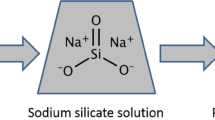
Abstract
Hydrochloric (HCl) acid is the most common stimulating fluid used in acidizing job due to its strong acidic property and low cost to create or enlarge existing wormhole within the reservoir. However, the HCl acid has rapid reaction with carbonate reservoir, and it is causing surface dissolution of the rock and lowering the penetration into the formation. Recent studies have shown the addition of nickel nanoparticles as catalyst to handle the problems in HCl acidizing. The nanoparticles are high-performance catalyst due to their high ratio of surface area to volume. The proposed method in this research is to mix the nanoparticles with the carbonate formation prior to the acid injection into the formation. The efficiency of the nanoparticles as catalyst depends on the thermodynamics property, which is surface energy of the materials used. The surface energy reduces as the size of particles become smaller. However, the effect of surface energy become insignificant on nanoparticles due to the small particles sizes, and the surface energy is based on the individual energy of the particles. Therefore, this research investigates the efficiency of silica, aluminum oxide, and zinc oxide besides nickel nanoparticles based on their thermodynamics property in accelerating the conversion of CO2 gas into carbonic acid. The approach consists of investigating the efficiency of nanoparticles in different concentrations of carbonate and mass of nanoparticles. Suitable nanoparticles are proposed based on efficiency and cost in retarding the HCl reactivity and rapid formation of in situ carbonic acid. The concentration of carbonic acid (H2CO3), bicarbonate ion (HCO3−), and carbonate ion (CO32−) is analyzed based on Henry’s law of solubility. The result shows that the silica has the best efficiency as catalyst in 6700 ppm Na2CO3 solution due to its high stability and dispersion in aqueous solution. The silica engages into rapid dissociation of water molecules and bind with OH− group to react with CO2 gas and form HCO3−. The nanoparticles reduce the reactivity of HCl through conversion of bicarbonate ions. However, ZnO gives better efficiency in 17,000 ppm of Na2CO3. The efficiency of silica in this concentration increased at 0.7 g, proving the minimum amount required as catalyst. In contrast, ZnO and Al2O3 have lower efficiency as acid retarder since changes in pH values affect the performance of the nanoparticles. The surface charge demonstrated by ZnO and Al2O3 depends on pH changes which makes these nanoparticles to perform inefficiently. The silica is chosen as the best catalyst due to high efficiency versus cost ratio.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbonates reservoirs possess heterogeneous properties in both horizontal and vertical directions, with natural fractures, permeability barriers, and huge array of porosity types (Izgec et al. 2008). Matrix acidizing is executed to create or enlarge existing wormhole by using hydrochloric acid (HCl) due to high dissolution into carbonate rock and lower cost (Mahmoud et al. 2010; Chang et al. 2008; Bartko et al. 2007; Huang et al. 2000). However, HCl has high corrosive property that tends to corrode well tubular and rapid reaction at the surface of carbonate rock (Izgec et al. 2008; Chang et al. 2008). The release of CO2 gas by-product between the reaction of HCl and carbonate rock has high adhesive force and forms barrier between acid and rock due to high adhesive force (Raj and Pal 2014). Expensive corrosion tubulars are unsuccessful in protecting the well at high temperature for longer time. The HCl acid also has high reaction with carbonate rock, which creates surface dissolution and low penetration (Raj and Pal 2014). Organic additives and modified acids are added to retard and reduce corrosiveness of HCl (Huang et al. 2000; Nasr-El-Din and Al-Mohammed 2006; Zakaria and Nasr-El-Din 2016; Amro 2006). These modified acids are used to corrosion inhibitor for wells besides improving the acid penetration into the formation. The drawback of organic acid is low dissolving capacity in the HCl, and longer time is taken to design suitable organic acid (Raj and Pal 2014). In conjunction with few researches on incorporating nanoparticles in alleviating these problems, this study proposes a detailed investigation on aluminum oxide (Al2O3), zinc oxide (ZnO), and silica (SiO2) nanoparticles as catalysts to retard the HCl reaction and accelerate the in situ conversion of CO2 gas into carbonic acid. The nanoparticles are high-performance catalyst due to their high ratio of surface area to volume. The objectives of this research are to (1) determine the efficiency of HCl reaction in carbonate with and without the presence of nanoparticles, (2) analyze and identify suitable nanoparticles in catalytic reaction to enhance the matrix acidizing, (3) interpret contributing properties of nanoparticles in the catalytic reaction, and (4) propose suitable nanoparticles based on cost and efficiency.
