Abstract
Carbonated water injection (CWI) might be an efficient alternate to CO2 injection technique. In CWI, CO2 exists as a dissolved phase and not as a free phase; thus, it eliminates some challenges encountered in CO2 injection such as poor sweep efficiency and gravity segregation. In CWI, the density and viscosity of water become higher than normal due to the CO2 dissolution, thereby reducing the gravity segregation and channeling effect. This article is a comprehensive review on how carbonated water flooding has evolved over the time and captured salient features on the mechanisms involved in its role in enhanced oil recovery. The aspects reviewed in this article include a brief comparison of conventional CO2 injection and carbonated water injection and the benefits thereof. Solubility of CO2 in water, brine and oil phases is discussed in detail with valid correlations. A brief history of the development of CWI in the laboratory and field information is captured from 1905s to the present followed by the possible mechanisms and principle of CWI reported by various authors. This article also captured the latest findings on the beneficial effect of hybridizing CWI with smart water technologies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction; CO2 injection and its limits
From the information available in public domain, it is unambiguous that tertiary or enhanced oil recovery (EOR) through CO2 flooding is a proven techno-economically efficient method of recovering additional oil from conventional light, medium as well as heavy oil reservoirs (Gao et al. 2010). The factors that contribute to the oil recovery are mainly related to lowering interfacial tension, swelling oil droplet volumes, reducing oil viscosity, and by mobilizing the lighter components of the oil, which are elaborated as follows.
CO2 coexists as gas and liquid in a single phase at its critical pressure (Pc) and temperature (Tc) (1070 psia and 87.9 °F, respectively). Above Pc and Tc (supercritical state) though its density is close to that of the liquid, its viscosity remains close to the viscosity of its gaseous phase (0.05–0.08 cp). These properties help in oil recovery by reducing the overall viscosity of oil and also reducing gravity override problem, compared to gaseous CO2 injection (Jarrell et al. 2002).
CO2 flooding is categorized mainly into two methods, miscible and immiscible flood. This classification depends on the reservoir rock and fluids properties at reservoir temperature and pressure conditions. When the reservoir is deep enough and the reservoir pressure exceeds the minimum miscibility pressure (MMP), CO2 and reservoir oil reach miscibility condition through a mechanism classified as multiple-contact miscibility (MCM) which is a dynamic and time-dependent process. This consists of vaporization gas-drive (in which intermediate hydrocarbon molecules vaporize into the CO2) and condensation gas-drive process (in which a portion of the injected CO2 dissolves into the oil (Merchant 2010). This combined mass transfer process helps CO2 and oil to become a single phase and drive the oil very efficiently (Diaz et al. 1996; Ju et al. 2012). If the oil is extremely light and of ultra-low viscosity, the first contact miscibility is also possible. Thus, miscibility mechanism depends predominantly on the crude oil composition (Kantzas et al. 2012). Though direct relationship between reservoir rock properties and CO2 miscibility is not yet established, reservoir rock properties can have a significant impact on overall performance of CO2 flooding performance, primarily attributed to the heterogeneity, permeability and overall porosity of the rock. Takahashi et al. (2003) demonstrated that CO2 breakthrough can occur much earlier in carbonates than in sandstones due to greater microscopic heterogeneity of carbonate rocks. This statement is supported by Bikkina et al. (2016) through a series of miscible CO2 coreflood on oil wet and water wet (whole and fractured core plugs). They observed that the miscible CO2 flooding recovered nearly 100% of the oil, in oil-wet homogeneous cores, while insignificant amount of oil was recovered from water-wet fractured cores. Under similar poro-perm conditions, they observed that miscible CO2 flooding performed significantly better in the oil-wet cores compared to the water-wet cores. During a field simulation and implementation study, Bhatti et al. (2019) have emphasized on the importance of reservoir wettability, heterogeneity and permeability properties and strongly suggested for inclusion as the screening criteria for miscible CO2 flooding.
There are also after effects of miscible CO2 flood which needs to be considered too. Reduction in porosity was observed in both secondary and tertiary CO2 flood modes, while permeability was seen to improve in tight carbonate rocks. Rock dissolution at high CO2 concentration in water phase impacts not only the poro-perm characteristics but also the wettability characteristics. The injection of super critical CO2 in tight carbonate rocks not only improved overall rock permeability but also changed the wettability toward a more water-wet state which favored improved recovery (Zekri et al. 2013). On the contrary, a recent work of Wang et al. (2019) evidenced that the CO2 flooding in sandstone rock may result in reduction in permeability with almost no change in porosity. The degree of permeability damage is found to be more after CO2-WAG flooding than that after only CO2 flooding. The damage analysis through PSD (pore size distribution) studies revealed that fines migration and blocking of small pore throats are responsible for the permeability damage.
Numerous laboratory and field studies proved that miscible gas flooding is way more efficient in terms of incremental oil recovery than immiscible flood (Agustssen and Grinestafr 2004; Cobanoglu 2001; Gao and Towler 2012; Sharma and Clements 1996). During the last two decades, miscible CO2 displacements have been well developed and applied, particularly in the US and some Chinese fields (Jishun et al. 2015; Liu 2013). The first ever CO2-miscible flood field pilot in Permian basin (Western Texas fields) was conducted in 1976. Since then, many large-scale CO2-miscible flood projects have been implemented with appreciable economic success (Verma 2015). Based on the published documents, the current “best practices” CO2-EOR technology generally recovers CO2 incremental oil of around 5–15% of OOIP from North American fields (Gao and Towler 2012), depending mainly on the reservoir rock and fluid characteristics and the flood pattern. Healy et al. (1994) reported about 9% incremental oil recovery from miscible flood recovery from Sacroc field, whereas incremental recovery resulting from the miscible CO2 flood is reported to be more than 15% of the OOIP in San Andres Unit (Stiles and Magruder 1992). This incremental recovery is below the industries expectation of > 80% of overall recovery (Merchant 2010), and thus leaves behind a large volume of oil in the reservoir. Analysis show that the major causes of below expectation recovery are due to (a) insufficient injection of CO2, (b) poor sweep efficiency, (c) poor displacement efficiency, (d) lack of CO2 contact with remaining oil resources and (e) inadequate management control (Verma 2015).
As reported by Summapo et al. (2013), reservoir heterogeneity is the major cause of poor CO2-miscible flood performance due mainly to higher unstable flood front and an early breakthrough of injected gas. This may result either from formation depositional sequence which defines the path preference or from natural fractures which facilitate CO2 channeling due to the high mobility of supercritical CO2 fluid. Injecting CO2 alone is found to cause early breakthrough of CO2 along fracture direction (Figuera et al. 2014), which necessitates corresponding modeling of injection/production adjustment strategies in advance (Luis et al. 2016). It is suggested that flood breakthrough in multilayered, multi-well CO2 flood system can be identified through pulse-neutron production logs combined with permeability from the magnetic resonance (Aryani et al. 2011). Unlike miscible flooding, gas breakthrough is more predominant in immiscible flooding in which gas moves through high permeable channels as evidenced in Yaoyingtai oil field (Yuncong et al. 2014). The conclusions were drawn from the changes in daily oil production, gas production rate, CO2 content and GOR from 40 oil data.
