO2 for an immiscible and miscible vertical flood in tertiary mode. As is evident, similar to tests 3 and test 4, the ultimate oil recovery and permeability alteration of tertiary injection experiments are significant in miscible flood. However, the results depict a smaller difference between the miscible and immiscible injection in tertiary tests. As the oil phase was drained out from main paths during secondary water injection, limited in placed oil phase was available to be recovered during tertiary CO2 injection.
The permeability in tests 5 and 6 does not depict a considerable change. Furthermore, due to more water saturation, the effect of chemical reactions is much greater than tests 1, 2 and 3. Although stronger chemical interactions should result in severe dissolution–precipitation process, the permeability almost remain intact in tests 5 and 6. As is expected, high concentration of chemical reactions is occurred in coarse and high permeable pores with greater transmission capacity. This could be compared with low transmission capacity of fine and low permeable pores which are the accordance places of main chemical interactions in tests 1, 2 and 3. When dissolution–precipitation process could reverse the effects of each other, even with severe chemical reactions, the permeability could remain intact. In Fig. 8, the test with immiscible injection regime (test 6) reveals a slight growth in permeability. Like test 4, this could be expressed with small amounts of CO2–water interaction in porous media.
Effect of salinity (test 3 and test 7)
The salinity of connate water could affect the CO2-water chemical reaction magnitude. Generally as the water salinity increase, solubility of CO2 in water decreased. It means, at the same condition with increasing the salinity of water, the less solubility is observed and subsequently, there is lower acid production and lower intensity of the chemical reaction (Duan and Sun 2000; Jarrell 2008). This is especially important in aquifer formations that are the target of CO2 injection for storage. But in oil reservoirs, with respect to the low saturation of connate water, its immobile nature and exposure in low permeable and dead end pores, the primary theory predicted no significant change in chemical reaction based on change in soluble salt during the CO2 injection process. Tests 3 and 7 were performed at the same condition (miscible regime with flow rate of 30 cc/h) in order to investigate the effect of salinity. In test 7, the salinity of connate water increased to 130,000 ppm.
Figure 9 displays the production rate under two different salinity of the brine. As mentioned, the lower reaction intensity in the seventh experiment could be attributed to the lower recovery rate. This question may arise here that as the increase in chemical reactions in the core could mean enhanced degradation and diminished permeability, how could it be considered as a reason to increase the recovery factor. Note that, according to the production diagrams, it is clear that a high percentage of recovery was obtained at the initial time intervals of the experiment; this is when the dissolution process was occurring in the core and when a large proportion of the particles have not yet precipitated or reduced the permeability. On the other hand, reduction of the permeability does not always mean diminished recovery capacity. During a CO2 flooding operation, the reaction between CO2 and the formation fluid can lead to the dissolution and reopening of the pores of core, which were otherwise blocked and could not be used to increase the recovery factor. At the same time, long-term precipitation can lead to a severe reduction of permeability and may affect the long-term recovery rate. This may be the case in near-wellbore areas that have been injected by CO2 for a long time, which can cause severe financial losses. Therefore we extended the experiment’s time to model the condition of near-wellbore areas, and monitor any possible hazard of pore plugging or injectivity reduction.
Considerable difference between two tests permeability alteration due to the possibility of more limited chemical reaction in test 7 is expected. As it is evident in Fig. 10, the permeability alteration in test 7 is negligible, however the permeability shows severe reduction in test 3, which has about 30% of the water salinity compared with test 7. Indeed, due to the lower solubility of CO2 in more salty water, the reaction intensity and the permeability changes are expected to be lower. It should be noted that visual tests such as CT scans can help to achieve more accurate results on permeability alteration for future studies.
By considering that the remaining parameters between the two experiments are constant and equal, this major difference can be attributed to the effect of water salinity and concluded that in this process, this parameter can play a decisive role along with other parameters.
Conclusions
We performed various experiments to investigate the effect of CO2 injection on permeability alteration of carbonate rocks. The results have been categorized in four parts: the injection rate alteration, miscible/immiscible displacement, secondary/tertiary recovery and change in salinity. Moreover, the results of experiments were compared and the main conclusions can be outlined as follows:
-
1.
CO2–water interaction has a considerable role in CO2 injection into carbonate samples and could drastically altered the rock properties. The severity of permeability reduction will be increased with incrimination of injection rate. This situation could be simulated with high velocity near wellbore area. The results also specified that the injection rate does not have a significant influence on the oil recovery at the early time or for the short core length samples.
-
2.
During miscible CO2 injection, we faced more intense chemical reaction and permeability reduction, which occurred because of more intense CO2–water interaction in miscible flood. As expected, the final recovery has a greater value in miscible flood, which was related to the accessibility of CO2 to the smaller pores of the core plug, lower capillary pressure and more efficient sweep.
-
3.
In the case of high water saturation oil reservoirs (water flooded oil reservoirs), the chemical reactions that occur after CO2 injection would be crucial. Furthermore, the severe dissolution–precipitation processes in coarse and high permeable pores could be neutralized by each other. This represents the complexity of permeability alteration pattern in tertiary recovery. This part of study represents a new open field for further studies in tertiary CO2-EOR projects.