Background
Carbonate reservoirs and matrix acidizing
Carbonate reservoir faces continuous diagenesis due to high-pressure increase on burial and results into physical compacted and natural fractures (London 2017). The severity of fractures is further increased by drilling and completion processes (Morsy et al. 2015). These complexities of carbonate formation are (1) heterogeneous in vertical and horizontal reservoir property, (2) natural fractures and permeability barriers, and (3) porosity ranging from intercrystalline to massive vugular and cavernous porosity (Raj and Pal 2014). The severity of fractured pore networks in carbonate reservoir increases the barrier for fluid flow (Jones 1975; Popov et al. 2007; Philip et al. Philip et al. 2005). Matrix acidizing is introduced by injecting acid stimulated fluid, i.e., HCl, into reservoir below fracture pressure to enhance reservoir conductivity and improve the flow rate through dissociation of the reservoir rock (Izgec et al. 2008; Chang et al. 2008; Raj and Pal 2014; Morsy et al. 2015; Company 2013; Gomez 2006; Kalia and Balakotaiah 2010; Al-Hadhrami and Blunt 2000; Rahim et al. 2014; Runtuwene et al. 2010). The HCl acid spreads efficiently in carbonate formation compared to homogeneous reservoir due to the flow properties, which varies from small (micron) to larger (centimeter) scale (Izgec et al. 2008). The wormhole operates to transport reservoir fluid within matrix into the wellbore by overcoming formation damage and low permeability (Rahim et al. 2014). This effectiveness is limited as HCl acid has strong acidic property, which corrodes the well tubular and reacts almost instantly with carbonate rock causing surface dissolution of formation (Chang et al. 2008). The instantaneous reaction fails to meet the main objective of matrix acidizing to create deeper penetration into the formation. CO2, which is a by-product of HCl reaction with carbonate formation as shown in Eq. (1), caused formation barrier due to high adhesive force (Raj and Pal 2014; Frenier and Hill 2002).
Carbonic acids are common alternatives to lower the reaction of HCl besides reducing the corrosiveness on well tubulars (Chang et al. 2008; Huang et al. 2000; Amro 2006; Buijse et al. Buijse et al. 2004; Yang et al. 2012). Despite the efficiency, carbonic acid buffers the pH of organic acid which causes incomplete dissociation of HCl. This defeats the sole reason of HCl usage since matrix acidizing cost increases as carbonic acid is much expensive compared to HCl for equivalent amount of rock dissolved (Chang et al. 2008; Raj and Pal 2014). Modified acid is investigated on improving the efficiency of matrix acidizing (Zakaria and Nasr-El-Din 2016; Al Otaibi et al. 2013; Carpenter 2014). The study does not comprehend the issue of CO2 gas release from the reaction between HCl acid and carbonate rock.
Nanoparticles in catalytic reaction
To alleviate these problems, studies and researches have included nanoparticles in oil recovery process to improve the oil flow (El-Diasty and Aly 2015; Ogolo et al. 2012; Bennetzen and Mogensen 2014; Idogun et al. 2016; Zhang et al. 2010; Negin et al. 2016). Nanoparticles are further developed as catalyst to solve the rapid reaction of HCl and CO2 gas production in matrix acidizing for carbonate reservoir (Raj and Pal 2014). The proposed research has used nickel nanoparticles to accelerate the conversion of in situ organic acid from CO2 gas, which reduces the need of expensive well tubulars and organic retarders (Ogolo et al. 2012; Bhaduri et al. 2015). Throughout the acidizing job, the nanoparticles do not undergo any changes physically and remain the same after the reaction has occurred. Once CO2 gas is released, NPs get into action as shown in Fig. 1.

(Raj and Pal 2014)
Conversion of CO2 gas into carbonic acid by nanoparticles
As soon as the reaction between HCl acid and carbonate rock releases CO2 gas, the nanoparticles convert the gaseous CO2 into carbonic acid (aqueous CO2) based on Eq. (2).
Referring to Eq. (3), the aqueous CO2 will then bind with water molecules to produce strong carbonic acid.
The generated carbonic acid will undergo further dissociation into hydrogen and bicarbonate ion in case of weaker concentration as shown in Eq. (4).
In case of weaker ionic bonding, the nanoparticles will further break the bicarbonate ion into hydrogen and carbonate ions based on Eq. (5).