Because of the reasons described above, direct gas injection and water-alternating-gas (WAG) injection might lead to commercial failure in heterogeneous layered and fractured formations to meet the targeted incremental oil within the projected economics. In addition, poor sweep implies lower storage capacity of CO2, which is an additional objective of CO2 injection, popularly known as carbon storage and sequestration (CCS) aiming at reducing the greenhouse effect.
Alternative method: Carbonated Water Injection (CWI)
To counter the issues mentioned above, carbonated water injection or CWI, that is injecting water saturated with CO2 instead of direct injection of CO2, is gaining rapid attention. Though this technique was first conceptualized in the 1930s, serious investigation began in the 1970s. It is found that because of lesser difference of viscosity and density with the crude oil, CWI has better sweep efficiency than supercritical CO2 (Sohrabi et al. 2008). Moreover, in reservoirs that have been water flooded, CWI can mitigate the negative effects of water shielding due to mixing with the resident water (Riazi et al. 2009). In the case of direct CO2 injection, diffusion of CO2 would take longer time because of low sweep efficiency and gravity segregation effects (Solomon 2007).
Technically, two major differences can be cited between CWI and conventional CO2 injection or water-alternating CO2 gas (WAG) injection. Firstly, the amount of CO2 that might be injected to the reservoir at certain temperature and pressure will be solubility limited, which implies no separate CO2-rich phase in the reservoir. The other point is that the displacement efficiency would not depend on the minimum miscibility pressure (MMP), because it is controlled by CO2 mass transfer between oil and CW resulting in no-transition zone in CWI (Dong et al. 2011).
CWI can be a very attractive method for CO2 sequestration too, which is becoming a hot topic in terms of environmental issues and can bring benefits in the form of reduction in greenhouse gases. It is reported by IPCC (Metz 2007), that to avoid the fast climate change and its side effects, global CO2 emissions should be cut by 50–80% in 30 years. Therefore, CO2 capturing and storage or CO2 sequestration (CCS) has great importance that should be considered.
In conventional CO2 sequestration processes, CO2 floats under the cap rock and there are possibilities of leakage through the micro-pores of the cap rock. This sometimes limits the number of reservoirs available for CO2 sequestration (Herzog 2000). On the contrary, CWI sequestration can be implemented without volume limitations with lesser risk of gas leakage through cap rock. Having a higher density and viscosity than resident water due to CO2 dissolution (Hebach et al. 2004), carbonated water will sink into the bottom of the reservoir, eliminating the risk of buoyancy-driven leakage which is usually caused by bulk phase gas injection (Burton and Bryant 2009). In addition, CO2 exists as a dissolved phase rather than a free phase, thus reducing the issues which are caused by the poor sweep efficiency and gravity segregation which are the drawbacks of typical direct gas injection. As a result, CWI provides a safer and better method of CCS compared to direct injection of CO2 (Anchliya et al. 2012).
Basic theory and fundamentals for CWI
CO2 phase behavior
The CO2 phase behavior is highly dependent on temperature and pressure of the reservoir. Figure 1 represents a phase diagram illustrating that CO2 injection can be sustained under different forms such as liquid, gas or supercritical fluid, all of which depend on the two factors P and T. Other properties that are affected by P and T include viscosity, where rising temperatures can reduce it significantly. Additional properties include gas compressibility factor and density. It is recommended for CO2 to be in the gaseous or supercritical form while injected. To specify, in supercritical condition, useful characteristics of gas and liquid phases coexist and its behaviors are similar to gaseous CO2 and liquid CO2 under certain circumstances. A supercritical CO2 provides the characteristics of liquid because of its similarity in density to the liquid state.
Carbon dioxide (CO2) pressure–temperature phase diagram (Whitson and Brulé 2000)
Solubility of CO2 in water, brine and oil phases
The amount of dissolved CO2 is one of the critical factors to every application including the CWI because it directly affects the data variation, resulting in its application efficiency. Therefore, the solubility of CO2 in water, brine and oil must be ascertained at the condition of its application. Several CO2 solubility studies in water and high- and low-salinity brines have been conducted for an extensive range of temperatures, pressures and ionic concentration in conjunction with different reservoir properties by many researchers (Bamberger et al. 2000; Chang et al. 1998; Chapoy et al. 2004; Gui et al. 2017; Liu et al. 2011; Valtz et al. 2004).
Solubility of CO2 was investigated both in pure water and in diverse brine solutions (Mg2+, K+, Na+, Ca2+, Cl−, and SO42−) at several different temperatures and at pressures up to 200 MPa (Duan et al. 2006). Solubility of CO2 and other injection gases in water and NaCl solutions is modeled at varying temperatures (0–350 °C), pressures (0.1–150 MPa) and ionic concentrations (0–4.5 mol/kg) by Mao et al. (2010) using Helmholtz free energy model, which can be extended to different ranges of the variables parameters. The correlation proposed by Enick and Klara (1990) on the CO2 solubility in brine at surface condition is found to be applicable at subsurface formation conditions too, including consideration of the dissolved solids in the brine. The major conclusion drawn from this work is that solubility is solely dependent on total dissolved solids, regardless of the salt type. Overall conclusion drawn from the above studies is that there could be a significant influence of brine salinity on CWI flooding performance; hence, it is necessary to execute solubility test with reservoir brine at reservoir temperature and pressure prior to performing injection.
Solubility Correlations
One of the most important parameters that affect gas solubility is Henry’s constant. It is defined as the limit of carbon dioxide’s fugacity to CO2 water ratio. \(K_{{{\text{H,CO}}_{2} }}\) has the dimension of pressure (Eq. 1) (Diamond and Akinfiev 2003).
where \(x_{{{\text{CO}}_{2} }}\)—the molar fraction of CO2 in water, \(f_{{{\text{CO}}_{2} }}\)—fugacity of CO2, \(K_{{{\text{H,CO}}2}}\)—Henry’s constant.
To verify the above correlation experimentally, Chang et al. (1998) studied the properties of carbonated water binary system including the CO2 solubility in water and brine and observed that the viscosity of CO2-saturated water remained unchanged. For solubility measurements, they used Eq. 2. given as follows for the estimation of CO2 solubility in distilled water and later adjusted to the salinity effect of the brine (Kechut et al. 2011) and found positive agreement between the measured values and the calculated values of solubility of CO2, implying that the correlation Eq. 2 is a reliable one.
where Rsb: solubility of CO2 in brine of salinity S (scf/STB), Rsw: solubility of CO2 of water (scf/STB), S: salinity of brine in weight % of solid and T: temperature (°F).