-
4.
Increasing the connate water salinity was a key variable in permeability evolution in porous media. It was concluded that raising the connate water salinity could result in significant decrease in severity chemical reactions in porous media. It can be concluded that the injection for improved oil recovery from the formation with saline connate water has less risk than low saline water rock.
References
Aycaguer A-C, Lev-On M, Winer AM (2001) Reducing carbon dioxide emissions with enhanced oil recovery projects: a life cycle assessment approach. Energy Fuels 15(2):303–308. https://doi.org/10.1021/ef000258a
Bachu S, Adams JJ (2003) Sequestration of CO2 in geological media in response to climate change: capacity of deep saline aquifers to sequester CO2 in solution. Energy Convers Manag 44(20):3151–3175. https://doi.org/10.1016/S0196-8904(03)00101-8
Beckwith R (2011) Carbon capture and storage: a mixed review. J Pet Technol. https://doi.org/10.2118/0511-0042-JPT
Bhatti AA, Raza A, Mahmood SM, Gholami R (2019) Assessing the application of miscible CO2 flooding in oil reservoirs: a case study from Pakistan. J Pet Explor Prod Technol 9(1):685–701. https://doi.org/10.1007/s13202-018-0504-x
Biyanto TR, Febriansyah LR, Abdillah AI, Perwira HY, Rizki RF, Bethiana TN, Irawan S (2019) Optimization of operating conditions of CO2-enhanced gas recovery and carbon sequestration. J Pet Explor Prod Technol 9(4):2689–2698. https://doi.org/10.1007/s13202-019-0669-y
Duan Z, Sun R (2003) An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. Chem Geol 193(3):257–271. https://doi.org/10.1016/S0009-2541(02)00263-2
Eliebid M, Mahmoud M, Shawabkeh R, Elkatatny S, Hussein IA (2017) Effect of CO2 adsorption on enhanced natural gas recovery and sequestration in carbonate reservoirs. J Nat Gas Sci Eng. https://doi.org/10.1016/j.jngse.2017.04.019
Fakher S, Ahdaya M, Elturki M, Imqam A (2019) An experimental investigation of asphaltene stability in heavy crude oil during carbon dioxide injection. J Pet Explor Prod Technol. https://doi.org/10.1007/s13202-019-00782-7
Ghani A, Khan F, Garaniya V (2015) Improved oil recovery using CO2 as an injection medium: a detailed analysis. J Pet Explor Prod Technol 5(3):241–254. https://doi.org/10.1007/s13202-014-0131-0
Ghedan SG (2009) Global laboratory experience of CO2-EOR flooding. In: SPE/EAGE reservoir characterization and simulation conference in Society of Petroleum Engineers: Abu Dhabi, UAE. https://doi.org/10.2118/125581-MS.
Hamid A, Raza A, Gholami R, Rezaee R, Bing CH, Nagarajan R (2017) Preliminary assessments of CO2 storage in carbonate formations: a case study from Malaysia. J Geophys Eng 14(3):533
Han J, Lee M, Lee W, Lee Y, Sung W (2016) Effect of gravity segregation on CO2 sequestration and oil production during CO2 flooding. Appl Energy 161:85–91. https://doi.org/10.1016/j.apenergy.2015.10.021
Hassani K, Rostami B, Ayatollahi S, Yassin MR (2016) Low salinity water injection at different reservoir rocks: similarities and differences. Spec Top Rev Porous Media 7(1):87–97
Hounsfield GN (1975) Method of and apparatus for examining a body by radiation such as X or gamma radiation. Patent specification 1283915. Bangalore: The Patent Office
Hunt PK, Engler P, Bajsarowicz C (1988) Computed tomography as a core analysis tool: applications, instrument evaluation, and image improvement techniques. J Pet Technol. https://doi.org/10.2118/16952-PA
Izgec O, Demiral B, Bertin HJ, Akin S (2006) Experimental and numerical modeling of direct injection of CO2 into carbonate formations. In: SPE annual technical conference and exhibition in Society of Petroleum Engineers: San Antonio, Texas, USA. https://doi.org/10.2118/100809-MS.