The catalytic reaction repeats as a cycle to ensure constant formation of carbonic acid while CO2 gas is released. Catalyst is preferred to convert CO2 gas into carbonic acid as lower activation energy is required. The efficiency of nanocomposite metal as catalyst depends on three criteria: (1) high surface area exposed to the reaction, (2) size and distribution of NPs should be uniform, and (3) nanoparticles presented in the substrate should be easily accessible to various chemical reagents (Patel et al. 2007). Nanoparticles act as good catalyst due to ratio of surface area to volume. Diffusion and reaction rate at nanoscale, nanomaterials, and nanodevices occur more rapidly compared to larger size of material (Ogolo et al. 2012; Bennetzen and Mogensen 2014; Chaturvedi et al. 2012). The efficiency of nanoparticles is a function of on their hydrophobicity (contact angle), size, shape, and degree of agglomeration since these parameters influence the behavior of NPs at air/water interface (Blute et al. 2009). This research requires further improvement since nickel nanoparticles are chosen due to its wide usage in industries as catalyst. Nickel also has strong electromagnetic property, which creates adhesive force, which causes blockage in pore channels (Wu and Chen 2003).
Method
Experimental setup
Figure 2 shows the experimental setup by wrapping 2-L beaker with cotton wool and aluminum foil to prevent heat lost to the surrounding. An airtight cap is made and sealed with high-density polyethylene (HDPE) materials to avoid air leakage. Four (4) holes are then drilled to place pH meter, gas pressure sensor, K-type thermowires, and burette. Silicon sealants and grease are applied to enclose the gaps. Leak detector is then poured around the cap as shown in Fig. 3 to observe any formation of bubble as an indicator of leakage.
Calibrations are done on gas pressure sensor, pH meter, and K-type thermometer prior to the acidizing runs.
Materials
The diluted sodium carbonate (Na2CO3) at 6700 ppm is obtained by mixing 600 ml of distilled water with 4 g of Na2CO3 powder. Meanwhile, the concentration of diluted sodium carbonate at 17,000 ppm is obtained by mixing 600 ml with 7 g of Na2CO3 powder. Initial concentration of hydrochloric (HCl) acid is diluted to achieve concentration at 5% based on Eqs. 7 and 8.
where Cp is concentration of HCl acid after dilution, Cm is molarity of HCl acid after dilution, mol, M is molecular weight of HCl, g/mol, and ρ is density of HCl acid, g/ml. Acidizing runs are conducted with and without the presence of nanoparticles. Manipulated variables used in this experiment are mentioned in Table 1.
The nanoparticles tabulated in Table 1 were chosen considering their proven performance as catalysts. Nickel oxide was studied by Raj and Pal (2014), and the outcome is quite promising.
Concentration of Carbonic Acid, Bicarbonate Ion and Carbonate Ion
Based on pH values measured in each run, the dissociation of hydrogen ions is calculated as shown in Eq. (8)
The concentration of carbonic acid (H2CO3) is calculated based on the partial pressure of CO2 gas, atm, and Henry’s law of solubility of CO2 in water, K0, L.atm/mol as shown in Eq. (9)
In Eq. (10), the concentration of bicarbonate ion (HCO3−) is calculated based on concentration of hydrogen ion and carbonic acid with Henry’s law of solubility of HCO3− ions, K1 L.atm/mol
Then, the concentration of carbonate ion (CO32−) is determined by using Eq. (11)
The study is using diluted sodium carbonate rather than carbonate core. This simplifies the measurement and hence the calculation.
Results and discussion
Acidizing runs with nanoparticles in 6700 ppm Na2CO3
Based on Fig. 4, SiO2 gives lower depletion in pH values for 0.01 g of nanoparticles in 6700 ppm concentration of diluted Na2CO3 solution. Meanwhile, the pH values dropped immediately after 100 s for Al2O3 NPs, while ZnO NPs gave pH depletion trend line similar to acidizing run without NPs.
In Fig. 5, SiO2 nanoparticles show slowest depletion in pH, while ZnO nanoparticles show rapid dissociation of HCl compared to Al2O3. Initially, ZnO nanoparticles give minimal reduction in pH till the values suddenly dropped below 3 at 2.5 min. Al2O3 nanoparticles show gradual decrease in pH before the reaction becomes slower.