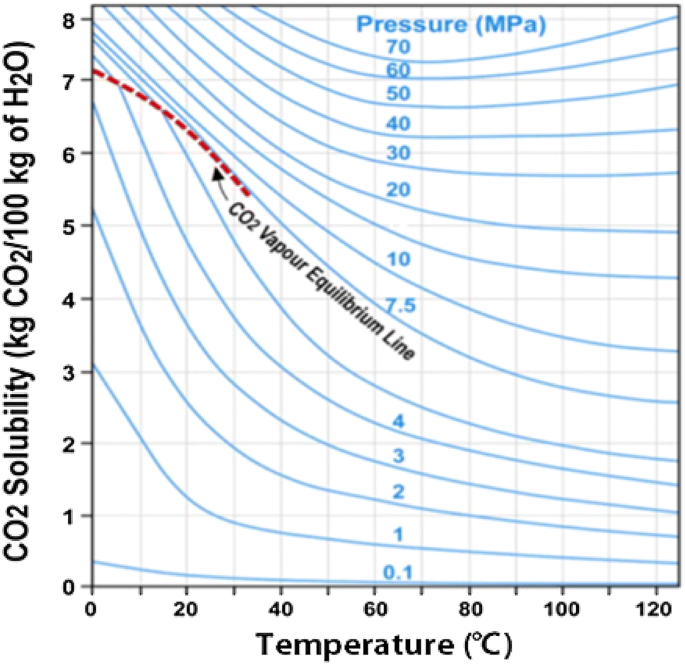
Although it was thought that CO2 solubility increases as pressure increases, however, below 65 °C, solubility decreases as temperature increases (Fig. 3). It can also be seen that the solubility goes up with increasing temperature when P > 30 MPa, while the solubility decreases with increasing temperature when P < 30 MPa (Perkins 2003).
Carbonate (CO32−), bicarbonate (HCO3−) and carbonic acid (H2CO3) are the main three ions that exist once CO2 is made soluble in water. Additionally, there are other possible ions which can contain inorganic carbon as well, but these concentrations are much less than that of the main three ions, so they are usually ignored during calculation (NaCO3−, NaHCO3, Na2CO3, MgCO3, MgHCO3+, etc.). For example, the reaction of an equilibrium state established by carbonic acid (H2CO3) can be expressed as (Eq. 3):
The relationships which demonstrate the relative carbonate ions concentrations are two mass balance equations and mass action equation given as follows:
Where the total dissolved carbon is obtained in analytical approach, log K1 and log K2 are relevant log equilibrium constants for the equilibrium and m and a are molality and activity of aqueous species, respectively.
The partial pressure of carbon dioxide and dissolved carbonate ions in solution can be related to each other as the following relationship:
With an increasing CO2 gas partial pressure, CO2 will be dissolved into the fluid, while with decreasing CO2 partial pressure in the gas CO2 will be evolved from the fluid. If the fluid turns more alkaline, CO2 will immediately start to evolve from it. The more the CO2 dissolves in the fluid, the more acidic it will become. Thus, more acidic solution might result in the dissolution of the rock, especially carbonate minerals from the rock surfaces (Perkins 2003).
Another important solubility parameter which may govern the success of CWI as regards phase transfer is the CO2 solubility in crude oil. The important properties identified are crude oil composition, temperature and saturation pressure (Emera and Sarma 2006; Jamaluddin et al. 1991; Srivastava et al. 1995).
Some mathematical correlations were established for CO2 solubility estimation in crude oil. However, these relations are restricted to certain ranges of fluid properties and conditions. Therefore, additional research on CO2 solubility and CO2-associated factors that affect oil swelling is required to find out the processes and mechanisms related to CO2-based EOR methods.
History of CWI laboratory work
In the late 1940s, initial research on CWI was performed by Monteclaire Research from the Oil Recovery Corporation (Adiputra et al. 2018). They reported that it was possible to reduce the residual oil saturation up to 15% of pore volume when carbonated water was flooded after conventional water flooding. From 1948 to 1952, Earlougher Engineering conducted carbonated water flood experiments with freshly sampled cores at low pressure ranges (800–1000 psig) with oil API gravity ranged from 28 to 50 API. Results indicated that CWI delivered a range of residual oil saturation depending on oil gravity. Additional recovery on tertiary mode after waterflood was achieved for light oil (up to 26%), whereas for heavier oil the additional recovery was as low as 2% of the pore volume (Lake et al. 1984). The mechanism behind oil recovery was accounted for the changes in both rock and fluid properties. Some experiments suggested that the result of additional oil recovery is due to a series of alterations in both the rock and fluid characteristics, not only relying on the oil swelling effect (Lake et al. 1984).
Martin (1951) reported that 12% oil recovery enhancement was resulted through carbonated water injection. He earlier pointed out that the recovery improvement might be correlated with the amount of carbonation in fluids (Martin 1950). Based on the work of Saxon Jr et al. (1951), Johnson et al. (1952) examined the effect of CWI on oil recovery and compared with brine flood. Using two different oil viscosities (1.42 mPa.s and 2.86 mPa.s), they concluded that CWI method could recover 15–25% of residual oil, while no recovery was observed using brine injection. The results also pointed out that oil recovery using CWI method is temperature dependent. At lower temperatures, the recovery was higher, due to higher solubility of CO2 (Fig. 2). High solubility of CO2 would cause further expansion in oil volume, thus resulting in better oil recovery. This observation was supported by coreflood experiments conducted by Holm (1959). The results show that keeping all parameters same, whereas 21% incremental oil recovery was achieved by CWI at 21.1 °C, the incremental recovery was limited to 19% at 37.8 °C, establishing a relation between reservoir temperature, CO2 solubility and incremental recovery.
CO2 solubility in water depends on temperature and CO2 pressure (Perkins 2003)
Several flooding experiments with sand pack were conducted for CWI by Falls (1986); Gorell and Falls (1986) and earlier by Van Dijk (1965). Conclusion drawn from these articles is that CW could bring about 12% to 23% of additional oil recovery after conventional water flooding, depending on oil viscosity. Panteleev and Tumasyan (1972) conducted a novel study on wettability changes in porous media. They observed faster water imbibition and higher oil recovery for CW compared to fresh water. CW imbibition produced 37.3% of oil compared with 26.5% oil recovery by fresh water imbibition. They also emphasized that higher CO2 concentration in water would result in further increase in oil recovery and imbibition rate.
Flumerfelt et al. (1993) continued the oil recovery studies from Perez et al. (1992). Kerosene oil, crude oil and low-permeability dolomite rock cores were used. They also studied the influence of surfactants during carbonated water injection. The results showed that CW with surfactant led to the recovery of additional 50% of residual oil, while 20% incremental oil was recovered with CW without surfactant, comparing with conventional WF. During the CWI experiment by Asghari et al. (2009), 16.9% of additional original oil in place was recovered from the consolidated core samples and around 14% of OOIP while using sand packs.
Since 2006, extensive research was conducted on CWI for oil recovery by the Herriot Watt Institute of Petroleum Engineering Centre. These experimental and numerical studies were carried out by different techniques and methods including the following: micro-model, core flooding, mathematical modeling and numerical simulations. They reported that the improved oil recovery through CWI was due to higher sweep efficiency, viscosity reduction, oil swelling and thus reconnection of the isolated oil droplet which all are attributed to better CO2 diffusion. Two samples tested resulted in 23.8% and 8.8% increment of oil recovery. They also suggested that the fundamental process is related to interactions between the fluid/fluid and fluid/solid during CWI. The studies suggested that significant improvement in oil recovery could be possible in both secondary and tertiary recoveries by using CWI (Sohrabi et al. 2011, 2015). Riazi et al. (2011) set up a mathematical model to investigate pore-scale mechanisms during flooding, which revealed that CWI might be an attractive method to store CO2 in certain reservoirs as well. Kechut et al. (2010) focused on maximizing CO2 concentration in CW and was able to enhance the concentration up to around 46%, which was significant in view of sequestration. Al Mesmari et al. (2016) conducted a series of direct visualization experiments using glass micro-models and also coreflood experiments, and the data were used for history matching and simulating the performance of CWI and identified the key parameters controlling the phase behavior of crude oil and CW. To account for the formation of the new phase, three-phase flow functions and relative permeability were incorporated in the model and measured the mass transfer and multi-phase flow behavior during CWI.