Izgec O, Demiral B, Bertin H, Akin S (2008) CO2 Injection into saline carbonate aquifer formations I: laboratory investigation. Transp Porous Media 72(1):1–24. https://doi.org/10.1007/s11242-007-9132-5
Jaber AK, Awang MB (2017) Field-scale investigation of different miscible CO2-injection modes to improve oil recovery in a clastic highly heterogeneous reservoir. J Pet Explor Prod Technol 7(1):125–146. https://doi.org/10.1007/s13202-016-0255-5
Jarrell PM (2008) Practical aspects of CO2 flooding. Society of Petroleum Engineers, Richardson
Lashkarbolooki M, Eftekhari MJ, Najimi S, Ayatollahi S (2017) Minimum miscibility pressure of CO2 and crude oil during CO2 injection in the reservoir. J Supercrit Fluids 127:121–128. https://doi.org/10.1016/j.supflu.2017.04.005
Ma J, Wang X, Gao R, Zeng F, Huang C, Tontiwachwuthikul P, Liang Z (2016) Study of cyclic CO2 injection for low-pressure light oil recovery under reservoir conditions. Fuel 174:296–306. https://doi.org/10.1016/j.fuel.2016.02.017
Mangalsingh D, Jagai T (1996) A laboratory investigation of the carbon dioxide immiscible process. In: SPE Latin America/Caribbean Petroleum Engineering Conference in Society of Petroleum Engineers: Port-of-Spain, Trinidad. https://doi.org/10.2118/36134-MS.
Moghadasi J, Müller-Steinhagen H, Jamialahmadi M, Sharif A (2004) Model study on the kinetics of oil field formation damage due to salt precipitation from injection. J Pet Sci Eng 43(3):201–217. https://doi.org/10.1016/j.petrol.2004.02.014
Nobakht M, Moghadam S, Gu Y (2007) Effects of viscous and capillary forces on CO2 enhanced oil recovery under reservoir conditions. Energy Fuels 21(6):3469–3476. https://doi.org/10.1021/ef700388a
Prues K, Xu T, Apps J, Garcia J (2003) Numerical modeling of aquifer disposal of CO2. https://doi.org/10.2118/83695-PA.
Pruess K, Xu T, Apps J, Garcia J (2001) Numerical modeling of aquifer disposal of CO2. In: SPE/EPA/DOE exploration and production environmental conference in Society of Petroleum Engineers: San Antonio, Texas, p 16. https://doi.org/10.2118/66537-MS.
Rostami B, Pourafshary P, Fathollahi A, Yassin MR, Hassani K, Khosravi M, Mohammadifard M, Dangkooban A (2018) A new approach to characterize the performance of heavy oil recovery due to various gas injection. Int J Multiph Flow 99:273–283. https://doi.org/10.1016/j.ijmultiphaseflow.2017.10.014
Saadat K, Rahimpour-Bonab H, Esfahani MR, Vali J (2011) Empirical correlation for porosity deduction from X-ray computed tomography (CT). Geopersia 1(2):47–54. https://doi.org/10.2259/jgeope.2011.23283
Sandeep VR, Chaudhuri A, Kelkar S (2016) Permeability and flow field evolution due to dissolution of calcite in a 3-D porous rock under geothermal gradient and through-flow. Transp Porous Media 112(1):39–52. https://doi.org/10.1007/s11242-016-0631-0
Seyyedsar SM, Farzaneh SA, Sohrabi M (2016) Experimental investigation of tertiary CO2 injection for enhanced heavy oil recovery. J Nat Gas Sci Eng 34:1205–1214. https://doi.org/10.1016/j.jngse.2016.08.020
Trivedi JJ, Babadagli T (2009) Oil recovery and sequestration potential of naturally fractured reservoirs during CO2 injection. Energy Fuels 23(8):4025–4036. https://doi.org/10.1021/ef900361n
Wang X, Gu Y (2011) Oil recovery and permeability reduction of a tight sandstone reservoir in immiscible and miscible CO2 flooding processes. Ind Eng Chem Res 50(4):2388–2399. https://doi.org/10.1021/ie1016046
Zarghami S, Boukadi F, Al-Wahaibi Y (2017) Diffusion of carbon dioxide in formation water as a result of CO2 enhanced oil recovery and CO2 sequestration. J Pet Explor Prod Technol 7(1):161–168. https://doi.org/10.1007/s13202-016-0261-7
Zekri ARY, Shedid SA, Almehaideb RA (2013) Experimental investigations of variations in petrophysical rock properties due to carbon dioxide flooding in oil heterogeneous low permeability carbonate reservoirs. J Pet Explor Prod Technol 3(4):265–277. https://doi.org/10.1007/s13202-013-0063-0
Zhao DF, Liao XW, Yin DD (2015) An experimental study for the effect of CO2-brine–rock interaction on reservoir physical properties. J Energy Inst 88(1):27–35. https://doi.org/10.1016/j.joei.2014.05.001
Zivar D, Pourafshary P (2019) A new approach for predicting oil recovery factor during immiscible CO2 flooding in sandstones using dimensionless numbers. J Pet Explor Prod Technol 9(3):2325–2332. https://doi.org/10.1007/s13202-019-0630-0
Acknowledgements
The authors would like to extend our appreciation to the Tehran University’s EOR laboratory team: Mr. Keyvan Kazemi and Mr. Daniyal Zeinabadi due to their sincere cooperation during the applicative steps of the experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okhovat, M.R., Hassani, K., Rostami, B. et al. Experimental studies of CO2-brine-rock interaction effects on permeability alteration during CO2-EOR. J Petrol Explor Prod Technol 10, 2293–2301 (2020). https://doi.org/10.1007/s13202-020-00883-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-020-00883-8