Referring to Fig. 6, SiO2 remained as the best catalyst to retard the reactivity of HCl as the amount of nanoparticles is increased to 0.4 g. ZnO gives almost similar reduction in pH as in SiO2, while rapid drop in pH values is observed for Al2O3 nanoparticles.
The SiO2 gives minimal reduction in pH when 0.7 g of nanoparticles is added into 6700 ppm Na2CO3 as shown in Fig. 7. The pH values reduced slightly for Al2O3 initially for 2 min and generated inconsistent pattern afterward due to stability issue. ZnO nanoparticles give better reaction compared to Al2O3 in reducing the HCl reaction.
Overall for 6700 ppm concentration of diluted sodium carbonate solution, silica shows the best efficiency as acid retarder, followed by zinc oxide and aluminum oxide nanoparticles. Silica has shown consistent performance in reducing the HCl reaction with sodium carbonate as the amounts are increased.
Acidizing runs with nanoparticles in 17,000 ppm Na2CO3
Based on Fig. 8, ZnO gives minimal reduction in pH of the solution for 0.01 g of nanoparticles added in 17,000 ppm Na2CO3. In contrast with acidizing runs for 6700 ppm Na2CO3, Al2O3 gives better performance as catalyst compared to silica nanoparticles. SiO2 nanoparticles give constant depletion in pH before the values decrease gradually after 100 s. For lower amount of nanoparticles in 17,000 ppm, ZnO gives the best efficiency as catalyst.
All the three nanoparticles gave low performance as the amount is increased to 0.1 g based on Fig. 9. For the first 130 s, ZnO, SiO2, and Al2O3 nanoparticles show constant pH reading. Aluminum oxide reduced the pH values first, followed by SiO2 and ZnO. ZnO gives constant pH of solution until the values dropped at 240 s. The depletion of pH is constant after 150 s for SiO2, while Al2O3 shows better performance than ZnO after 200 s. Overall, Silica gave consistent performance compared to ZnO and Al2O3 although the nanoparticles did not retard the reactivity of HCl with diluted sodium carbonate solution.
Silica shows slight reduction in pH compared to acidizing runs without nanoparticles in Fig. 10. ZnO gives rapid depletion in pH values compared to silica. The lowest efficiency of catalytic reaction is demonstrated by Al2O3. By increasing the amount of silica in 17,000 ppm Na2CO3, the efficiency of the nanoparticles as catalyst can be increased.
The pH values remained almost constant with the presence of 0.7 g silica in 17,000 ppm Na2CO3 solution as shown in Fig. 11. Al2O3 demonstrated consistent pH trend for the first 180 s before the values dropped rapidly. Initially, ZnO gave rapid drop in pH values before the reduction becomes minimal after 200 s.
Performance of nanoparticles in 6700 ppm and 17,000 ppm Na2CO3
The efficiency of metal oxide nanoparticles as catalyst depends on the surface charge demonstrated as pH of solution changes. Based on thermodynamics law, the surface energy decreases as size of particles decreases. However, the effect of surface charge becomes insignificant as size of particles reduces in nanoscale. The surface charge demonstrated by nanoparticles is larger compared to bulk material due to size-dependent lattice parameter (Nanda et al. 2003). The reduction in particle size causes the surface energy demonstrated by individual particles in nanoscale become significant and yields larger energy.
Initially, Al2O3 nanoparticles show negative charge since the pH is high and attracted more H+ from HCl, which resulted in low reaction with Na2CO3. As the solution reached neutral pH, the surface charge for these nanoparticles becomes zero and caused HCl to dissociate H+ rapidly (Gulicovski et al. 2008). This solution turns acidic immediately due to abundance of hydrogen ions reacting with Na2CO3. The reaction is further accelerated by Al2O3 as the surface charge becomes positive in low pH. At this point, the nanoparticles attract hydroxyl (OH−) ion from water molecules to form HCO3− through bonding with CO2 gas.