More recently, Zou et al. (2018) established that CWI was more effective than water flooding in tight reservoirs. Coreflood experiments conducted on tight core samples from Ordos Basin showed that CWI promoted higher oil recovery in comparison with WF in both secondary and tertiary injection modes by 20.3% and 11.3%, respectively. Qu et al. (2018) also conducted recovery experiments on tight sandstone core samples with a permeability range of 0.1 to 0.2 mD, and compared CWI recoveries with CO2-WAG and surfactant flooding. They claimed lesser incremental recovery for surfactant (2.05%) and WAG (4.53%) compared to CWI which resulted in 7.22% incremental oil recovery.
History of CWI Field work
In 1957, the first field test for CWI was conducted in Allegany County, New York. The outcome of using CWI was reported as successful. The production rate changed significantly from 92 to 1260 barrels/acre/year. This was followed by several other successful implementations of CWI field trials (Christensen 1961; Hickok and Ramsay 1962). In 1958, injection of CO2 and water in the same tubing was begun. Production increased in 1959 to 123,000 STB, higher than the total oil produced from 1905 to 1934. Additionally, CWI resulted in an estimated 37% increase in net oil production compared to conventional water flooding in the same reservoir.
Another large-scale CWI implementation in the Domes Unit on 90 wells reported techno-economic success. During the simultaneous CO2 and water injection, about 30% of pore volume equivalent of CW was injected, followed by water flooding. The reported incremental oil increment was about 9% of OOIP (Riazi et al. 2011).
Possible mechanisms and principle of CWI
Since 1950, carbonated water flooding has been considered as a promising flooding technique, because of enhanced mobility of oil when high-concentration CO2 is dissolved in the flood water. Mobility (M) is explained as the ratio of permeability of a porous medium (keff) to that of fluid’s viscosity (μ). If there is decrease in oil viscosity, the oil mobility will be higher and vice versa. It is established that the mechanisms of CWI for EOR are on the basis of changing the physical properties of oil. The proposed mechanisms so far are as follows: (1) CO2 dissolution in oil leading to oil swelling and subsequent viscosity reduction. This in turn leads to reconnection of the isolated oil droplets and fluid flow diversion (sweep efficiency improvement), (2) evolution of solution gas from the oil caused by CO2 dissolution, (3) wettability alteration due to CO2 mass transfer (Sohrabi et al. 2015), and (4) oil swelling also leads to improved relative permeability to the oil, thus providing a better mobility (Riazi et al. 2009).
CO2 solubility in oil phase is one of the main parameters that affect the EOR performance. This is because it directly affects the oil viscosity and swelling, which enhances oil production (Abedini and Torabi 2013; Mosavat 2014). In addition, one of the key trapping mechanisms which govern CO2 storability in high-salinity environment is its solubility, where the majority of CO2 is trapped by dissolving in the formation brine (Ennis-King and Paterson 2005; Lindeberg and Wessel-Berg 1997).
Perez et al. (1992) carried out studies on CO2-saturated water imbibition at different pressures and temperature. In these experiments, CO2 was considered as the prime factor that causes an increase in the recovery of oil, compared to other factors normally considered as the major reason for oil recovery in conventional water flooding process. The authors proposed several possible mechanisms such as an increase in oil mobility, increase in carbonate core permeability, oil swelling and a gas-drive mechanism.
In general, it is found that all possible contributions of CWI have favorable impact on oil recovery. For instance, oil swelling has two effects. One of them is the swelling factor of oil which is inversely proportional to residual oil left in the formation. The second is that more volume will be occupied by swollen oil droplets, which means higher relative permeability because of enhanced oil saturation and further reduction in mobility ratio. In CWI, CO2 is spread in the reservoir more evenly and thereby avoids the CO2 breakthrough, thereby enhancing the sweep efficiency throughout the formation. Wettability alteration is also a possible contribution by CO2-saturated water flooding which can shift the surface to a more water-wet state (Dong et al. 2011).
Oil swelling and CO2 mass transfer
Several researchers have conducted experiments on CO2-saturated water flooding to study the impact of CO2 diffusion and the recovery process, including the studies on the performance of certain parameters that affect CWI efficacies. In addition, the effect on mass transfer during carbonated water injection is also studied. There are several proposed mechanisms in terms of CWI that will be discussed in the following chapters.
An experimental study on sand packs using medium-viscosity crude oil and CWI as flooding fluid was performed by Dong et al. (2011). Results indicated that injecting CO2-saturated brine performs better as a displacing fluid both in secondary and tertiary modes with a reduction in residual oil saturation from 0.03 PV to 0.35 PV, compared to conventional water flooding. The mechanism of such successful outcome is explained by CO2 migration into the oil phase from the water phase without forming a separate CO2-rich phase. The mass transfer of CO2 into oil phase is substantial since it is more soluble in the oil than water (3 to 7 times higher solubility) under the same pressure and temperature conditions. Due to high CO2 mass transfer into the oil phase, oil becomes less viscous and thus enhances the oil–water mobility ratio. The relative permeability of oil might also improve due to the oil swelling. All these subsequent processes together might have resulted in greater or improved oil recovery than conventional water flooding.
In 1981, extensive PVT studies were performed by Miller and Jones in order to determine how oil physical characteristics might be changed with CO2 saturation. They concluded that whereas the viscosity of oil without CO2 saturation increases with increasing pressure, both density and viscosity of CO2-saturated oil significantly decrease with increasing pressure (Miller and Jones 1981). In order to investigate the effect of CWI as a method of EOR and storing CO2, Sohrabi et al. (2011) performed laboratory core flooding experiments focusing on oil viscosity, rock wettability and brine salinity. This study reported that in both secondary and tertiary recovery modes, oil recovery was increased. When compared with tertiary recovery (31% reduction in Soi.), the secondary recovery (35% reduction in Soi) gave more and earlier incremental oil recovery. Through this study, they explained that the main mechanism is mass transfer of CO2 into oil, since CO2 has higher solubility in oil than in water. Thus, the viscosity of oil would be decreased bringing the oil mobility to a more favorable range.
Earlier, Nevers (1964) developed a mathematical model to examine the influence of changing oil phases during CWI, which revealed that CO2 dissolution and resulting reduction in oil viscosity are the main parameters that can explain the oil recovery mechanism effectively. Recently, Riazi et al. (2011) developed another mathematical model where it demonstrated the dynamic process during oil swelling and its redistribution into oil at pore-scale system. Their results pointed out that the solubility and molar density of CO2 in water are the most important factors influencing oil swelling during CWI (Mosavat 2014). The investigation of CWI performance on micro-model showed that the oil recovery is optimized due to viscosity reduction, while oil swelling is caused by CO2 mass transfer between brine and the oil-in-place phase.
Riazi et al. (2009) performed experimental studies on the CO2-enriched water flooding as an EOR method. The study consisted of direct flow visualization by high-pressure transparent porous media followed by mathematical modeling. They noticed that during CWI, the oil started to swell due to CO2 mass transfer into oil through CW. The CO2 diffusion into oil caused reconnection of trapped oil ganglia which started to mobilize subsequently. After performing the second WF, the residual oil saturation was 33.39%, indicating a 15.74% additional oil recovery after CWI. The data showed that CWI could add up to 16% additional oil recoveries. They assumed that the main mechanisms by which CWI recovers residual oil are enhanced sweep efficiency due to oil swelling and reconnection of the isolated oil droplet and the subsequent redistribution of fluid in consequence of CO2 diffusion. Mosavat (2014) presented that CWI could improve the conventional water flooding by recovering around 19.0% of OOIP during the secondary stage and 12.5% of OOIP in the tertiary stage. This result shows that the CWI might be more effective over conventional water flooding for residual oil recovery from oil reservoirs.
Studies performed by Sohrabi et al. (2008) on glass micro-models show that oil can be swelled even by 105% with CWI (Fig. 3). Figure 4 shows the time dependency of oil swelling, shown in three different time steps, strongly indicating occurrence of oil swelling during CWI. The number of areal oil phase pixels according to time is plotted in Fig. 5, where the estimated increase in swelling was found to be around 22.4%. These visual evidences supported the earlier theories of CO2 mass transfer from CW to oil leading to oil swelling and viscosity reduction resulting in: (a) increasing oil relative permeability by reconnection of isolated oil ganglia and (b) improving sweep by diversion of flood water. Viscosity reduction mechanisms also play an important role in intermediate and viscous oil systems due to improved mobility ratio.
Comparison of the oil volume before and after CWI. Image analysis observed that a massive 105% oil swelling was captured by image analysis (Sohrabi et al. 2008)
Oil swelling due to CO2 diffusion through CW into oil phase (Sohrabi et al. 2008)
The oil droplet volume vs time (Sohrabi et al. 2008)
Wettability Alteration
Sohrabi et al. (2008) performed fluid flow studies at high-pressure condition with two-dimensional glass micro-model and observed that as more CW was injected, more amount of CO2 diffused into trapped oil that was left in pores after normal waterflood displacement. The micro-model was used to investigate the wettability effect. As the surface became more water-wet, the water thickness on the pore surface was seen to increase. The capillary forces changed the fluid interfaces shape in the porous medium, and based on this, they proposed that the change was due to decreasing interfacial tension at oil/brine interface and the wettability alteration toward water wet condition (Sohrabi et al. 2009).
Sohrabi et al. (2015) also studied the fluid interface shape to examine the change in wettability of rock surface. Figure 6a shows that the oil phase has been broken apart and spread out as the water film surrounding the oil ganglia, which means more oil-wet phase after several hours of normal WI. However, after CWI (Fig. 6b), the oil/water interfaces demonstrated a more spherical shape, which implies more water-wetness (less oil-wet). The capillary forces altered the fluids’ interface shape. The fluid interface is determined through wettability and interfacial tension between oil and water. Since the IFT is not so prominent due to CO2 dissolution, wettability alteration ranks higher as the possible cause of capillary forces alteration. The explanation for wettability alteration is given as: Due to decreases in the aqueous phase pH (in CW), changes in surface charges on the water/oil and water/rock interfaces occur leading to subsequent changes in the wettability of the system. Also, this alteration might be caused by dissolution of CO2 in oil and destabilizing the polar components of oil. The destabilized polar components can disperse through the water layers and adsorb onto the rock surface which increases the tendency of the pore system to become water-wet.
A magnified image of the micro-model demonstrates two different conditions of wettability: a more oil wet after WI and b less oil wet after CWI (Sohrabi et al. 2015)
Evolution of the New Phase
Creation and growth of new gas phase from solution gas within the oil was first seen in the micro-model tests when CW was injected for an extended period of time in dead-oil experiments (Mosavat 2014). Its mechanisms for additional oil recovery are similar to that of oil swelling but to a much larger extent by: (a) oil displacement and reconnection of trapped oil and (b) restriction of water flow path and its diversion toward un-swept parts of the porous medium which results in improved oil recovery. Figure 7 demonstrates micro-model images of the primary WI and tertiary CWI. The CWI was able to promote higher oil recovery and reduces residual oil saturation after primary WF. Results indicate about 7.7% of residual oil saturation has been reduced in the tertiary mode with CWI when compared with the primary water flooding.
Chronological progress of the primary WI followed by tertiary CWI (Mosavat 2014)
Figure 8 demonstrates the ability to capture the trapped oil ganglia in tertiary CWI for recovering isolated oil after primary WI. In Fig. 8a, the residual oil sticks to the oil-wet surface and becomes trapped oil, and after tertiary CWI (Fig. 8b) less residual oil in grains is seen, which means improvement in sweep. This is because, injection of carbonated water and hence the mass transfer of CO2 from brine to oil brings the changes from an oil-wet to water-wet wettability, which helps in the enhancement of oil recovery. The process behind this is the dissolution of CO2 into the oil system which leads to reduction in viscosity and oil swelling, thus improving the sweep efficiency and finally increasing the oil recovery.
Trapped oil production during CWI. a Before tertiary CWI and b after tertiary CWI (Mosavat 2014)
The above images are clear evidence of the expansion of isolated oil ganglia and their reconnection which could be attributed to be the main principle for improvement in oil recovery with CWI. When the system is first water flooded, the oil saturation reaches Sor conditions. During the subsequent injection of CW, mass transfer occurs between CO2 and residual oil in place, causing an expansion of oil particles resulting in enhanced oil recovery.
Reaction between rock and CW: Rock dissolution process
The presence of CO2 in the formation leads to various chemical reactions between rock, aquifer and CO2 which might affect the petro-physical properties of the reservoir formation. The effect of injected CO2 depends on several factors such as the rock chemistry, injected fluid type, injection strategy and the physical conditions of the reservoir. It is known that when CO2 is injected into carbonate formation, it leads to the dissolution of carbonate minerals because of the acidic nature of CW. Dissolution of the rock leads to an initial increase in formation permeability; subsequently, transportation of these minerals and later precipitation lead to decrease in permeability and effective porosity (Bowker and Shuler 1991; Grigg and Svec 2006; Shiraki and Dunn 2000; Wellman et al. 2003).
Izgec et al. (2008) observed that with the injection of CO2, porosity of core plug is changed with corresponding changes in permeability. CaCO3 and MgCO3 are the carbonate minerals that can easily react with carbonated waters. The reactions that can occur with carbonated water are:
When CaCO3, MgCO3 or FeCO3 exists in the rock, the water-soluble bicarbonates might form by the following reactions:
The aforementioned reactions might be the reason for rock dissolution in the carbonate reservoir matrix. The rock dissolution will definitely result in the change in petro-physical properties of the rock by creating new flow paths and increasing the rock permeability. However, the presence of CO2 in sandstone rocks might lead to the reduction in permeability because of dissolution of released cementing particles and later precipitation. If the released particle sizes are more than the pore throat size, they might obstruct the pore throats, thus reducing the rock permeability (Sayegh et al. 1990). The other possible reactions are:
where M2+ = generic carbon, l = liquid, aq = aqueous, s = solid.