Meanwhile, ZnO nanoparticles in water show a different property as the surface of the oxide is hydrolyzed and forms buildup of hydroxide layer (Reed 1986; Xu and Wang 2011). The changes in pH are minimal since the dissociation of ZnO into Zn2+ is most stable due to ionic equilibrium between zinc ion and zinc hydroxide. As ZnO binds with hydroxyl group from water in the beginning, the pH values drop gradually. Between pH at 4 and 6 for each case, ZnO gives unstable electrostatic charge due to buildup of unstable colloidal particles of Zn(OH)2 as a result of ionization (Degen and Kosec 2000). The depletion of ph becomes constant at the end of each run since ZnO nanoparticles have achieved stable surface charge. It is observed that:
Nanoparticles acts as good corrosion inhibitor
Nanoparticles are capable to be good retarder for acidic mud
Nanoparticles are affected by thermal condition
Overall, SiO2 shows the best performance as catalyst due to its stability in dispersing within the sodium carbonate solution (Kissa 1999; Asay and Kim 2005; Yalamanchili et al. 1996; Metin et al. 2011; Zhuravlev 1987). Once the nanoparticles are mixed with diluted sodium carbonate solution, silica immediately engages with dissociation of hydrogen and hydroxyl ions from water molecules to form silanol group (Si–OH) (Hofmann and Wilm 1934). The silanol group has strong hydrophilic properties that binds into hydrogen (H+) ions which were dissociated from HCl to prevent the ions from fully spent. The reaction is further improved due to larger surface area of hydrophilic tail exposed to the reaction between HCl and sodium carbonate.
Efficiency versus cost ratio of nanoparticles in 6700 ppm and 17,000 ppm Na2CO3
For the acidizing run without the addition of nanoparticles, the total bicarbonate ion remained in the solution is divided with cost of HCl to yield efficiency versus cost ratio (mol/USD). The efficiency of nanoparticles in both cases is evaluated based on the remaining concentration bicarbonate ion in the solution and cost of each nanoparticle.
Based on Fig. 12, silica gives highest efficiency versus cost ratio. Silica shows higher concentration of HCO3− ion remaining in the solution due to rapid ionization between water and CO2 gas which was released from the reaction between HCl and Na2CO3. Low conversion of CO2 gas into carbonic acid since HCl acid is not fully spent in the reaction. ZnO and Al2O3 nanoparticles show low efficiency versus cost ratio. Lower amount of bicarbonate ions remaining in the solution for these nanoparticles indicates that abundance of CO2 gas has been released as rapid result between HCl and Na2CO3.
Referring to Fig. 13, Al2O3 and ZnO show the best efficiency versus cost. These values dropped gradually in 0.1 g of nanoparticles added into Na2CO3 solution. However, silica shows slightly improved efficiency compared to acidizing run in 0.01 g. The efficiency of Al2O3 is highest in 0.4 g of nanoparticles added in the solution, whereas silica and zinc oxide show similar efficiency. For 0.7 g of nanoparticles in 17000 ppm of Na2CO3, silica shows the best efficiency compared to ZnO and Al2O3. Overall, the performance of Al2O3 fluctuates for each case; meanwhile, ZnO nanoparticles demonstrate reducing efficiency. Silica, on the other hand, gives improving efficiency as the amount of nanoparticles in Na2CO3 solution is increased.
Conclusion
The efficiency of nanoparticles in catalyzing carbon dioxide gas into carbonic acid is further studied by using SiO2, Al2O3, and ZnO in varying concentration of Na2CO3. Concentration of carbonic acid (H2CO3), bicarbonate ion (HCO3−), and carbonate ion (CO32−) is analyzed based on Henry’s law of solubility. Overall, silica shows the best efficiency as catalyst in 6700 ppm Na2CO3 solution due to high stability and dispersion in aqueous solution. Silica engages into rapid dissociation of water molecules and binds with OH− group to react with CO2 gas and form HCO3−. The nanoparticles reduce the reactivity of HCl through conversion of bicarbonate ions. However, ZnO gives better efficiency in 17 000 ppm of Na2CO3. The efficiency of silica in this concentration increased at 0.7 g, proving the minimum amount required as catalyst. In contrast, ZnO and Al2O3 have lower efficiency as acid retarder since changes in pH values affect the performance of the nanoparticles. The surface charge demonstrated by ZnO and Al2O3 depends on pH changes which make these nanoparticles to perform inefficiently. Overall, silica is chosen as the best catalyst due to high efficiency versus cost ratio as illustrated in Figs. 12 and 13.