Several experimental studies have been performed to examine the changes in the rock petro-physical properties by the injection of CO2-saturated water (Sayegh et al. 1990). Kono et al. (2014) conducted experimental studies to show how the carbonated water can change the carbonate rock properties in the reservoirs of Middle East, through core flooding at reservoir conditions, measuring porosities and permeabilities. The carbonate mineral dissolution studies were conducted on scanning electron microscope (SEM) and liquid chromatography also. Results obtained (without the compaction effects) showed an increase in porosity by a 3.6% in the first 50 pore volume injected, and a further increase in up to 6.0% from 50 to 100 pore volume injected.
Further Development; Hybrid CWI
With the advent of low-salinity water and smart water flooding techniques, CO2-saturated water flooding seems to be an even more efficient EOR option. The synergic effect of these two EOR techniques could be even more promising. Alizadeh et al. (2011) investigated the benefits of carbonated smart water injection (CSWI) in Berea sandstone core plugs above miscible pressure. Distilled water was used to make the aqueous phase where it contained 2 wt% of CaCl2, 12 wt% of NaI. In addition, 0.01 wt% of NaN3 was used as biocides. The density of brine was measured as 1.116 g/ml at 20 °C and atmospheric pressure. The CO2-saturated brine contacted with all sizes of isolated oil blobs in the core. Figure 9 shows the comparison between the CT images of core slices and their respective oil saturation after brine imbibition to those at the end of the first and second carbonated smart brine flooding processes. Results indicated that the first CSWI could recover around 51% of the trapped oil and reduce the oil saturation from 41% to about 20%. This is a noticeable reduction in the residual oil in place. The third row in Fig. 9 demonstrates the oil saturation at the end of the second CSWI process, and not much difference in oil saturation could be seen. The average residual oil saturation was about 17.3%, leading to an additional recovery of about 4% OOIP compared to the first stage. Therefore, it was not recommended to reach high differential pressure drop as it will not reduce oil saturation further. Another point to consider is the economical and operational difficulties that would increase by reaching high levels of CO2 saturation in brine without significant advantage as the bulk of oil recovery takes place in the minor saturation levels of CO2 in brine.
CT images showing oil saturation distribution. First row: oil saturation distribution after imbibition; second row: first saturated brine flooding; third row: second saturated brine flooding (Alizadeh et al. 2011)
The slice-averaged oil saturation along the length of the core at the end of fresh brine imbibition and CO2-saturated brine flooding at 90 and 180 psig inlet pressures are shown in Fig. 10. The difference between the two top distributions again indicates the success of the first CSWI process in promoting higher oil recovery (Alizadeh et al. 2011).
Slice-averaged residual oil saturation (Alizadeh et al. 2011)
In a recent work by (Kilybay et al. 2016), CSWI was performed on three carbonate core plugs from Abu Dhabi. The core plugs were flooded with three fluids in the following order: (1) seawater, (2) seawater with four times sulfate (SW4S) and (3) SW4S saturated with CO2 (carbonated smart water). Results indicated that smart water saturated with CO2 can recover a significant amount of immobile oil left after tertiary recovery with SW4S smart water. Whereas tertiary recovery with smart water injection resulted in 4.8–9.5% additional recovery, carbonated smart water injection in quaternary mode resulted in 5.7% to 13.6% additional oil recovery. This is attributed to the impact of CO2 mass transfer from brine to oil causing viscosity drop, local flow diversion and trapped oil swelling. Carbonate dissolution and pore enlargement were also proven through NMR porosity and ICP-MS studies. Figure 11 exhibits the plots of displacement efficiency of brine flooding versus pore volume (PV) injected for three different core flooding experiments conducted. From this study, it is established that CSWI could be a novel and promising EOR technique among the latest EOR methods. It reduces the requirement for high-pressure system, the problems of gravity segregation and poor sweep efficiency.
Coreflood recovery results on secondary, tertiary and quaternary modes (Kilybay et al. 2016)
Conclusion
This paper comprehensively reviewed CO2 EOR techniques in its primitive and modern form including immiscible, miscible and dissolved forms. The salient features are:
CO2 injection in heterogeneous layered and fractured formations might lead to techno-economical failure due to poor sweep and increasing OPEX.
CWI promoted higher oil recovery in the majority of experiments due to CO2 mass transfer to oil which consequently resulted in lowering oil viscosity, swelling of trapped oil, enhanced flow diversion and improved sweep efficiency.
CWI in high concentration did not provide a significant advantage over low concentration.
In certain oil fields, CWI injection improved the well’s water intake rate and was successfully deployed as a well stimulation technique.
Results from carbonated smart water injection proved that an initial injection provided up to 51% additional recovery of residual oil in place, while a second injection only recovered 4%.
A novel method of injecting carbonated water saturated with seawater that contains 4 times sulfate proved to be successful and provided up to 13% additional recovery in quaternary mode, emphatically proving the impact of CO2 in reducing residual oil saturation even at low oil saturation.
References
Abedini A, Torabi F (2013) Parametric study of the cyclic CO2 injection process in light oil systems. Ind Eng Chem Res 52(43):15211–15223
Adiputra E, Mucharam L, Rahmawati SD (2018) Experimental evaluation of carbonated water injection to increase oil recovery using spontaneous imbibition. In: Negash B et al (eds) Selected topics on improved oil recovery. Springer, Singapore, pp 33–44
Agustssen H, Grinestafr G (2004) A Study of IOR by CO2 Injection in the Gullfaks Field. Offshore Norway, SPE, p 89338
Al Mesmari A, Mahzari P, Sohrabi M (2016) An improved methodology for simulating oil recovery by carbonated water injection: impact of compositional changes. SPE-181630
Alizadeh AH, Ioannidis M, Piri M (2011) CO2-saturated brine injection: an effective process for mobilization and recovery of waterflood residual oil. International Symposium of the Society of Core Analysts, Austin
Anchliya A, Ehlig-Economides CA, Jafarpour B (2012) Aquifer management to accelerate CO2 dissolution and trapping. SPE J 17(03):805–816
Aryani A, Mohamed F, Obeidi A, Brahmakulam JV, Ramamoorthy R (2011) Pulsed neutron monitoring of the first CO2 EOR pilot in the Middle East. SPE-141490
Asghari K, Araghi MM, Ahmadloo F, Nakutnyy P (2009) Utilization of CO for improving the performance of waterflooding in heavy oil recovery. Paper presented at the Canadian international petroleum conference. https://doi.org/10.2118/2009-130
Bamberger A, Sieder G, Maurer G (2000) High-pressure (vapor-liquid) equilibrium in binary mixtures of (carbon dioxide + water or acetic acid) at temperatures from 313 to 353 K. J Supercrit Fluids 17(2):97–110
Bhatti AA, Raza A, Mahmood SM, Gholami R (2019) Assessing the application of miscible CO2 flooding in oil reservoirs: a case study from Pakistan. J Pet Explor Prod Technol 9:685–701
Bikkina P, Wan J, Kim Y, Timothy JK, Tokunaga TK (2016) Influence of wettability and permeability heterogeneity on miscible CO2 flooding efficiency. Fuel 166:219–226
Bowker KA, Shuler PJ (1991) Carbon dioxide injection and resultant alteration of the Weber Sandstone, Rangely Field, Colorado. AAPG Bull 75(9):1489–1499
Burton M, Bryant SL (2009) Eliminating buoyant migration of sequestered CO2 through surface dissolution: implementation costs and technical challenges. SPE Reservoir Eval Eng 12(03):399–407
Chang Y-B, Coats BK, Nolen JS (1998) A compositional model for CO2 floods including CO2 solubility in water. SPE Reservoir Eval Eng 1(2):155–160
Chapoy A, Mohammadi A, Chareton A, Tohidi B, Richon D (2004) Measurement and modeling of gas solubility and literature review of the properties for the carbon dioxide–water system. Ind Eng Chem Res 43(7):1794–1802
Christensen R (1961) Carbonated waterflood results–Texas and Oklahoma. Paper presented at the annual meeting of rocky mountain petroleum engineers of AIME. https://doi.org/10.2118/66-ms
Cobanoglu M (2001) A numerical study to evaluate the use of WAG as an EOR method for oil production improvement at B. Kozluca field, Turkey. SPE-72127
Diamond LW, Akinfiev NN (2003) Solubility of CO2 in water from 1.5 to 100 C and from 0.1 to 100 MPa: evaluation of literature data and thermodynamic modelling. Fluid Phase Equilib 208(1–2):265–290
Diaz D, Bassiouni Z, Kimbrell W, Wolcott J (1996) Screening criteria for application of CO2 miscible displacement in waterflooded reservoirs containing light oil. SPE-35431
Dong Y, Dindoruk B, Ishizawa C, Lewis EJ (2011) An experimental investigation of carbonated water flooding. SPE-145380
Duan Z, Sun R, Zhu C, Chou IM (2006) An improved model for the calculation of CO2 solubility in aqueous solutions containing Na+, K+, Ca2+, Mg2+, Cl−, and SO42−. Mar Chem 98(2–4):131–139
Emera M, Sarma H (2006) A genetic algorithm-based model to predict co-oil physical properties for dead and live oil. J Can Pet Technol 47(2):52–61
Enick RM, Klara SM (1990) CO2 solubility in water and brine under reservoir conditions. Chem Eng Commun 90(1):23–33
Ennis-King J, Paterson L (2005) Role of convective mixing in the long-term storage of carbon dioxide in deep saline formations. SPE J 10(3):349–356
Falls A (1986) Analysis of an idealized carbonated waterflood in the South Wasson/Clearfork Formation: Shell Internal Research Report
Figuera LA, Al-basry AH, Al-Hammadi KE, Al-Yafei A, Sakaria D, Tanakov MY (2014) Complex phased development for CO2 EOR in oil carbonate reservoir, Abu Dhabi Onshore. SPE-171967
Flumerfelt R, Li X, Cox J, Hsu WF (1993) A cyclic surfactant-based imbibition/solution gas drive process for low-permeability, fractured reservoirs. SPE-26373
Gao P, Towler B (2012) Integrated investigation of enhanced oil recovery in South Slattery Minnelusa Reservoir, part 2: CO2 miscible injection. Pet Sci Technol 30(24):2543–2551
Gao P, Towler BF, Pan G (2010) Strategies for evaluation of the CO2 miscible flooding process. SPE-138786
Gorell S, Falls A (1986) Status of carbonated waterflooding research: shell internal research report
Grigg RB, Svec RK (2006) CO2 transport mechanisms in CO2/brine coreflooding. SPE-103228
Gui X, Wang W, Gao Q, Yun Z, Fan M, Chen Z (2017) Measurement and correlation of high pressure phase equilibria for CO2 + alkanes and CO2 + crude oil systems. J Chem Eng Data 62(11):3807–3822
Healy R, Holstein E, Batycky J (1994) Status of miscible flooding technology. Paper presented at the 14th world petroleum congress. WPC-26169
Hebach A, Oberhof A, Dahmen N (2004) Density of water + CO2 at elevated pressures: measurements and correlation. J Chem Eng Data 49(4):950–953
Herzog H (2000) The economics of CO2 separation and capture. Open source
Hickok CW, Ramsay Jr HJ (1962) Case histories of carbonated waterfloods in Dewey-Bartlesville field. SPE-333
Holm LW (1959) Carbon dioxide solvent flooding for increased oil recovery. SPE-1250
Izgec O, Demiral B, Bertin H, Akin S (2008) CO2 injection into saline carbonate aquifer formations I: laboratory investigation. Transp Porous Media 72(1):1–24
Jamaluddin A, Kalogerakis N, Chakma A (1991) Predictions of CO2 solubility and CO2 saturated liquid density of heavy oils and bitumens using a cubic equation of state. Fluid Phase Equilib 64:33–48
Jarrell PM, Fox CE, Stein MH, Webb SL (2002) Practical aspects of CO2 flooding. SPE Monogr Ser 22:214–216
Jishun Q, Haishui H, Xiaolei L (2015) Application and enlightenment of CO2 flooding in the United States of America. Pet Explor Dev 42(2):232–240
Johnson W, Macfarlane R, Breston J, Neil D (1952). Laboratory experiments with carbonated water and liquid carbon dioxide as oil recovery agents. Prod Mon 17
Ju B, Wu YS, Qin J, Fan T, Li Z (2012) Modeling CO2 miscible flooding for enhanced oil recovery. Pet Sci 9(2):192–198
Kantzas A, Bryan, J, Taheri S (2012). Fundamentals of fluid flow in porous media. Ch-Pore size distribution. Open Source
Kechut NI, Riazi M, Sohrabi M, Jamiolahmady M (2010) Tertiary oil recovery and CO2 sequestration by carbonated water injection (CWI). SPE-139667
Kechut NI, Sohrabi M, Jamiolahmady M (2011) Experimental and numerical evaluation of carbonated water injection (CWI) for improved oil recovery and CO2 storage. SPE-143005
Kilybay A, Ghosh B, Thomas NC, Aras P (2016) Hybrid EOR technology: carbonated water and smart water improved recovery in oil wet carbonate formation. SPE-182567