References
Al-Hadhrami HS, Blunt MJ (2000) Thermally induced wettability alteration to improve oil recovery in fractured reservoirs. Soc Pet Eng. https://doi.org/10.2118/59289-ms
Al Otaibi FM, Kokal SL, Chang Y, AlQahtani JF, AlAbdulwahab AM (2013) Gelled emulsion of CO-water-nanoparticles. Soc Pet Eng. https://doi.org/10.2118/166072-ms
Amro MM (2006) Extended matrix acidizing using polymer-acid solutions. Soc Pet Eng. https://doi.org/10.2118/106360-ms
Asay DB, Kim SH (2005) Evolution of the adsorbed water layer structure on silicon oxide at room temperature. J Phys Chem B 109:16760–16763
Bartko KM, Chang FF, Behrmann LA, Walton IC (2007) Effective matrix acidizing in carbonate reservoir: Does perforating matter? Soc Pet Eng. https://doi.org/10.2118/105022-ms
Bennetzen MV, Mogensen K (2014) Novel applications of nanoparticles for future enhanced oil recovery. Int Pet Technol Conf. https://doi.org/10.2523/iptc-17857-ms
Bhaduri GA, Alamiry MAH, Šiller L (2015) Nickel nanoparticles for enhancing carbon capture. J Nanomater. https://doi.org/10.1155/2015/581785
Blute I, Pugh RJ, van de Pas J, Callaghan I (2009) Industrial manufactured silica nanoparticle sols. 2: surface tension, particle concentration, foam generation and stability. Colloids Surf A 337:127–135
Buijse M, de Boer P, Breukel B, Burgos G (2004) Organic acids in carbonate acidizing. Soc Pet Eng. https://doi.org/10.2118/82211-pa
Carpenter C (2014) Gelled emulsions of CO2, water, and nanoparticles. Soc Pet Eng. https://doi.org/10.2118/0714-0135-jpt
Chang FF, Nasr-El-Din HA, Lindvig T, Qui XW (2008) Matrix acidizing of carbonate reservoirs using organic acids and mixture of HCl and organic acids. Soc Pet Eng. https://doi.org/10.2118/116601-ms
Chaturvedi S, Dave PN, Shah NK (2012) Applications of nano-catalyst in new era. J Saudi Chem Soc 16:307–325
Company CR (2013) Matrix acidizing 101: the history, process safety behind a widely used technique. Retrieved from http://content.collierresources.com/resources/Acidizing_01.pdf
Degen A, Kosec M (2000) Effect of pH and impurities on the surface charge of zinc oxide in aqueous solution. J Eur Ceram Soc 20:667–673
El-Diasty AI, Aly AM (2015) Understanding the mechanism of nanoparticles applications in enhanced oil recovery. Soc Pet Eng. https://doi.org/10.2118/175806-ms
Frenier WW, Hill DG (2002) Effect of acidizing additives on formation permeability during matrix treatments. Soc Pet Eng. https://doi.org/10.2118/73705-ms
Gomez JN (2006) Design, set-up, and testing of a matrix acidizing apparatus. MSc thesis submitted to Texas A&M University
Gulicovski JJ, Čerović LS, Milonjić SK (2008) Point of zero charge and isoelectric point of alumina. Mater Manuf Processes 23:615–619
Hofmann EK, Wilm UD (1934) Röntgeno-graphische und kolloidchemische Untersuchungen über Ton. Angew Chem 47:539–558
Huang T, Ostensen L, Hill AD (2000) Carbonate matrix acidizing with acetic acid. Soc Pet Eng. https://doi.org/10.2118/58715-ms
Idogun AK, Iyagba ET, Ukwotije-Ikwut RP, Aseminaso A (2016) A review study of oil displacement mechanisms and challenges of nanoparticle enhanced oil recovery. Soc Pet Eng. https://doi.org/10.2118/184352-ms
Izgec O, Keys RS, Zhu D, Hill AD (2008) An integrated theoretical and experimental study on the effects of multiscale heterogeneities in matrix acidizing of carbonates. Soc Pet Eng. https://doi.org/10.2118/115143-ms
Jones FO (1975) A laboratory study of the effects of confining pressure on fracture flow and storage capacity in carbonate rocks. Soc Pet Eng. https://doi.org/10.2118/4569-pa
Kalia N, Balakotaiah V (2010) Wormholing in perforated completions. Soc Pet Eng. https://doi.org/10.2118/127347-ms
Kissa E (1999) Dispersions: characterizations testing, and measurement. Marcel Dekker, New York
London RLIC (2017) wwwf.imperial.ac.uk