Kono F, Kato A, Shimokawara M, Tsushima K (2014) Laboratory measurements on changes in carbonate rock properties due to CO2-saturated water injection. SPE-172013
Lake L, Carey G, Pope G, Sepehrnoori K (1984) Isothermal, multiphase, multicomponent fluid flow in permeable media. Situ. 8(1):1–40
Lindeberg E, Wessel-Berg D (1997) Vertical convection in an aquifer column under a gas cap of CO2. Energy Convers Manag 38:229–234
Liu R (2013) A miscible CO2 injection project and evaluation in Daqing, China. Journal of Petroleum Technology and Alternative Fuels. 4(6):113–118
Liu Y, Hou M, Yang G, Han B (2011) Solubility of CO2 in aqueous solutions of NaCl, KCl, CaCl2 and their mixed salts at different temperatures and pressures. J Supercrit Fluids 56(2):125–129
Luis F, Al Hammadi K, Tanakov M (2016) Case study of CO2 injection to enhance oil recovery into the transition zone of a tight carbonate reservoir. SPE-183203
Mao S, Duan Z, Hu J, Zhang D (2010) A model for single-phase PVTx properties of CO2–CH4–C2H6–N2–H2O–NaCl fluid mixtures from 273 to 1273 K and from 1 to 5000 bar. Chem Geol 275(3–4):148–160
Martin JW (1950) The use of carbon dioxide for increasing the recovery of oil
Martin JW (1951) Additional oil production through flooding with carbonated water. Prod Mon 15(7):18–22
Merchant DH (2010) Life Beyond 80: A look at conventional WAG recovery beyond 80% HCPV injected in CO2 tertiary floods. SPE-139516
Metz B (2007) Special report on carbon dioxide capture and storage. http://www.ipcc.ch/pub/reports.html
Miller JS, Jones RA (1981) A laboratory study to determine physical characteristics of heavy oil after CO2 saturation. SPE-9789
Mosavat N (2014) Utilization of carbonated water injection (CWI) as a means of improved oil recovery in light oil systems: pore-scale mechanisms and recovery evaluation. http://hdl.handle.net/10294/5816
Nevers DN (1964) Calculation method for carbonated water injection. SPE-569
Panteleev V, Tumasyan A (1972) Capillary imbibition of carbonated water into an oil-saturated porous medium. Neft Khoz 5:64–66
Perez J, Poston S, Sharif Q (1992) Carbonated water imbibition flooding: an enhanced oil recovery process for fractured reservoirs. SPE-24164
Perkins E (2003) Fundamental geochemical processes between CO2, water and minerals. Alberta Innovates–Technology Futures
Qu X, Lei Q, He Y, Chen Z, Yu H (2018) Experimental investigation of the EOR performances of carbonated water injection in tight sandstone oil reservoirs. Earth Environ Sci 208:12054
Riazi M, Sohrabi M, Jamiolahmady M, Ireland S (2009) Oil recovery improvement using CO2-enriched water injection. SPE-121170
Riazi M, Sohrabi M, Jamiolahmady M (2011) Experimental study of pore-scale mechanisms of carbonated water injection. Transp Porous Media 86(1):73–86
Saxon Jr J, Breston J, Macfarlane R (1951). Laboratory tests with carbon dioxide and carbonated water as flooding mediums. Prod Mon 16
Sayegh SG, Krause FF, Girard M, DeBree C (1990) Rock/fluid interactions of carbonated brines in a sandstone reservoir: Pembina Cardium, Alberta, Canada. SPE Form Eval 5(4):399–405
Sharma AK, Clements LE (1996) From simulator to field management: optimum WAG application in a West Texas CO2 flood—a case history. SPE-36711
Shiraki R, Dunn TL (2000) Experimental study on water–rock interactions during CO2 flooding in the Tensleep Formation, Wyoming, USA. Appl Geochem 15(3):265–279
Sohrabi M, Riazi M, Jamiolahmady M, Ireland S, Brown C (2008) Carbonated water injection for oil recovery and CO2 storage. Paper presented at the Sustainable energy UK conference: Meeting the Science and Engineering challenge, Oxford, UK
Sohrabi M, Riazi M, Jamiolahmady M, Ireland S, Brown C (2009) Mechanisms of Oil Recovery by Carbonated Water Injection. Presented at the International Symposium of the Society of Core Analysts. Jan-2009
Sohrabi M, Riazi M, Jamiolahmady M, Kechut N, Ireland S, Robertson G (2011) Carbonated water injection (CWI)—a productive way of using CO2 for oil recovery and CO2 storage. Energy Procedia 4:2192–2199
Sohrabi M, Emadi A, Farzaneh SA, Ireland S (2015) A thorough investigation of mechanisms of enhanced oil recovery by carbonated water injection. SPE-175159
Solomon S (2007) Carbon dioxide storage: Geological security and environmental issues–Case study on the sleipner gas field in Norway. Bellona report, 128
Srivastava R, Huang S, Dyer S (1995) Measurement and prediction of PVT properties of heavy and medium oils with carbon dioxide. UNITAR, New York
Stiles L, Magruder J (1992) Reservoir management in the means San Andres Unit. J Pet Technol 44(4):469–475
Summapo S, Srisuriyachai F, Athichanagorn S (2013) Evaluation of CO2 flooding in multi-layered heterogeneous reservoir. Paper presented at the 11th international conference on mining, materials and petroleum engineering, Chiang Mai, Thailand
Takahashi S, Hayashi Y, Takahashi S, Yazawa N, Sarma H (2003) Characteristics and impact of asphaltene precipitation during CO2 injection: an investigative analysis through laboratory tests and compositional simulation. SPE-84895
Valtz A, Chapoy A, Coquelet C, Paricaud P, Richon D (2004) Vapour-liquid equilibria in the carbon dioxide-water system, measurement and modelling from 278.2 to 318.2 K. Fluid Phase Equilib 226:333–344
Van Dijk C (1965) Carbonated water flood Shell Internal Research Report R 1189
Verma MK (2015) Fundamentals of carbon dioxide-enhanced oil recovery (CO2-EOR). US Department of the Interior, US Geological Survey, Washington, DC
Wang Q, Yang S, Han H, Wang L, Qian K, Pang J (2019) Experimental investigation on the effects of CO2 displacement methods on petrophysical property changes of ultra-low permeability sandstone reservoirs near injection wells. Energies 12:327–347
Wellman TP, Grigg RB, McPherson BJ, Svec RK, Lichtner PC (2003) Evaluation of CO2-brine-reservoir rock interaction with laboratory flow tests and reactive transport modeling. SPE-80228
Whitson CH, Brulé MR (2000) Phase behavior. Society of Petroleum Engineer, Richardson
Yuncong G, Mifu Z, Jianbo W, Chang Z (2014) Performance and gas breakthrough during CO2 immiscible flooding in ultra-low permeability reservoirs. Pet Explor Dev 41(1):88–95
Zekri ARY, Shedid SA, Almehaideb RA (2013) Experimental investigations of variations in petrophysical rock properties due to carbon dioxide flooding in oil heterogeneous low permeability carbonate reservoirs. J Pet Explor Prod Technol 3:265–277
Zou J, Liao X, Shen X (2018) An experimental study on carbonated water injection of core samples from tight oil reservoirs from ordos basin. Paper presented at the SPE-191474
Acknowledgements
The authors acknowledge the Khalifa University of Science and Technology for the support and encouragement provided in undertaking this study.
Author information
Authors and Affiliations
Contributions
GB, SJ and AA contributed to investigation and resources; GB, SJ and AA contributed to original draft preparation; GB and AA contributed to writing, review and editing; and GB contributed to supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bisweswar, G., Al-Hamairi, A. & Jin, S. Carbonated water injection: an efficient EOR approach. A review of fundamentals and prospects. J Petrol Explor Prod Technol 10, 673–685 (2020). https://doi.org/10.1007/s13202-019-0738-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-019-0738-2