Mahmoud MA, Nasr-El-Din HA, De Wolf C, LePage JN (2010) An effective stimulation fluid for deep carbonate reservoirs: a core flood study. Soc Pet Eng. https://doi.org/10.2118/131626-ms
Metin CO, Lake LW, Miranda CR, Nguyen QP (2011) Stability of aqueous silica nanoparticle dispersions. J Nanopart Res 13:839–850
Morsy S, Hetherington CJ, Sheng JJ (2015) Effect of low-concentration HCl on the mineralogy, physical and mechanical properties, and recovery factors of some shales. J Unconv Oil Gas Resour 9:94–102
Nanda KK, Maisels A, Kruis FE, Fissan H, Stappert S (2003) Higher surface energy of free nanoparticles. Phys Rev Lett 91:106102
Nasr-El-Din HA, Al-Mohammed AM (2006) Reaction of calcite with surfactant-based acids. Soc Pet Eng. https://doi.org/10.2118/102838-ms
Negin C, Ali S, Xie Q (2016) Application of nanotechnology for enhancing oil recovery: a review. Petroleum 2(4):324–333
Ogolo NA, Olafuyi OA, Onyekonwu MO (2012) Enhanced oil recovery using nanoparticles. Soc Pet Eng. https://doi.org/10.2118/160847-ms
Patel AC, Li S, Wang C, Zhang W, Wei Y (2007) Electrospinning of porous silica nanofibers containing silver nanoparticles for catalytic applications. Chem Mater 19:1231–1238
Philip ZG, Jennings JW, Olson JE, Laubach SE, Holder J (2005) Modeling coupled fracture-matrix fluid flow in geomechanically simulated fracture networks. Soc Pet Eng. https://doi.org/10.2118/77340-pa
Popov P, Qin G, Bi L, Efendiev Y, Ewing RE, Kang Z, Li J (2007) Multiscale methods for modeling fluid flow through naturally fractured carbonate karst reservoirs. Soc Pet Eng. https://doi.org/10.2118/110778-ms
Rahim Z, Al-Anazi H, Ahmed M, Al-Kanaan A, El-Mofty W (2014) Matrix acidizing innovation surpasses competing methods in Saudi carbonate. Soc Pet Eng. https://doi.org/10.2118/0514-0032-jpt
Raj N, Pal TV (2014) Enhancing efficiency of HCl based stimulating fluids by creating in-situ carbonic acid using nickel nanoparticles. International Petroleum Technology Conference. https://doi.org/10.2523/iptc-17814-ms
Reed JS (1986) Introduction to the principles of ceramic processing. Wiley, Hoboken
Runtuwene M, Fasa MH, Rachmawati FD, Wijanarko M, Kadarsyah A, Komalasari N, Gurnito N (2010) Crosslinked acid as an effective diversion agent in matrix acidizing. Soc Pet Eng. https://doi.org/10.2118/133926-ms
Wu SH, Chen DH (2003) Synthesis and characterization of nickel nanoparticles by hydrazine reduction in ethylene glycol. J Colloid Interface Sci 259:282–286
Xu S, Wang ZL (2011) One-dimensional ZnO nanostructures: solution growth and functional properties. Nano Res 4:1013–1098
Yalamanchili MR, Atia AA, Miller JD (1996) Analysis of interfacial water at a hydrophilic silicon surface by in-situ FTIR/internal reflection spectroscopy. Langmuir 12:4176–4184
Yang F, Nasr-El-Din HA, Harbi BA (2012) Acidizing sandstone reservoirs using HF and organic acids. Soc Pet Eng. 1:1. https://doi.org/10.2118/157250-ms
Zakaria AS, Nasr-El-Din HA (2016) A novel polymer-assisted emulsified-acid system improves the efficiency of carbonate matrix acidizing. Soc Pet Eng. https://doi.org/10.2118/173711-pa
Zhang T, Davidson D, Bryant SL, Huh C (2010) Nanoparticle-stabilized emulsions for applications in enhanced oil recovery. Soc Pet Eng. https://doi.org/10.2118/129885-ms
Zhuravlev LT (1987) Concentration of hydroxyl groups on the surface of amorphous silicas. Langmuir 3:316–318
Acknowledgements
The authors wish to acknowledge the Petroleum Engineering Department and the Institute of Hydrocarbon Recovery of Universiti Teknologi PETRONAS for the support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Selvaraj, G., Maulianda, B., Wee, S.C. et al. Experimental study of nanoparticles as catalyst in enhancing matrix acidizing for carbonate reservoir. J Petrol Explor Prod Technol 10, 1145–1153 (2020). https://doi.org/10.1007/s13202-019-0684-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-019-0684-z